Master cGMP Regulations: Key Steps for Compliance Success

Overview
This article delineates the essential steps for achieving compliance with current Good Manufacturing Practices (cGMP), underscoring the critical role of quality management systems, personnel training, and meticulous documentation. It elucidates actionable strategies, including:
- Conducting gap analyses
- Developing standard operating procedures
- Fostering open communication with regulatory bodies
These measures are designed to enhance product safety and ensure adherence to stringent regulatory standards. By implementing these practices, organizations can effectively navigate compliance challenges and elevate their operational excellence.
Introduction
Navigating the intricate landscape of current Good Manufacturing Practices (cGMP) is essential for organizations within the pharmaceutical industry, where the imperatives of quality and safety reign supreme. This article explores the critical steps necessary for achieving cGMP compliance, providing invaluable insights into the processes that guarantee products adhere to rigorous regulatory standards. Yet, as regulations evolve and compliance challenges persist, the pressing question remains: how can companies effectively implement these practices and avoid falling behind in a competitive environment?
Understand Current Good Manufacturing Practices (cGMP)
The cgmp regulations are essential guidelines enforced by the FDA, ensuring that pharmaceutical products are consistently produced and controlled in accordance with stringent quality standards. These regulations encompass all facets of production, from raw materials to the facilities and equipment utilized, guaranteeing that products are safe for human consumption. Key components of cGMP include:
- Quality Management Systems: A robust quality management system is vital for maintaining compliance. Organizations must implement comprehensive frameworks that facilitate continuous monitoring and improvement of processes. This is highlighted by the FDA's New Inspection Protocol Project (NIPP), which aims to enhance inspection efficiency and quality management in pharmaceutical manufacturing.
- Personnel Training: Adequate training in cGMP principles is crucial for all employees to ensure compliance with regulations. Fostering a culture of quality that emphasizes continuous improvement can significantly enhance compliance outcomes. This is evidenced by the rise in non-compliant samples, which reached 35% in fiscal year 2021, up from 16% the previous year.
- Facility Requirements: Manufacturing facilities must comply with specific design and operational standards to prevent contamination and maintain product integrity. The FDA's enforcement actions in 2025 underscore the importance of adhering to these standards, as numerous sites received 'Import Alerts' for non-compliance.
Understanding these components is essential for any organization seeking to successfully navigate the complexities of cgmp regulations and ensure the safety and effectiveness of their pharmaceutical products.
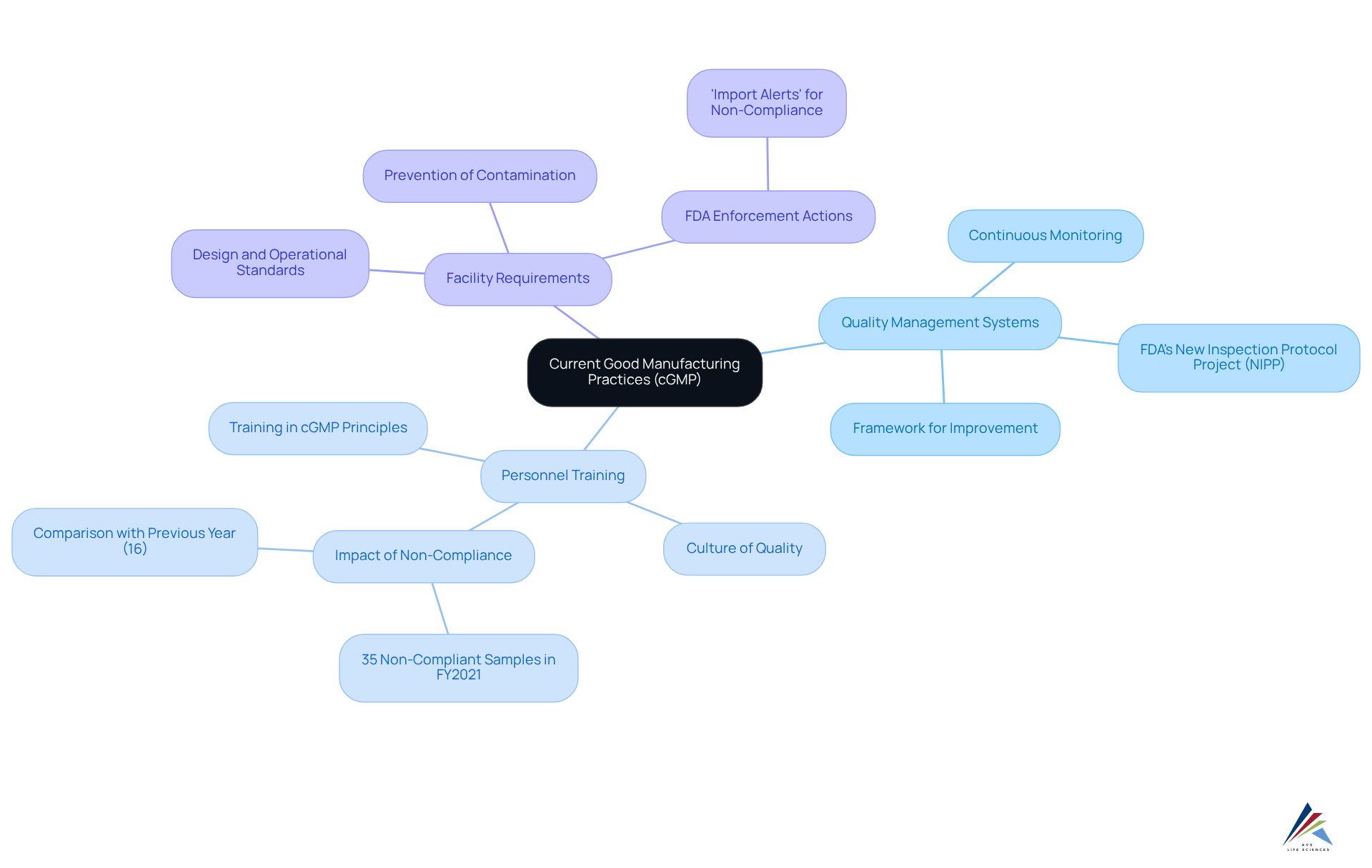
Implement Key Steps for cGMP Compliance
To successfully implement cGMP compliance, adhere to these essential steps:
- Conduct a Gap Analysis: Systematically evaluate current practices against cGMP requirements to identify adherence gaps. This analysis is crucial; missing even a single guidance document can significantly delay progress compared to competitors. Engaging with AVS Life Sciences provides tailored consulting solutions that address gaps in compliance with cGMP regulations.
- Develop a Quality Management System (QMS): Establish a robust QMS that encompasses policies, procedures, and records to ensure adherence to regulatory standards. A strong QMS is vital for ensuring product safety, efficacy, and compliance with cGMP regulations throughout the product lifecycle. Companies embracing digital transformation have reported up to 80% reductions in documentation time, enhancing efficiency and data integrity. AVS Life Sciences offers comprehensive GXP regulatory services to support the development of an effective QMS.
- Train Employees: Regular instruction on cGMP principles and updates is essential to ensure that all staff members understand their responsibilities in upholding standards. With 40% of experienced quality professionals expected to retire by 2030, is increasingly critical to mitigate potential staffing shortages and ensure compliance with cGMP regulations. AVS Life Sciences can assist in creating training programs tailored to your organization's needs.
- Establish Standard Operating Procedures (SOPs): Create SOPs for all critical processes to standardize operations and ensure consistency. This practice minimizes risks associated with manufacturing and enhances overall quality. AVS Life Sciences can help develop SOPs that align with industry best practices.
- Implement Quality Control Measures: Regularly test and validate processes to confirm they meet established quality standards. This proactive approach is essential, as non-compliance with cGMP regulations can lead to severe consequences, such as product recalls and legal penalties. AVS Life Sciences offers quality adherence and validation solutions to ensure your processes meet regulatory expectations.
- Conduct Internal Audits: Schedule regular audits to evaluate adherence and identify areas for enhancement. These audits are crucial for sustaining a proactive strategy towards cGMP regulations, as quality culture shortcomings are identified as a root cause in approximately 40% of major regulatory actions. AVS Life Sciences offers GMP audits that focus on key areas such as APIs, drug products, and testing facilities.
- Engage with Regulatory Bodies: Maintain open communication with regulatory agencies to stay informed about changes in cGMP regulations. With more than 230 new or modified cGMP regulations released since 2020, remaining informed is essential for success in adhering to these regulations. AVS Life Sciences can facilitate these communications to ensure your organization remains compliant with the latest regulations.
For more detailed guidance and to initiate your journey with AVS Life Sciences, refer to our user manual.
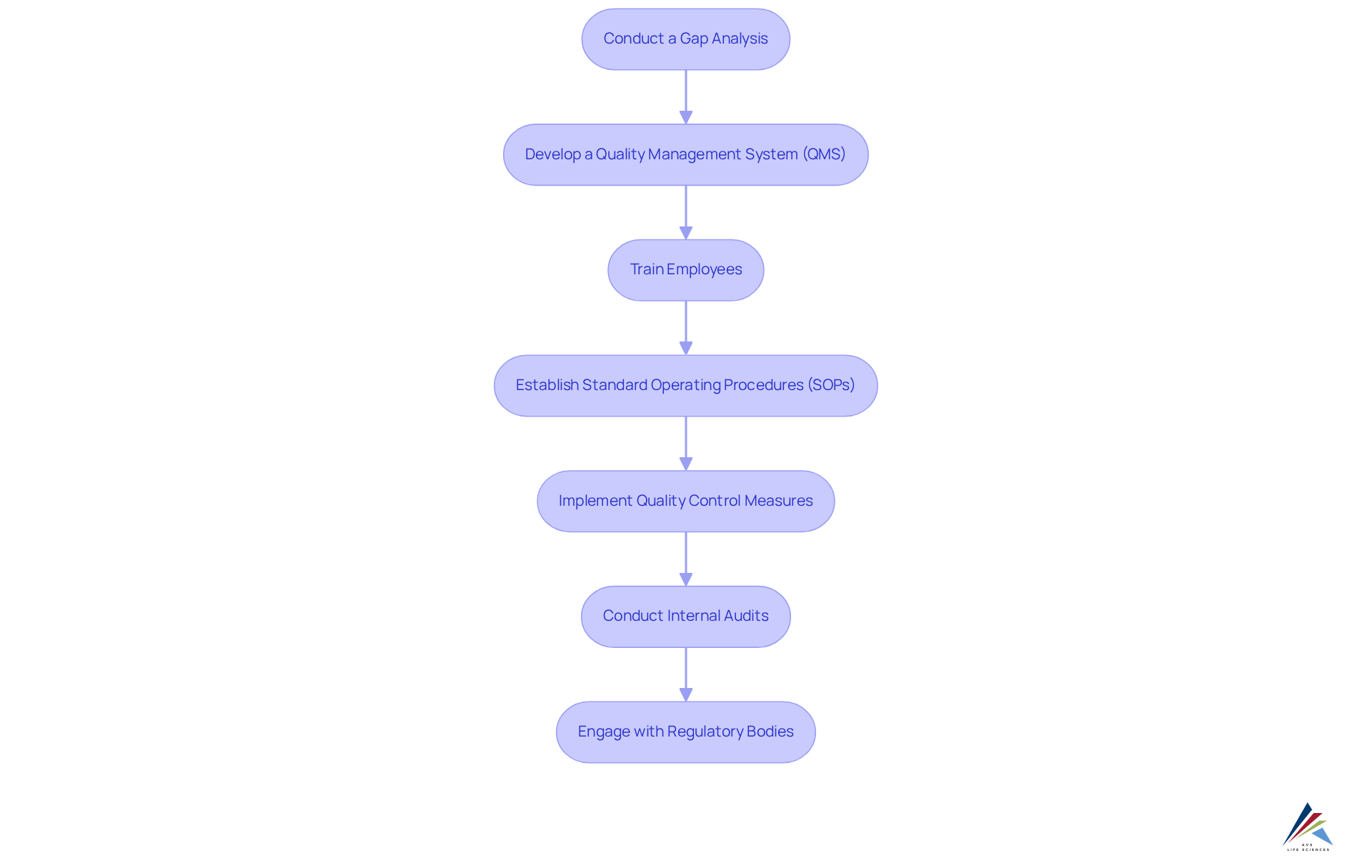
Establish Effective Documentation and Audit Trails
Efficient records and audit trails are critical for demonstrating adherence to current Good Manufacturing Practices (cGMP). To establish robust systems, consider the following key steps:
- Create a Document Control System: Implement a comprehensive system to manage the creation, review, approval, and distribution of documents. This system should integrate seamlessly with commonly used software like Microsoft Office to enhance usability and efficiency.
- Maintain Accurate Records: Ensure that all manufacturing processes, quality control tests, and employee training sessions are documented with precision. Accurate record-keeping is crucial, as the FDA mandates that data must be attributable, legible, complete, original, and accurate (ALCOA).
- Implement Audit Trails: Utilize electronic systems that automatically log changes to documents and processes. This feature offers a transparent record of actions performed, essential for compliance, and can significantly reduce the likelihood of human error in record-keeping.
- Consistently Examine Records: Regularly assess records to verify they remain up to date and comply with evolving regulations. The most common industry standard for these reviews is every two years, aligning with best practices in the pharmaceutical sector.
- Train Staff on Record-Keeping Practices: Educate employees on the and the methods for maintaining it efficiently. Regular training sessions can strengthen effective record-keeping practices and ensure compliance with regulatory requirements.
- Acknowledge Technological Challenges: Recognize that implementing electronic document management systems may pose technological challenges, particularly for smaller organizations. Addressing these challenges is essential for sustaining efficient record-keeping practices.
- Reference International Standards: Align record-keeping practices with international guidelines, such as the European Union’s GMP guidelines outlined in EudraLex Volume 4, which provide detailed requirements for record-keeping practices.
Moreover, organizations should integrate the stages of computer system validation as outlined in the GAMP 5 Guide. Each step—from planning and defining user requirements to Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—must be meticulously documented. Documenting validation results is critical to demonstrate that the system meets all specified requirements and is ready for release. By adhering to these steps, organizations can enhance their documentation procedures, ensuring conformity with cGMP regulations and improving overall operational efficiency.
![]()
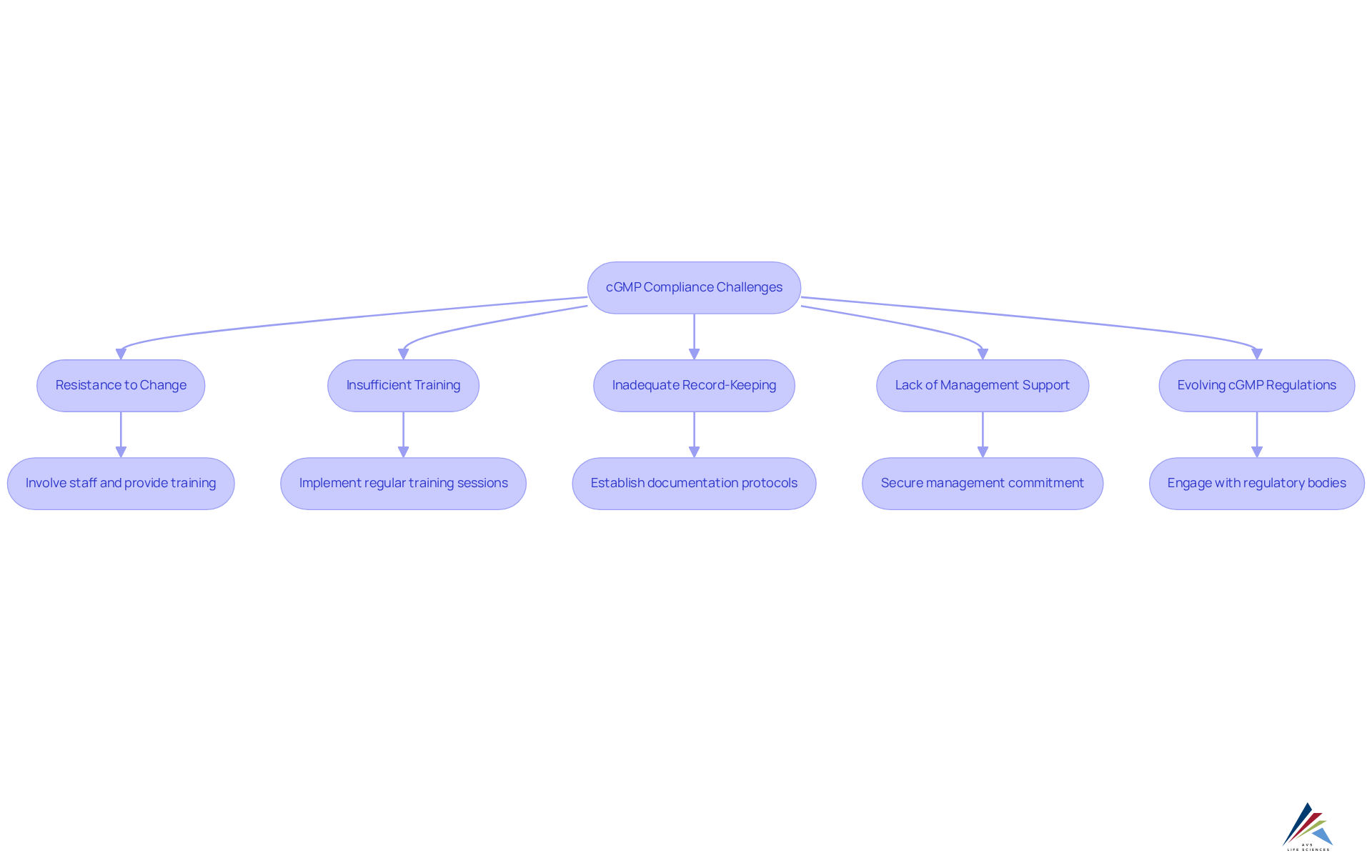
Troubleshoot Common cGMP Compliance Challenges
Organizations frequently encounter significant obstacles in achieving . Common issues arise, but effective troubleshooting strategies can pave the way for compliance.
- Resistance to Change: Employees often resist adopting new processes due to fear or uncertainty.
Solution: Actively involve staff in the change process and provide comprehensive training to foster a sense of ownership and understanding. - Insufficient training can lead to regulatory failures, as employees may not fully grasp cgmp regulations.
Solution: Implement regular training sessions and refresher courses to ensure that staff remain informed about the latest cgmp regulations and practices. - Inadequate Record-Keeping: Erratic record-keeping can result in adherence failures and complications during regulatory examinations.
Solution: Establish clear documentation protocols and conduct regular audits to ensure compliance and accuracy in record-keeping. - Lack of Management Support: Without leadership buy-in, regulatory initiatives may struggle to gain traction.
Solution: Secure commitment from management by demonstrating the tangible benefits of cgmp regulations compliance, such as improved product quality and enhanced safety. - Evolving cgmp regulations: Keeping pace with changing cgmp regulations can be daunting for organizations.
Solution: Subscribe to industry updates and actively engage with regulatory bodies to stay informed about the latest changes and requirements.
By addressing these challenges with targeted strategies, organizations can enhance their compliance efforts and ultimately improve their operational integrity.
Conclusion
Mastering current Good Manufacturing Practices (cGMP) is not merely a regulatory requirement; it is essential for ensuring the quality and safety of pharmaceutical products. Understanding and implementing the key components of cGMP regulations allows organizations to cultivate a culture of compliance that not only meets regulatory standards but also enhances overall operational integrity. This unwavering commitment to quality is vital for maintaining consumer trust and safeguarding public health.
To achieve cGMP compliance, organizations must undertake several fundamental steps. These include:
- Conducting thorough gap analyses
- Developing robust quality management systems
- Investing in comprehensive employee training
It is imperative to maintain accurate documentation and audit trails while addressing common challenges such as resistance to change and inadequate record-keeping. Each of these elements is pivotal in navigating the complexities of cGMP regulations and ensuring that pharmaceutical products are consistently produced to the highest standards.
The significance of cGMP compliance transcends mere regulatory adherence; it embodies a commitment to excellence that can lead to improved product quality, reduced risk of recalls, and enhanced consumer safety. Organizations are encouraged to actively engage with regulatory bodies, stay informed about updates to cGMP regulations, and invest in continuous training and improvement. By taking these proactive steps, companies can not only meet compliance standards but also position themselves as leaders in the pharmaceutical industry, dedicated to delivering safe and effective products to consumers.
Frequently Asked Questions
What are Current Good Manufacturing Practices (cGMP)?
Current Good Manufacturing Practices (cGMP) are essential guidelines enforced by the FDA to ensure that pharmaceutical products are consistently produced and controlled according to stringent quality standards, covering all aspects of production from raw materials to facilities and equipment.
Why are quality management systems important in cGMP?
Quality management systems are vital for maintaining compliance with cGMP regulations. They provide comprehensive frameworks for continuous monitoring and improvement of processes, which is emphasized by the FDA's New Inspection Protocol Project (NIPP) aimed at enhancing inspection efficiency and quality management.
How important is personnel training in cGMP compliance?
Adequate training in cGMP principles is crucial for all employees to ensure compliance with regulations. Fostering a culture of quality that emphasizes continuous improvement can significantly enhance compliance outcomes, as evidenced by the increase in non-compliant samples from 16% to 35% in fiscal year 2021.
What are the facility requirements under cGMP?
Manufacturing facilities must meet specific design and operational standards to prevent contamination and maintain product integrity. The FDA has emphasized the importance of these standards, as demonstrated by enforcement actions in 2025 where numerous sites received 'Import Alerts' for non-compliance.
Why is understanding cGMP components essential for organizations?
Understanding the components of cGMP is essential for organizations to navigate the complexities of the regulations effectively and ensure the safety and effectiveness of their pharmaceutical products.
