Master 21 CFR Part 211: Essential Steps for Compliance Officers

Overview
This article outlines the essential steps for compliance officers to master 21 CFR Part 211, highlighting the critical importance of:
- Quality assurance
- Documentation
- Personnel training
- Facility standards in pharmaceutical manufacturing
It further elaborates on compliance elements and strategies, such as:
- Conducting gap analyses
- Developing Standard Operating Procedures (SOPs)
- Fostering open communication
These strategies are vital for adhering to regulatory requirements and ensuring product safety and quality. By implementing these practices, compliance officers can effectively navigate the complexities of regulatory standards, ultimately enhancing the integrity of their operations.
Introduction
Navigating the complexities of pharmaceutical regulations presents a formidable challenge for compliance officers, particularly in adhering to 21 CFR Part 211. This pivotal regulation delineates the current Good Manufacturing Practices (cGMP) essential for ensuring that drug products are both safe and effective. By exploring the critical steps for compliance, officers can deepen their understanding of regulatory requirements and implement strategies that not only safeguard public health but also uphold organizational integrity. Given the increasing scrutiny from regulatory bodies and the severe consequences associated with non-compliance, how can compliance officers effectively address the challenges posed by these stringent guidelines?
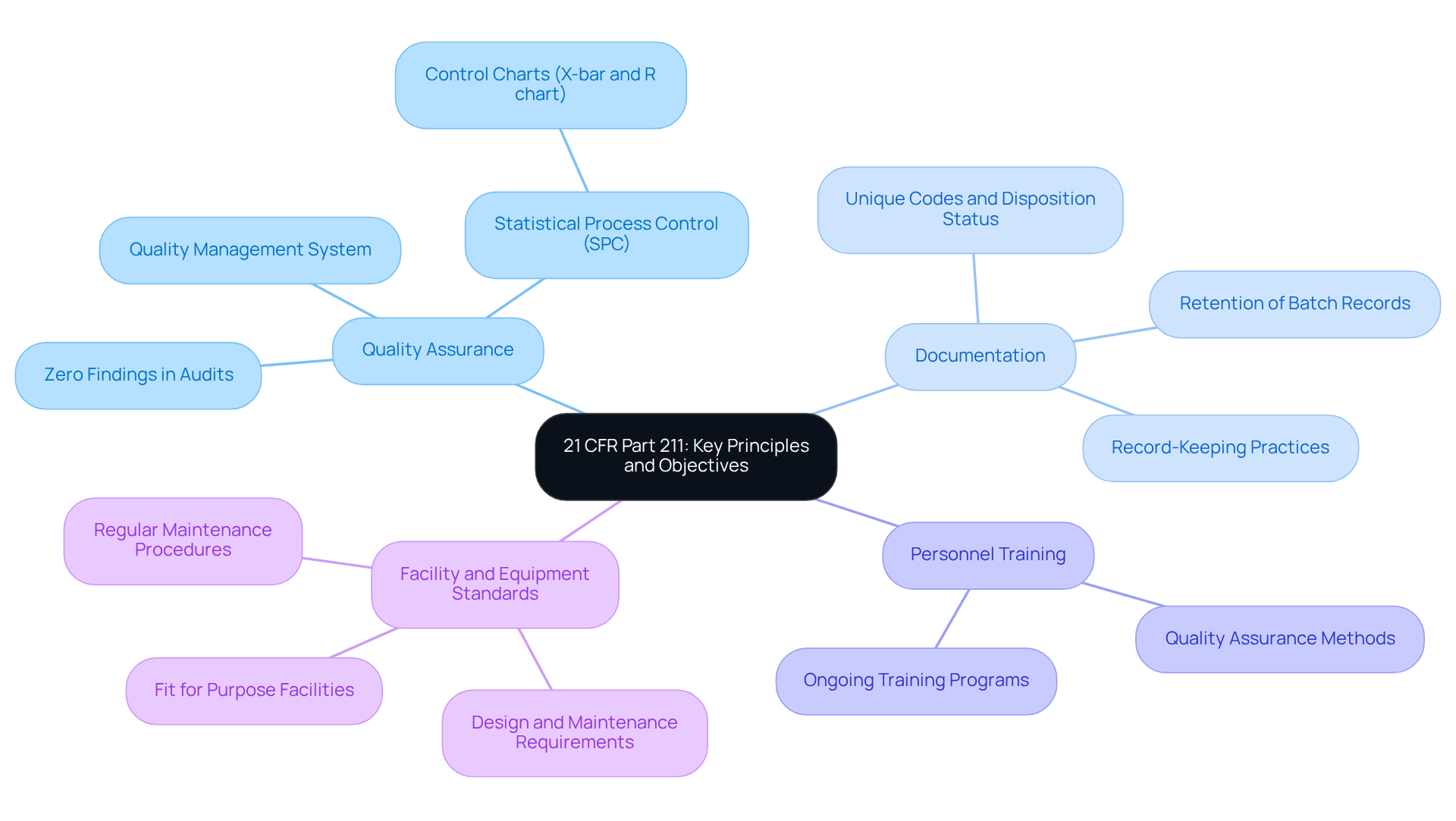
Understand 21 CFR Part 211: Key Principles and Objectives
The current Good Manufacturing Practices (cGMP) that pharmaceutical manufacturers must adhere to in order to ensure the quality and safety of drug products are delineated in 21 CFR Part 211. The core principles are as follows:
- Quality Assurance: Establishing is crucial for ensuring that products consistently meet their intended specifications. This proactive approach not only mitigates risks but also enhances overall product standards. The application of Statistical Process Control (SPC) techniques, including control charts like the X-bar and R chart, aids in identifying variations and ensuring compliance. AVS Life Sciences exemplifies this commitment to quality assurance, having achieved zero findings during audits, which showcases their expertise in managing compliance-driven projects.
- Documentation: Accurate record-keeping is vital for traceability and accountability in manufacturing processes. Each lot in every shipment must be accompanied by a unique code and disposition status, ensuring that all necessary documentation is readily accessible for regulatory inspections. The significance of maintaining comprehensive records is underscored by the fact that failure to address issues noted in Form 483 can result in severe consequences, including warning letters and potential recalls. Furthermore, batch records must be preserved for at least one year after expiry or three years after distribution if exempt from expiry dating, as mandated by cGMP requirements. AVS Life Sciences emphasizes excellent documentation practices and adherence to Standard Operating Procedures (SOPs), guaranteeing alignment with these stringent requirements.
- Personnel Training: Adequate training for all staff is essential to uphold standards and regulations. Ongoing training in quality assurance methods, including the concepts outlined in ICH Q10, equips personnel to effectively manage adherence projects and tackle oversight challenges. AVS Life Sciences provides customized consulting services designed to enhance personnel training, ensuring that teams are well-prepared to navigate the complexities of regulatory adherence.
- Facility and Equipment Standards: Compliance with specific design and maintenance requirements for facilities and equipment is non-negotiable in drug manufacturing. Facilities must be fit for purpose, with regular maintenance procedures instituted to ensure cleanliness and functionality, as mandated by cGMP requirements, specifically those outlined in 21 CFR Part 211, Subpart C. AVS Life Sciences has successfully guided clients in upgrading their facilities, such as transitioning from a Biosafety Level 1 to a Level 2 GMP facility, demonstrating their capability in managing complex engineering and validation projects.
Understanding these principles is crucial for compliance officers to effectively implement and oversee regulatory strategies within their organizations. The commitment to quality assurance not only protects public health but also cultivates a culture of accountability and continuous improvement, as evidenced by AVS Life Sciences' impressive 80% repeat business rate.
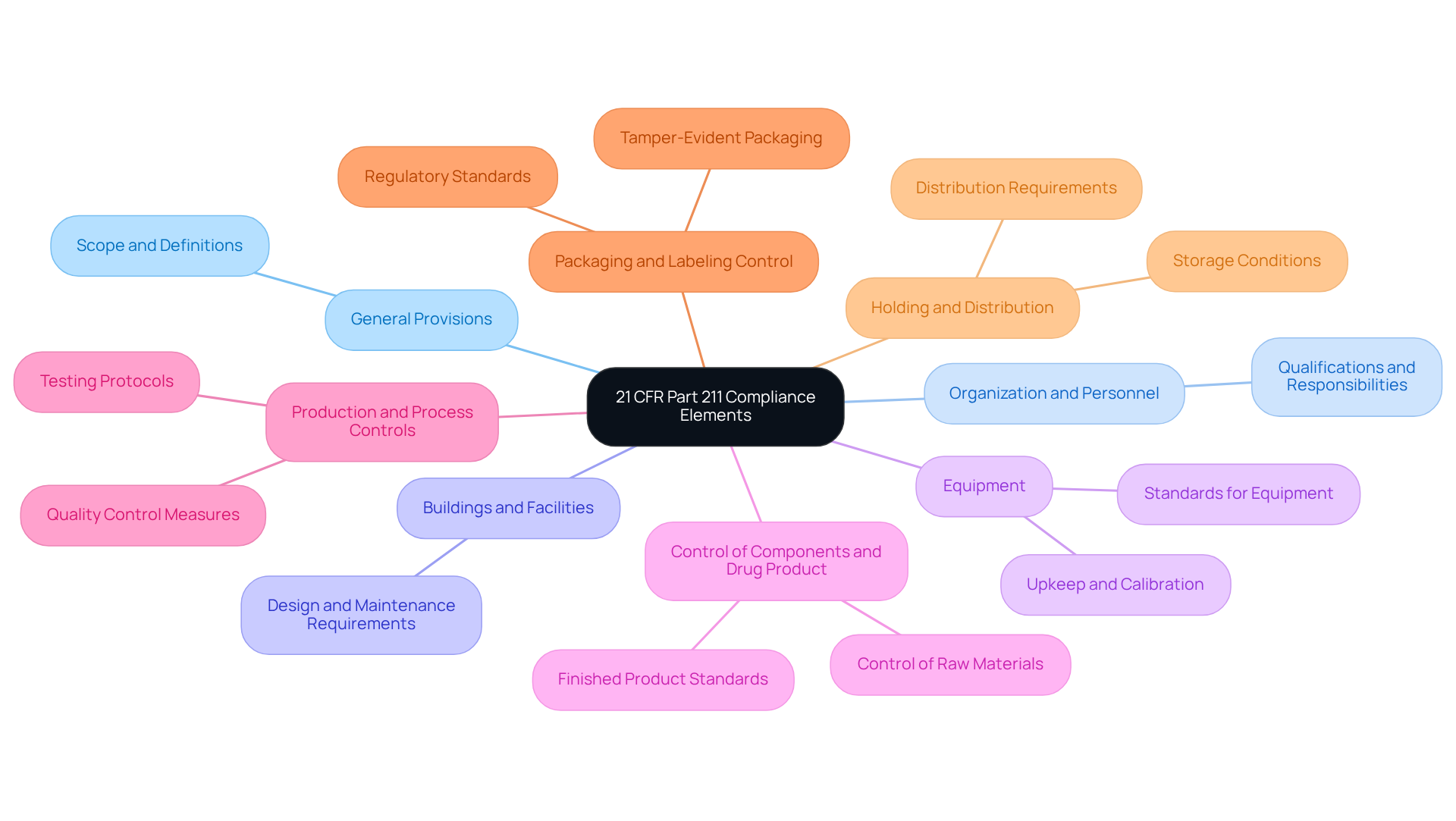
Identify the Requirements of 21 CFR Part 211: Essential Compliance Elements
The essential compliance elements of 21 CFR Part 211 are foundational for pharmaceutical manufacturing and encompass several critical subparts:
- Subpart A - General Provisions: This section outlines the scope and definitions relevant to the regulation, establishing a robust framework for compliance.
- Subpart B - Organization and Personnel: It specifies the qualifications and responsibilities of personnel involved in drug manufacturing, ensuring that only trained and competent individuals engage in critical processes.
- Subpart C - Buildings and Facilities: This subpart details the requirements for the design, construction, and maintenance of manufacturing facilities, emphasizing the necessity of a controlled environment to prevent contamination.
- Subpart D - Equipment: It addresses the standards for equipment utilized in the manufacturing process, including upkeep and calibration, which are essential for maintaining consistent product excellence.
- Subpart E - Control of Components and Drug Product: This section mandates the control of raw materials and finished products, ensuring that all components meet specified standards before use.
- Subpart F - Production and Process Controls: It outlines the necessary controls to ensure consistent product quality, including rigorous testing protocols to identify and manage impurities and deviations from specifications.
- Subpart G - Packaging and Labeling Control: This subpart ensures that packaging and labeling meet regulatory standards, including tamper-evident packaging requirements to enhance consumer safety.
- Subpart H - Holding and Distribution: It addresses the requirements for the storage and distribution of drug products, guaranteeing that products are maintained under appropriate conditions throughout their lifecycle.
Each of these elements plays a critical role in ensuring adherence to 21 CFR Part 211. A comprehensive grasp and execution of these regulations are crucial for oversight personnel to navigate the intricacies of pharmaceutical production and uphold high standards of quality and safety. and audits, potentially resulting in warning letters, fines, or shutdowns. Additionally, adherence to cGMP guidelines is a requirement for approvals and market entry.
AVS Life Sciences provides extensive GXP compliance services, including various types of GMP audits that focus on critical areas such as APIs, drug products, and testing facilities. Their proficiency is exemplified in a transformative case study where they successfully enhanced a biotechnology GMP facility, ensuring assurance and regulatory adherence throughout the process. Good documentation practices (GDocP) are essential for effective quality management in the pharmaceutical industry. As Angel Buendia, Knowledge Manager, aptly stated, 'Documentation is the backbone of adherence to 21 CFR Part 211.
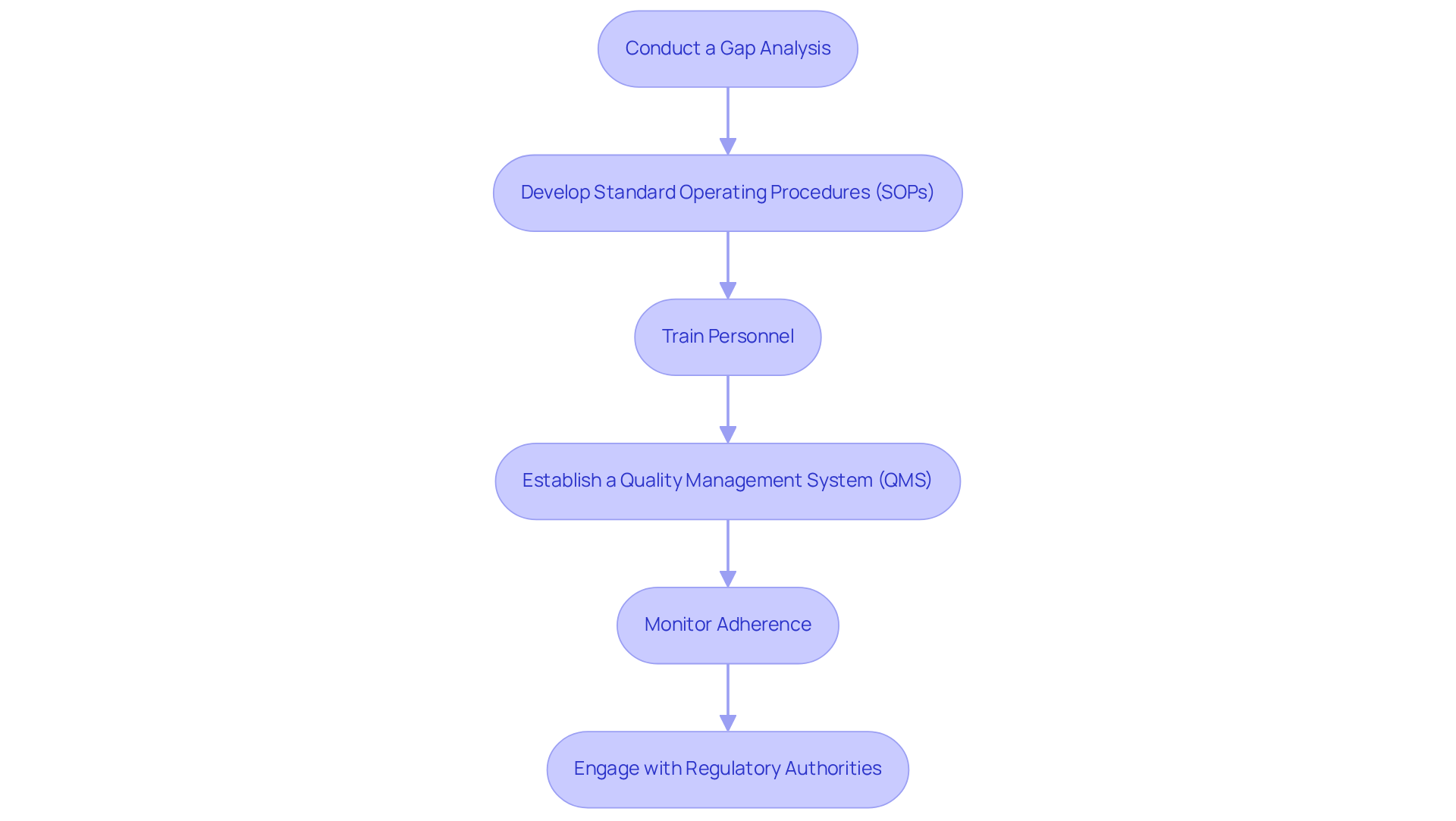
Implement Compliance Strategies: Step-by-Step Approach for Pharmaceutical Officers
To implement compliance strategies effectively, it is essential to follow these critical steps:
- Conduct a Gap Analysis: Begin by evaluating current practices against the requirements of 21 CFR Part 211 to identify areas that need improvement. This analysis should focus on pinpointing discrepancies in documentation, processes, and personnel qualifications. For example, AVS Life Sciences conducted a gap analysis for a pharmaceutical manufacturer transitioning from a Biosafety Level 1 to a Level 2 GMP facility, revealing critical differences that needed to be addressed.
- Develop Standard Operating Procedures (SOPs): Create comprehensive SOPs for all processes related to manufacturing, quality control, and documentation. These SOPs must be clear, concise, and tailored to meet regulatory standards, ensuring that all employees understand their roles in compliance.
- Train personnel by providing thorough training for all employees on the new SOPs and emphasizing the significance of adhering to 21 CFR Part 211. Ongoing education is vital to ensure that personnel remain updated on regulatory demands and best practices.
- Establish (QMS): Implement a robust QMS that includes regular audits, documentation reviews, and corrective actions. This system should facilitate ongoing monitoring of adherence and ensure that any deviations are promptly addressed. AVS Life Sciences exemplifies this by assisting clients with the installation and qualification of new equipment, thereby ensuring adherence to GMP standards.
- Monitor Adherence: Regularly evaluate adherence metrics and conduct internal audits to confirm conformity to established procedures. This proactive approach aids in recognizing potential issues before they escalate into significant regulatory failures. Non-compliance with cGMP can lead to fines, recalls, and even closure, underscoring the importance of this step.
- Engage with Regulatory Authorities: Foster open communication with regulatory bodies to remain informed about changes in regulations and best practices. This engagement not only aids in compliance but also cultivates a collaborative relationship with regulators. As Charles Ahn wisely stated, "You’ve got to be very careful if you don’t know where you are going, because you might not get there."
By adhering to these steps, compliance officers can establish a solid compliance framework that aligns with the requirements of 21 CFR Part 211, ultimately enhancing the quality and safety of pharmaceutical products. Additionally, leveraging the expertise of AVS Life Sciences can provide invaluable support in navigating these complex regulatory environments, as demonstrated in their successful project that enabled a client to manufacture medication with lentivirus vector material.
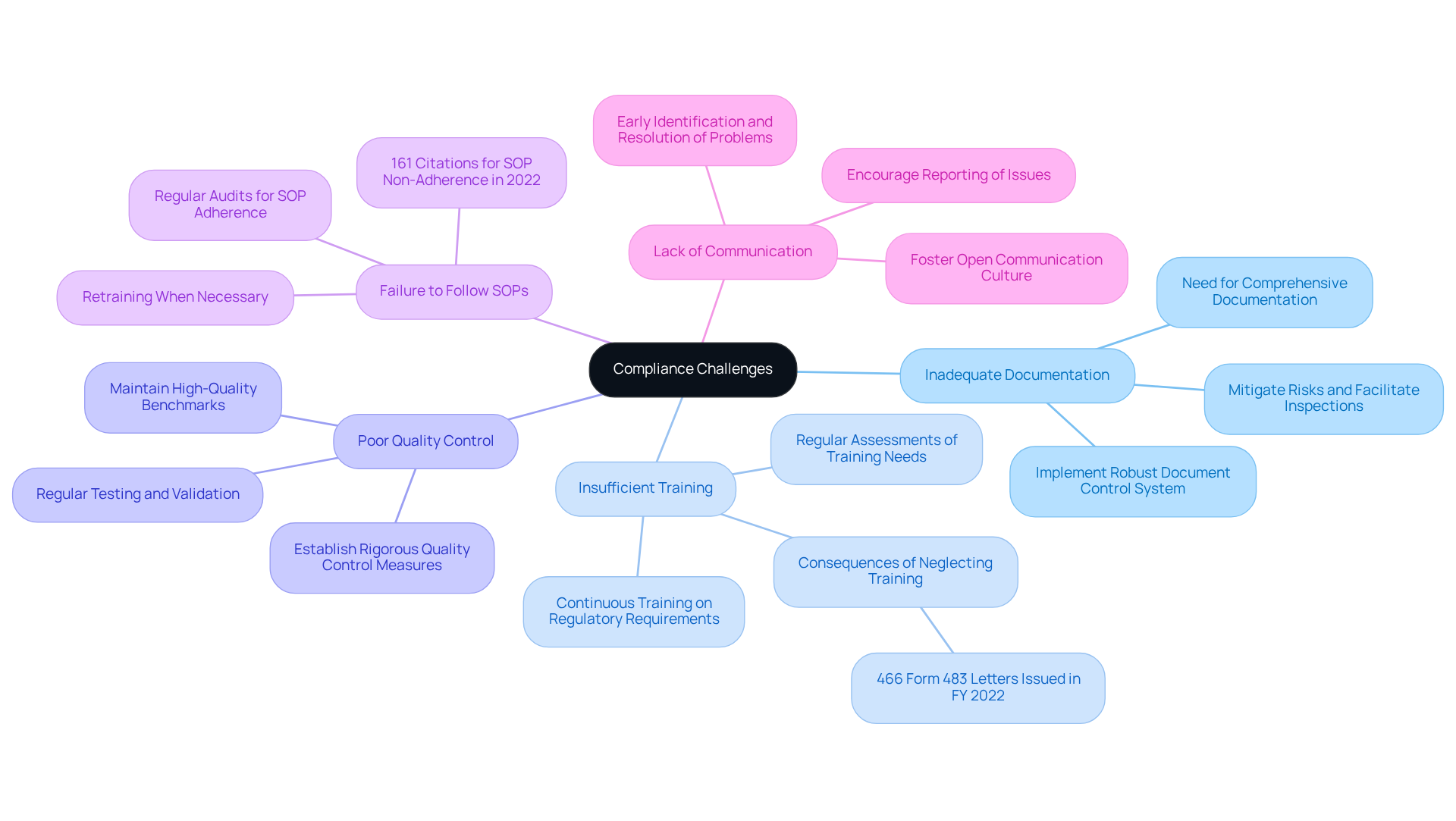
Overcome Compliance Challenges: Troubleshooting Common Issues
Compliance officers frequently encounter significant challenges in adhering to the regulations outlined in 21 CFR Part 211, particularly concerning documentation and training. Addressing these prevalent issues with effective strategies is essential for maintaining compliance and enhancing operational integrity.
- Inadequate Documentation: A staggering 58% of inspections were conducted in facilities outside the US, underscoring the critical need for comprehensive documentation. Implementing a robust document control system is essential for managing revisions and approvals, ensuring that all processes are thoroughly documented and easily retrievable. This proactive approach not only mitigates risks but also facilitates smoother inspections.
- Insufficient Training: Regular assessments of training needs are vital. Offering continuous training to employees regarding regulatory requirements can significantly reduce risks. Organizations that invest in training often observe marked improvements in adherence outcomes; for instance, the 466 Form 483 letters issued by the FDA in FY 2022, many stemming from inadequate training protocols, highlight the consequences of neglecting this area.
- Poor Quality Control: Establishing rigorous quality control measures is crucial. This includes regular testing and and products to ensure they meet established standards. The FDA's emphasis on scientifically sound laboratory controls underscores the importance of maintaining high-quality benchmarks, which are indispensable for compliance.
- Failure to Follow SOPs: Regular audits are necessary to ensure adherence to Standard Operating Procedures (SOPs). Offering retraining when necessary can help strengthen adherence. In 2022, the FDA issued 161 citations for procedures not being in writing or not fully followed, illustrating the consequences of neglecting SOP adherence. Such statistics serve as a reminder of the importance of diligence in compliance practices.
- Lack of Communication: Fostering a culture of open communication within the organization is essential. Encouraging staff to report adherence issues without fear of repercussions can lead to the early identification and resolution of potential problems, ultimately enhancing compliance efforts.
By proactively addressing these challenges, compliance officers can significantly enhance their organization's compliance posture, reduce the risk of regulatory violations, and contribute to a culture of quality and safety in drug manufacturing.
Conclusion
Mastering 21 CFR Part 211 is essential for compliance officers tasked with ensuring the safety and quality of pharmaceutical products. This regulatory framework provides a detailed roadmap for maintaining high manufacturing standards, emphasizing the significance of quality assurance, thorough documentation, personnel training, and adherence to facility and equipment specifications. Understanding and implementing these principles not only protects public health but also fosters a culture of continuous improvement within organizations.
The article outlines several key elements that compliance officers must navigate, including the critical subparts of 21 CFR Part 211, which address everything from general provisions to production controls and distribution requirements. Each of these components plays a pivotal role in ensuring that pharmaceutical manufacturing processes meet regulatory expectations. Moreover, the importance of effective compliance strategies, such as:
- Conducting gap analyses
- Developing standard operating procedures
- Engaging with regulatory authorities
cannot be overstated. These steps are vital in overcoming common challenges like inadequate documentation and insufficient training.
Ultimately, the commitment to adhering to 21 CFR Part 211 is not merely a regulatory obligation but a fundamental aspect of upholding public trust in the pharmaceutical industry. By proactively addressing compliance challenges and implementing best practices, compliance officers can significantly enhance their organization's operational integrity and contribute to the overall safety and efficacy of drug products. Embracing these principles and strategies will pave the way for a more compliant and successful future in pharmaceutical manufacturing.
Frequently Asked Questions
What is 21 CFR Part 211?
21 CFR Part 211 outlines the current Good Manufacturing Practices (cGMP) that pharmaceutical manufacturers must follow to ensure the quality and safety of drug products.
What are the core principles of 21 CFR Part 211?
The core principles include Quality Assurance, Documentation, Personnel Training, and Facility and Equipment Standards.
Why is Quality Assurance important in pharmaceutical manufacturing?
Quality Assurance is crucial for establishing a robust quality management system that ensures products consistently meet their intended specifications, mitigates risks, and enhances overall product standards.
How does Statistical Process Control (SPC) contribute to quality assurance?
SPC techniques, such as control charts like the X-bar and R chart, help identify variations in manufacturing processes and ensure compliance with quality standards.
What role does documentation play in compliance with 21 CFR Part 211?
Accurate documentation is vital for traceability and accountability, ensuring that each lot has a unique code and disposition status for regulatory inspections. Comprehensive records help prevent severe consequences, such as warning letters or recalls.
How long must batch records be preserved according to cGMP requirements?
Batch records must be retained for at least one year after expiry or three years after distribution if exempt from expiry dating.
Why is personnel training essential in pharmaceutical manufacturing?
Adequate training ensures that staff uphold standards and regulations, equipping them to manage adherence projects and tackle oversight challenges effectively.
What does AVS Life Sciences offer for personnel training?
AVS Life Sciences provides customized consulting services designed to enhance personnel training in quality assurance methods.
What are the requirements for facility and equipment standards under cGMP?
Facilities must meet specific design and maintenance requirements to ensure cleanliness and functionality, and regular maintenance procedures must be instituted.
How can AVS Life Sciences assist with facility upgrades?
AVS Life Sciences has experience in guiding clients through complex engineering and validation projects, such as upgrading facilities from Biosafety Level 1 to Level 2 GMP.
Why is understanding these principles important for compliance officers?
Understanding these principles is crucial for compliance officers to effectively implement and oversee regulatory strategies, protect public health, and foster a culture of accountability and continuous improvement.
