LIMS Meaning: Key Functions and Importance in Laboratories

Overview
The article articulates that Laboratory Information Management Systems (LIMS) are indispensable software solutions that significantly enhance laboratory operations. By effectively managing samples, data, and workflows, LIMS ensure compliance and quality management. This assertion is substantiated by a thorough exploration of LIMS functionalities, including:
- Sample tracking
- Workflow automation
- Regulatory compliance
Collectively, these features not only bolster efficiency but also mitigate errors within laboratory environments. As compliance challenges continue to evolve, the implementation of LIMS emerges as a critical strategy for laboratories aiming to achieve operational excellence.
Introduction
Laboratories today encounter an escalating demand for efficiency, accuracy, and compliance, positioning Laboratory Information Management Systems (LIMS) as an indispensable asset. These advanced software solutions not only streamline operations but also ensure adherence to stringent regulatory standards, ultimately protecting the integrity of scientific research.
Yet, with a myriad of systems available, how can laboratories ascertain which LIMS aligns best with their distinct needs and challenges? This article explores the essence of LIMS, its critical functions, and its significant role in enhancing laboratory performance and regulatory compliance.
Define LIMS: Understanding Laboratory Information Management Systems
The term 'LIMS meaning' refers to a Laboratory Information Management System, which serves as a vital software-based solution designed to manage samples, related information, and testing workflows, particularly in the life sciences sector. By functioning as a centralized platform, it significantly enhances the efficiency and accuracy of operations, which is critical for upholding quality management and ensuring regulatory compliance.
These systems are indispensable for tracking samples from their entry into the laboratory through testing and analysis, ensuring that all information is systematically organized and readily accessible. This software, often referred to as a Laboratory Information System (LIS) or Laboratory Management System (LMS), embodies the lims meaning by playing a pivotal role in automating various laboratory processes.
By reducing human errors and improving data reliability, LIMS assists facilities in adhering to stringent regulatory requirements and quality control practices, establishing itself as an essential resource for Pharmaceutical Compliance Officers.
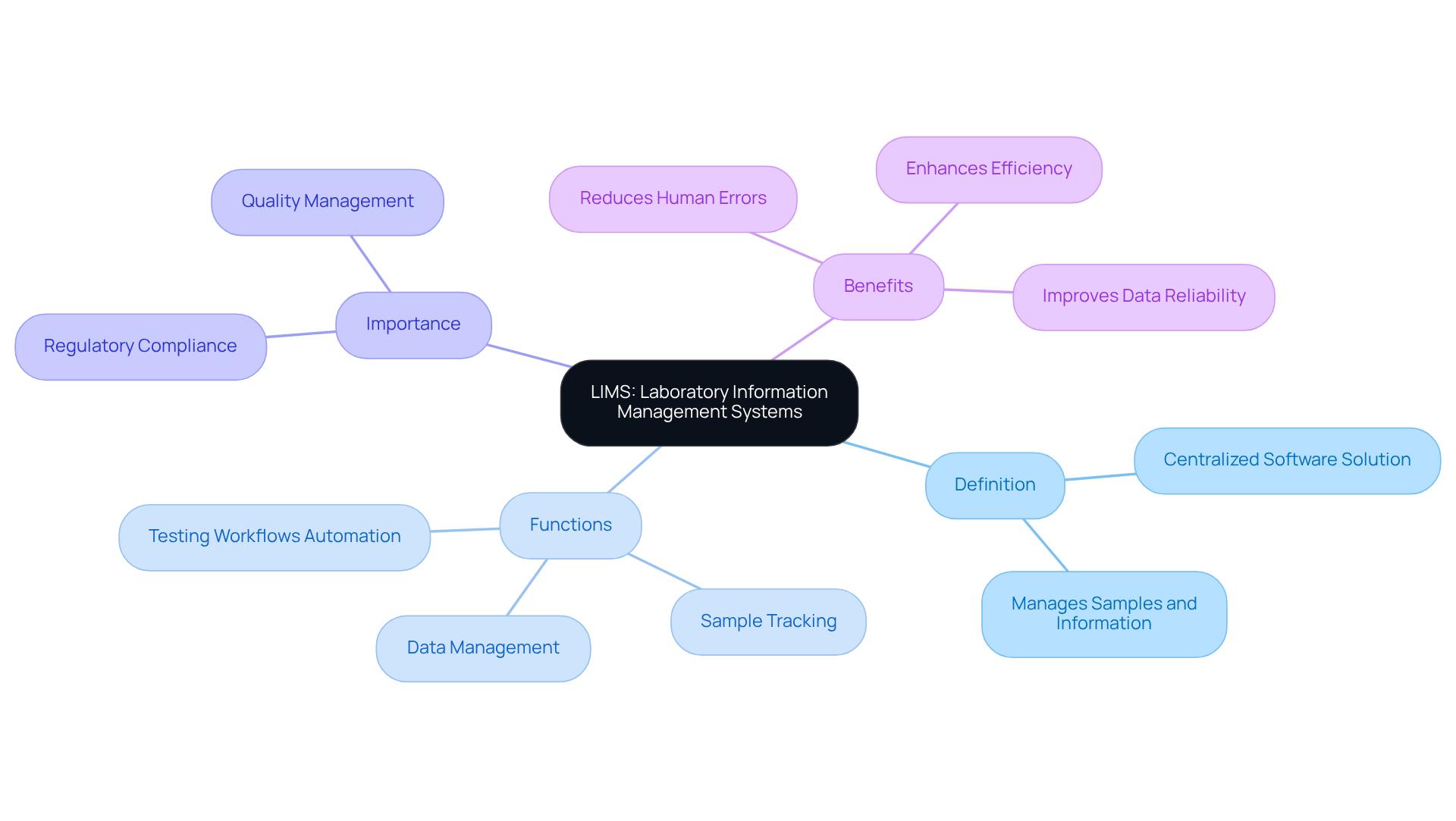
Explore Key Functions of LIMS in Laboratory Operations
Laboratory Information Management Systems (LIMS) are pivotal in optimizing laboratory operations through several key functions:
- Sample Management: LIMS meticulously monitors samples throughout their lifecycle—from receipt to disposal—ensuring accurate record-keeping and traceability.
- Workflow Automation: By automating repetitive tasks, LIMS significantly enhances productivity and mitigates the risk of human error, allowing staff to focus on more complex tasks.
- Data Management: The system centralizes data storage, facilitating easier access and analysis of test results, which is essential for informed decision-making and comprehensive reporting.
- Quality Control and Assurance: LIMS plays a crucial role in maintaining high standards by implementing checks and balances throughout the testing processes, ensuring compliance with regulatory requirements.
Collectively, these functions foster a more organized, efficient, and compliant workspace, ultimately driving the laboratory towards excellence in performance and regulatory adherence.
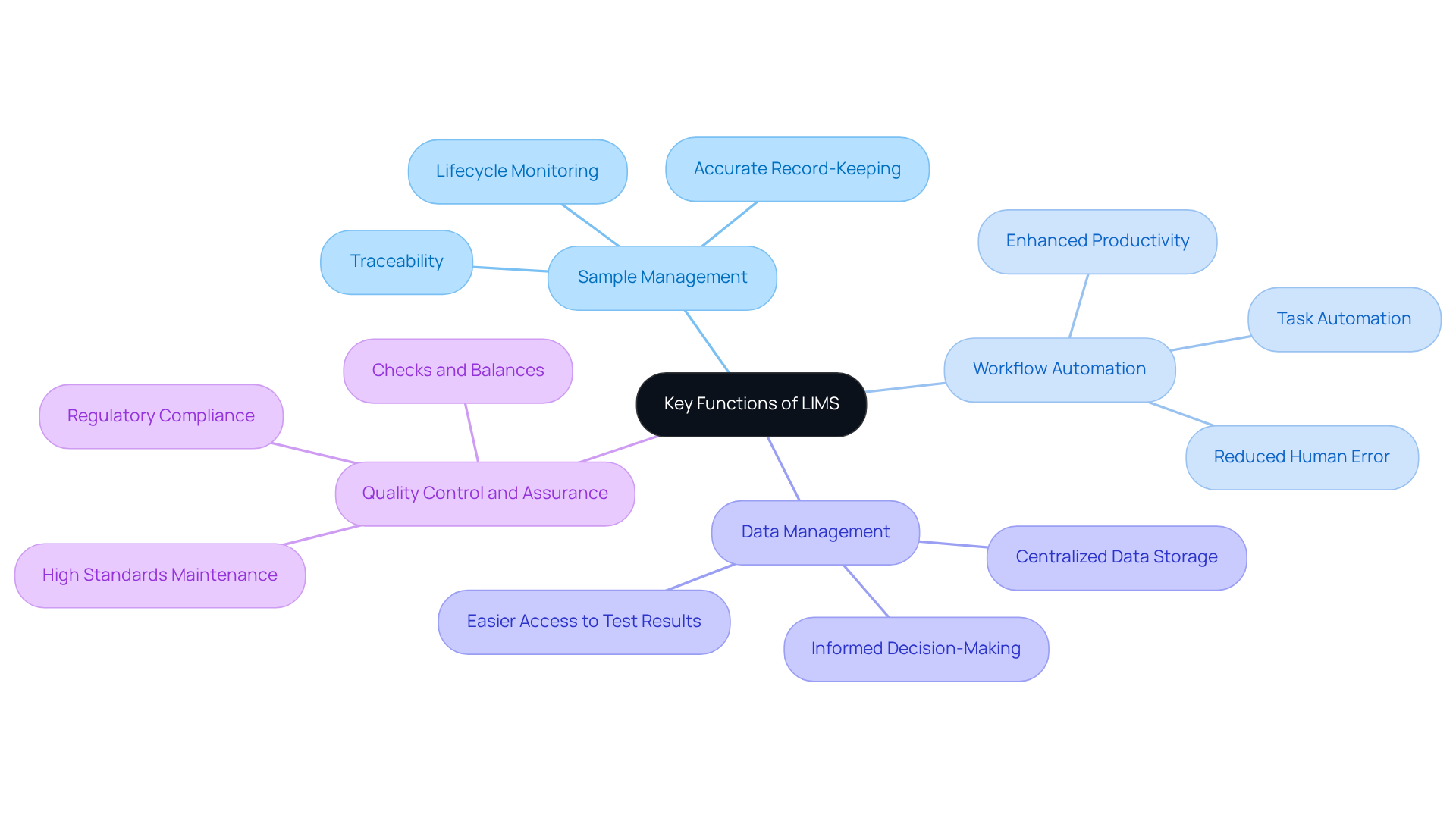
Assess the Importance of LIMS for Compliance and Quality Management
The significance of Information Management Systems (IMS) in research facilities extends far beyond mere operational efficiency; it serves as a fundamental pillar for ensuring compliance and managing standards. Regulatory bodies, including the FDA and ISO, mandate that testing facilities maintain precise records and adhere to stringent quality standards. LIMS plays a pivotal role in facilitating this compliance through several essential functions:
-
Ensuring Data Integrity: LIMS systems are meticulously designed with secure data entry, comprehensive audit trails, and robust access controls, all vital for preserving the accuracy and reliability of laboratory data. This is particularly critical in regulated environments where meticulous documentation of validation is necessary to satisfy regulatory authorities.
-
Streamlining Compliance Processes: By automating documentation and reporting, the system significantly enhances the efficiency with which facilities meet regulatory requirements, thereby mitigating the risk of non-compliance. For instance, AVS Life Sciences effectively assisted a leading biotechnology company in upgrading their manufacturing space to a Level 2 GMP facility. During this transition, the client encountered challenges such as ensuring the proper installation of equipment and addressing anomalies in test results stemming from barcode scanner issues. This collaboration not only streamlined their compliance workflows but also allowed the client to concentrate on developing medicines for serious diseases. The implementation of Laboratory Information Management Systems (LIMS) in such environments can lead to substantial improvements in operational efficiency, illustrating the LIMS meaning through the example of a pharmaceutical control laboratory that achieved a 30% reduction in manual data entry and a 40% faster production of Certificates of Analysis (CoA).
-
Improving Quality Control: The system supports management initiatives by enabling facilities to establish standard operating procedures (SOPs) and monitor compliance with these protocols. This capability is essential for maintaining high-quality standards and ensuring that testing facilities can swiftly adapt to evolving regulatory demands. The insights gained from AVS Life Sciences' partnership with their biotech client also highlighted the importance of ongoing assessments of business procedures to identify deficiencies that could lead to questionable test outcomes, further emphasizing the LIMS meaning in the context of enhancing assurance standards.
In conclusion, IMS is not merely a data management tool; it is an essential component that ensures facilities operate within the regulatory frameworks governing their activities. As regulatory requirements evolve, continuous updates and improvements to laboratory information management systems are crucial to uphold compliance and guarantee the integrity and security of lab systems, ultimately enhancing both adherence and management standards.
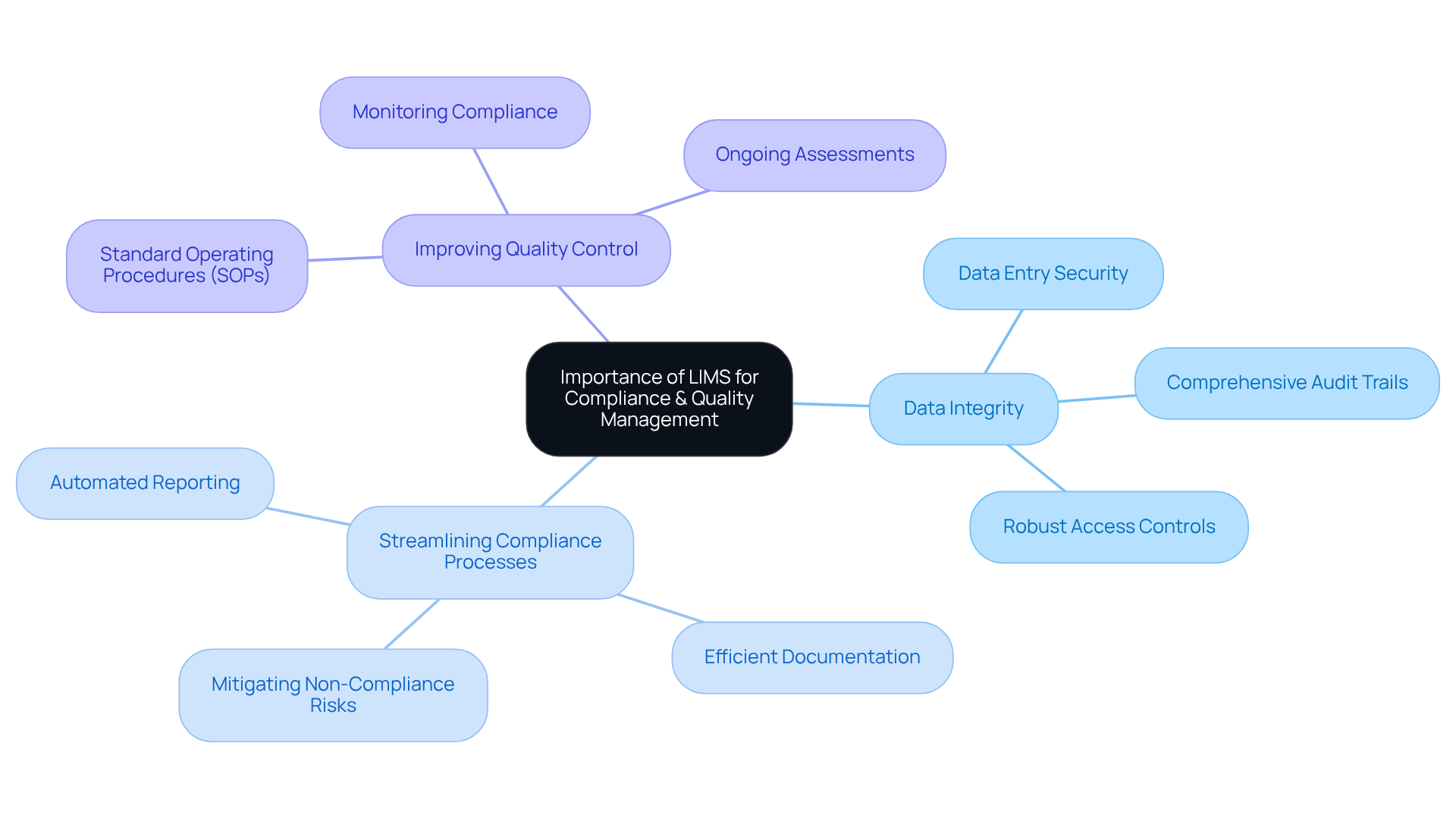
Examples of LIMS in Action
Numerous laboratories across various sectors have successfully adopted laboratory information management systems (LIMS), reflecting the LIMS meaning, to enhance their operations. Consider the following examples:
-
Pharmaceutical Laboratories: A leading biotechnology company in San Francisco upgraded its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility with the assistance of AVS Life Sciences. This transformation not only ensured compliance with regulatory standards but also enhanced their quality assurance processes. The introduction of the laboratory information management system during this upgrade enabled improved monitoring of samples and information, ultimately resulting in a more efficient drug development process and a significant decrease in time-to-market for new products.
-
Clinical Laboratories: A clinical lab implemented a laboratory information management system to handle patient samples and test outcomes more effectively. The automation of data entry and reporting led to a significant decrease in turnaround times for test results, thereby enhancing patient care.
-
Environmental Testing Laboratories: An environmental lab adopted a laboratory information management system to ensure compliance with environmental regulations. The system helped maintain accurate records of sample collection and analysis, which were crucial for regulatory audits.
These examples illustrate the versatility and effectiveness of LIMS meaning in enhancing laboratory efficiency, compliance, and overall quality management, which drives the need for organizations to consider such solutions for their operational success.
Conclusion
The essence of Laboratory Information Management Systems (LIMS) is their remarkable ability to streamline laboratory operations, enhance data reliability, and ensure compliance with regulatory standards. As a cornerstone of modern laboratory management, LIMS not only organizes and tracks samples but also automates workflows, ultimately driving efficiency and accuracy in testing environments.
Key insights from the discussion underscore the multifaceted functions of LIMS, including:
- Sample management
- Workflow automation
- Data centralization
- Robust quality control
These features collectively contribute to a more organized and compliant laboratory setting, enabling facilities to meet stringent regulatory demands while maintaining high-quality standards.
As laboratories continue to evolve, embracing the capabilities of LIMS becomes increasingly vital. The integration of such systems not only facilitates operational excellence but also empowers organizations to adapt to changing regulations and improve overall quality management. Investing in LIMS transcends a mere technological upgrade; it represents a strategic move toward achieving sustained compliance and enhanced laboratory performance.
Frequently Asked Questions
What does LIMS stand for?
LIMS stands for Laboratory Information Management System.
What is the primary purpose of a LIMS?
The primary purpose of a LIMS is to manage samples, related information, and testing workflows, particularly in the life sciences sector.
How does a LIMS improve laboratory operations?
A LIMS enhances the efficiency and accuracy of laboratory operations by serving as a centralized platform for organizing and accessing information, which is critical for quality management and regulatory compliance.
What are some alternative names for LIMS?
LIMS can also be referred to as a Laboratory Information System (LIS) or Laboratory Management System (LMS).
In what ways does LIMS contribute to sample tracking?
LIMS tracks samples from their entry into the laboratory through testing and analysis, ensuring that all information is systematically organized and readily accessible.
How does LIMS help reduce errors in laboratory processes?
LIMS reduces human errors and improves data reliability, which helps facilities adhere to stringent regulatory requirements and quality control practices.
Who benefits from the use of LIMS in a laboratory setting?
Pharmaceutical Compliance Officers and laboratory personnel benefit from LIMS as it helps ensure compliance with regulations and improves the overall quality of laboratory operations.
