LIMS Definition: Understanding Its Role in Pharmaceutical Compliance
Overview
Laboratory Information Management Systems (LIMS) are essential software solutions that significantly enhance pharmaceutical compliance by effectively managing samples, data, and workflows. These systems ensure adherence to regulatory standards, including GXP and FDA guidelines. By automating processes, LIMS reduce human error and create detailed audit trails. This functionality collectively improves data integrity and operational efficiency in laboratories, ultimately fostering a culture of regulatory excellence. As compliance challenges continue to evolve, the implementation of LIMS emerges as a critical strategy for organizations aiming to uphold the highest standards in the industry.
Introduction
The landscape of pharmaceutical compliance is increasingly complex, as regulations evolve to uphold the highest standards of safety and efficacy. Central to this transformation is the Laboratory Information Management System (LIMS), a robust software solution that not only streamlines laboratory workflows but also strengthens data integrity and regulatory adherence. As organizations endeavor to navigate the intricate web of compliance requirements, a pivotal question emerges: how can LIMS enhance operational efficiency while simultaneously serving as a critical ally in overcoming regulatory challenges?
Define LIMS: Core Concepts and Functionality
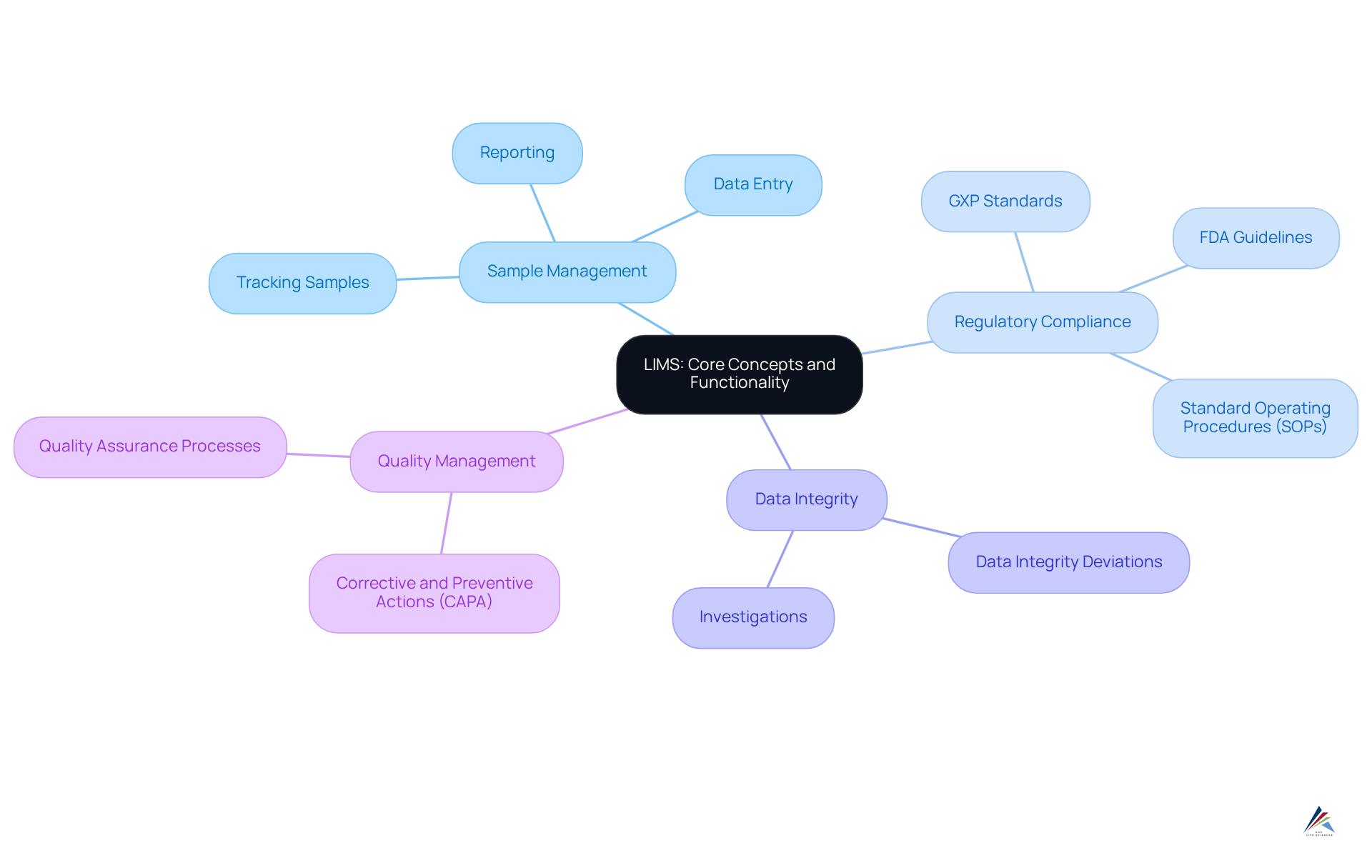
The LIMS definition refers to a sophisticated software solution designed to manage samples, associated data, and workflows efficiently. This system facilitates the tracking of samples through various stages of testing and analysis, ensuring that information is meticulously documented and readily accessible. By integrating with laboratory devices, it streamlines essential procedures such as data entry, sample tracking, and reporting, thereby significantly enhancing laboratory productivity.
In the context of pharmaceutical regulations, the LIMS definition emphasizes that laboratory information management systems are crucial for maintaining data integrity, ensuring traceability, and supporting compliance with regulatory obligations, including adherence to GXP standards and FDA guidelines. The implementation of robust Standard Operating Procedures (SOPs) and effective documentation practices empowers organizations to navigate the complexities of adeptly.
Moreover, the system effectively addresses Data Integrity Deviations, Investigations, and Corrective and Preventive Actions (CAPA), thereby contributing to a comprehensive quality management framework within the life sciences sector. By leveraging such systems, organizations not only streamline their operations but also reinforce their commitment to regulatory excellence.
Explore LIMS in Pharmaceutical Compliance: Importance and Applications
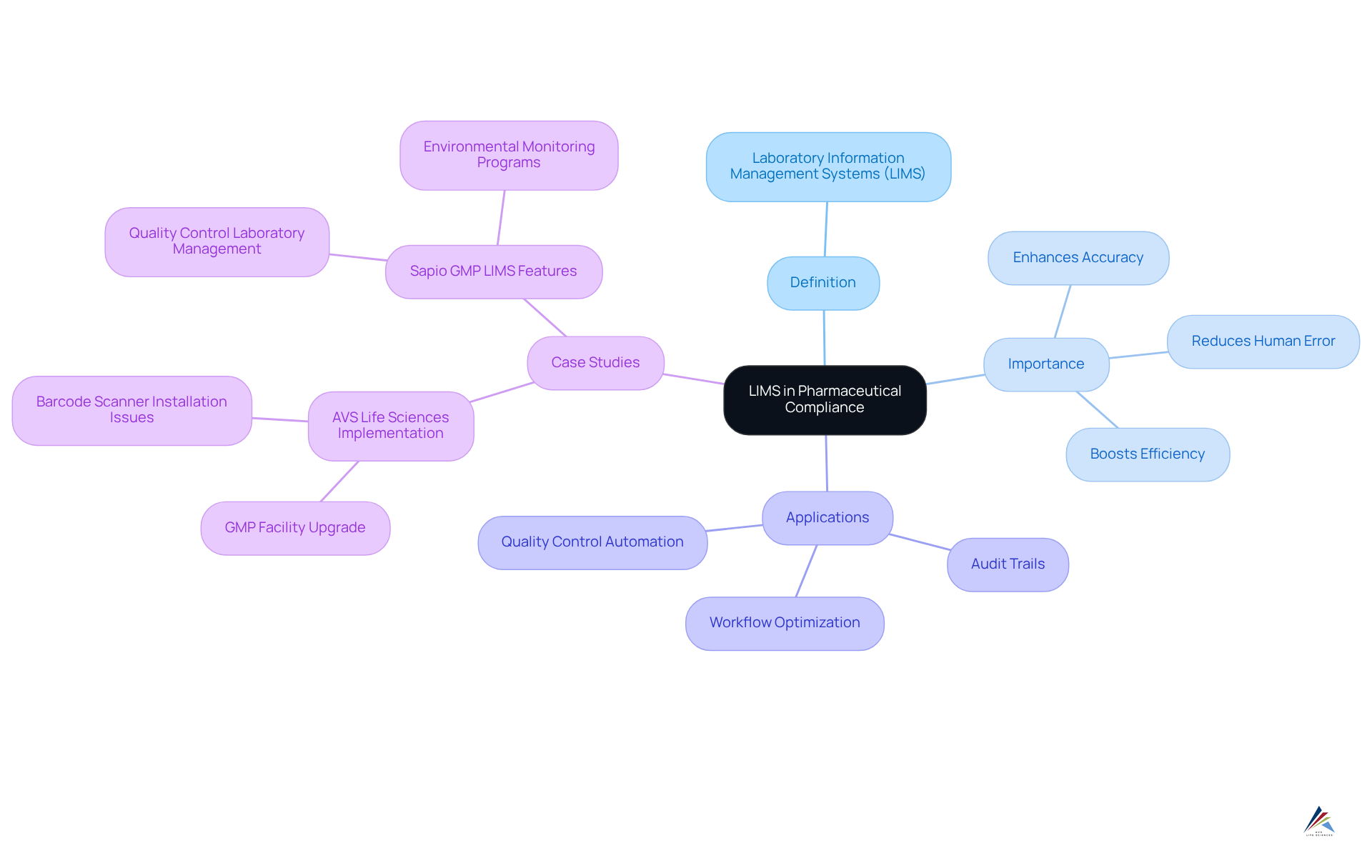
The lims definition indicates that Laboratory Information Management Systems (LIMS) are crucial for pharmaceutical compliance, ensuring strict adherence to Good Manufacturing Practices (GMP) and various regulatory standards. The LIMS definition illustrates how automating information gathering and administration can significantly reduce human error and enhance accuracy—an essential factor for successful regulatory submissions.
For instance, these systems can automatically generate detailed audit trails that document every modification made to data, thereby ensuring transparency and accountability throughout testing processes. Furthermore, LIMS optimize workflows, guaranteeing that all operations align with established protocols. This capability not only boosts efficiency but also during inspections and audits.
The LIMS definition highlights how the integration of such systems in pharmaceutical laboratories has proven to elevate adherence rates; research indicates that organizations utilizing LIMS experience a notable reduction in regulatory-related challenges, ultimately fostering a culture of quality and regulatory observance.
A transformative case study involving AVS Life Sciences exemplifies this: the company successfully upgraded a biotechnology GMP facility, enhancing quality assurance and meeting regulatory standards. During this upgrade, challenges such as the incorrect installation of barcode scanner cameras were identified, which initially resulted in anomalies in test results. This experience underscored the importance of comprehensive testing and quality management, further advocating for the effective implementation of LIMS in regulatory efforts.
Additionally, the recent acquisition of ValidPath enhances AVS Life Sciences' capabilities in quality engineering, further supporting the successful execution of LIMS in compliance initiatives.
Trace the Evolution of LIMS: Historical Development and Advancements
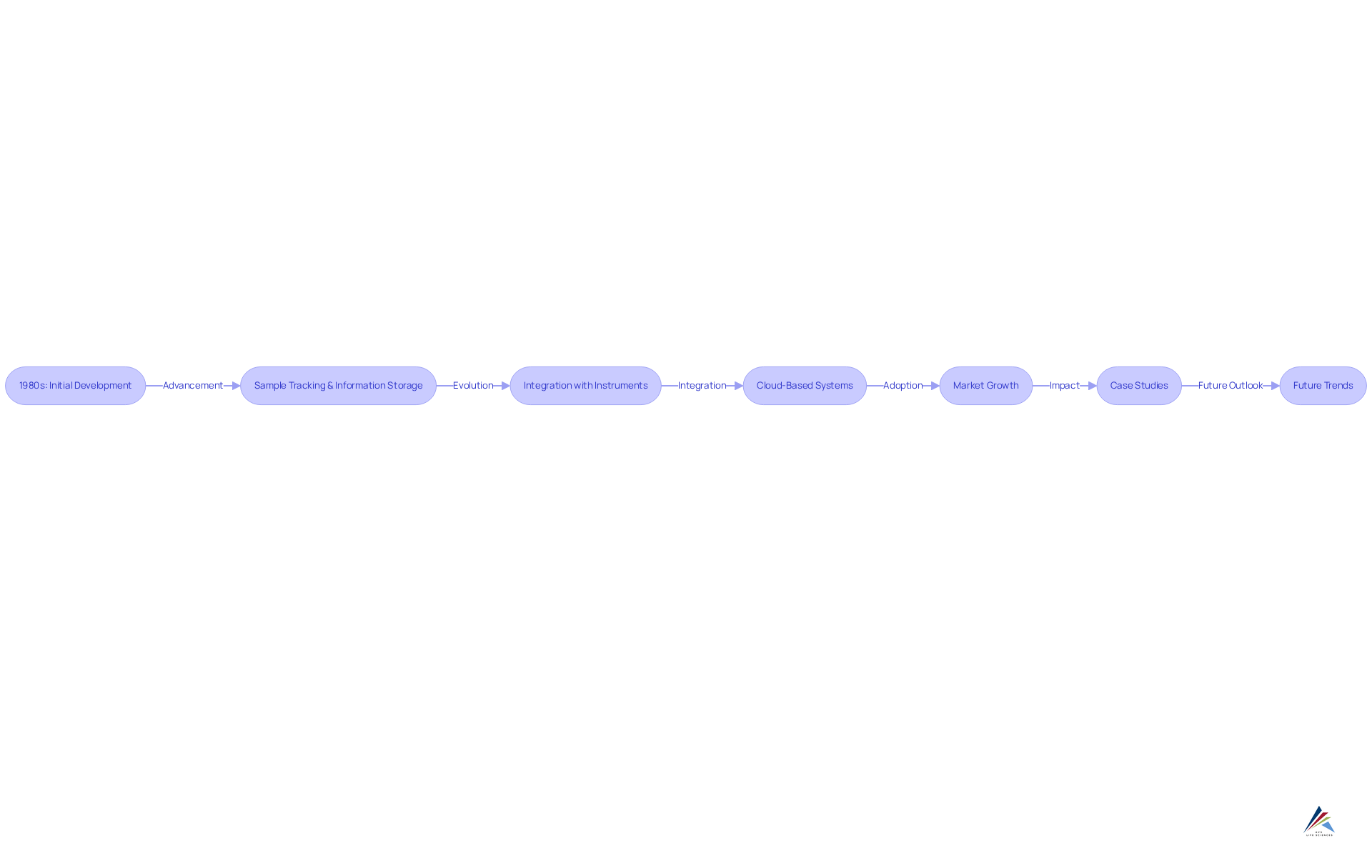
The development of Information Management Solutions commenced in the 1980s, driven by research facilities' need to enhance information management and optimize processes. Initially, these systems focused on fundamental functions such as sample tracking and information storage. However, as technology advanced, information management systems evolved into sophisticated solutions that seamlessly integrate with scientific instruments, support complex workflows, and provide advanced data analytics capabilities. The introduction of cloud-based management systems has significantly improved accessibility and collaboration, enabling research facilities to operate more efficiently while adhering to stringent regulatory standards.
Statistics indicate that the global informatics market for laboratories is projected to grow from $3.62 billion in 2023 to $5.25 billion by 2028, primarily fueled by the increasing adoption of laboratory information management systems (LIMS), as outlined in the LIMS definition, in pharmaceutical facilities. This growth underscores the vital role that the plays in modern laboratory operations, particularly in maintaining data integrity and compliance within the pharmaceutical sector.
Case studies exemplify this evolution:
- Adcock's achievement as India's first 100% paperless facility highlights the transformative potential of digitalization in operational processes.
- During their transition, Adcock confronted challenges related to partial automation, which they adeptly navigated to establish a new benchmark in laboratory operations.
- Similarly, the development of independent laboratory information management systems in the 1980s marked a significant shift towards automating complex information processing tasks, which aligns with the LIMS definition by reducing reliance on paper records and enhancing overall efficiency.
As laboratory information management systems continue to advance, they are expected to incorporate cutting-edge technologies such as artificial intelligence and machine learning, addressing persistent concerns regarding data security and regulatory compliance. This trajectory reflects the growing complexity of research operations and the pharmaceutical sector's heightened emphasis on maintaining high standards of quality and compliance. AVS Life Sciences plays a critical role in this landscape by providing comprehensive quality management and regulatory compliance solutions that align with the advancements in information management systems, ensuring that facilities can meet the evolving demands of the industry.
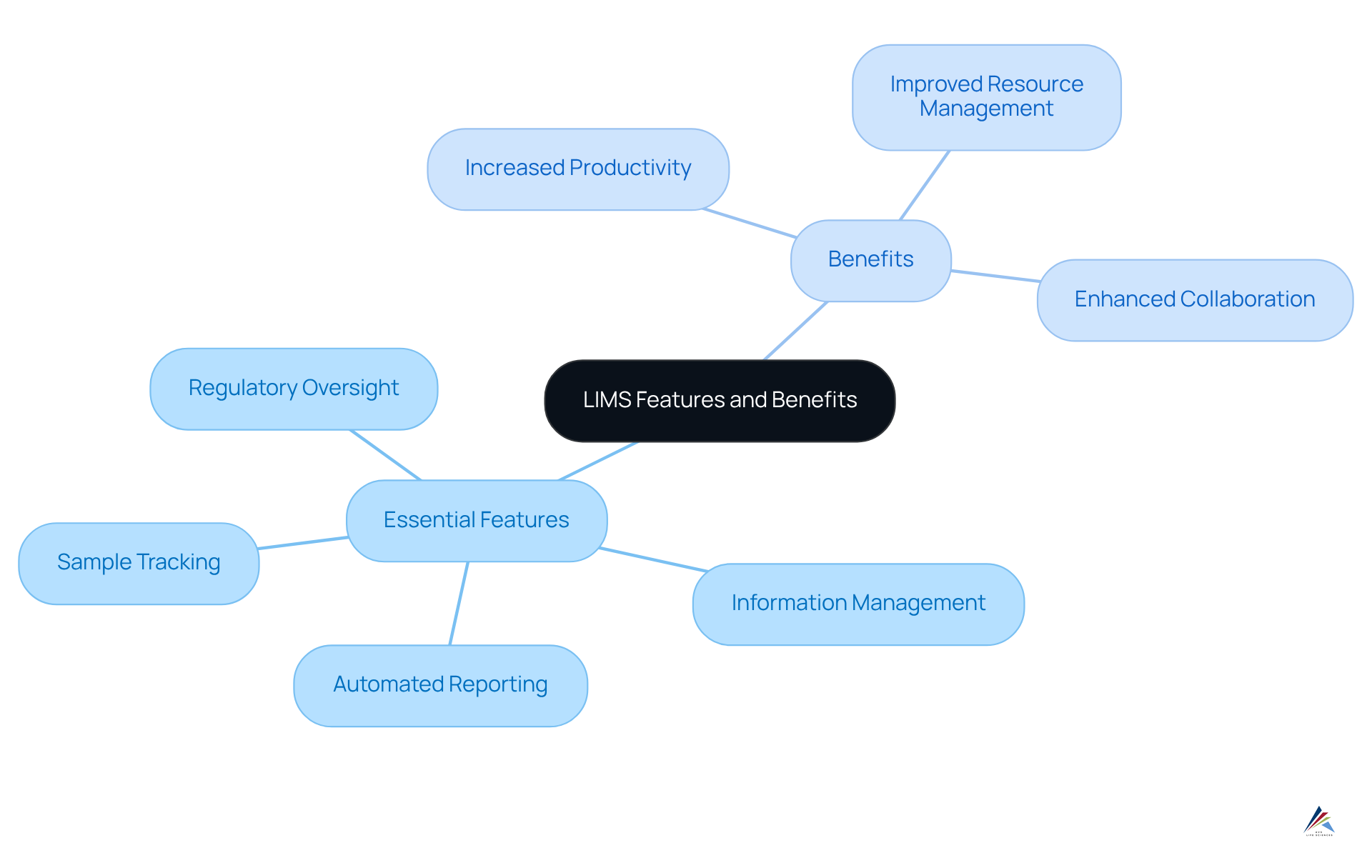
Identify Key Features and Benefits of LIMS: Enhancing Efficiency and Compliance
Essential aspects of the system encompass sample tracking, information management, automated reporting, and regulatory oversight. These elements significantly enhance operational efficiency within the research environment. Notably, automated reporting features streamline the creation of regulatory documents, which are crucial during audits, thereby reducing the time and effort required for manual documentation. Furthermore, the system facilitates instant data retrieval, empowering facilities to promptly address regulatory inquiries and adapt to evolving standards.
According to the LIMS definition, the advantages of adopting laboratory information management systems extend beyond mere compliance; they encompass increased productivity, improved resource management, and enhanced collaboration among laboratory personnel. For instance, AVS Life Sciences, with over 300 skilled professionals globally, exemplifies how effective implementation of laboratory information management systems can lead to superior quality outcomes in pharmaceutical development. This comprehensive approach underscores the critical role of the LIMS definition in navigating the intricacies of regulatory environments, contributing to AVS's remarkable 80% repeat business rate. This statistic highlights the concerning LIMS and compliance monitoring.
Conclusion
The role of Laboratory Information Management Systems (LIMS) in pharmaceutical compliance is undeniably pivotal. It serves as a sophisticated tool that enhances data management, sample tracking, and regulatory adherence. By effectively integrating technology into laboratory processes, LIMS not only streamlines operations but also fortifies organizations against compliance challenges, ensuring that they uphold the highest standards of quality and integrity.
The importance of LIMS is underscored through various aspects, including its ability to:
- Automate workflows
- Generate detailed audit trails
- Support adherence to Good Manufacturing Practices (GMP)
Case studies, such as the transformation of AVS Life Sciences and the achievements of Adcock in establishing a paperless facility, highlight the tangible benefits that come from implementing robust LIMS solutions. These systems not only reduce human error but also foster a culture of accountability and excellence in regulatory compliance.
Embracing LIMS is not merely a technological upgrade; it is a strategic imperative for pharmaceutical organizations aiming to navigate the complexities of regulatory landscapes. As the industry evolves and the demand for stringent compliance increases, investing in LIMS will be crucial for fostering innovation, enhancing operational efficiency, and ultimately ensuring the delivery of safe, effective products to the market. The time to prioritize LIMS as a cornerstone of pharmaceutical compliance is now, as its benefits extend far beyond compliance to influence the very fabric of laboratory excellence.
Frequently Asked Questions
What is a LIMS?
A LIMS, or Laboratory Information Management System, is a sophisticated software solution designed to manage samples, associated data, and workflows efficiently in a laboratory setting.
What are the core functionalities of a LIMS?
A LIMS facilitates the tracking of samples through various stages of testing and analysis, integrates with laboratory devices, and streamlines procedures such as data entry, sample tracking, and reporting, thereby enhancing laboratory productivity.
How does a LIMS support regulatory compliance?
A LIMS is crucial for maintaining data integrity, ensuring traceability, and supporting compliance with regulatory obligations, including adherence to GXP standards and FDA guidelines.
What role do Standard Operating Procedures (SOPs) play in LIMS?
The implementation of robust Standard Operating Procedures (SOPs) and effective documentation practices within a LIMS empowers organizations to navigate the complexities of regulatory compliance adeptly.
How does a LIMS address Data Integrity Deviations?
A LIMS effectively addresses Data Integrity Deviations, Investigations, and Corrective and Preventive Actions (CAPA), contributing to a comprehensive quality management framework within the life sciences sector.
What benefits do organizations gain from using a LIMS?
By leveraging a LIMS, organizations streamline their operations and reinforce their commitment to regulatory excellence in the laboratory environment.
