Is Your Manufacturer Registered? Why It Matters for Your Brand

Overview
Manufacturer registration is essential for ensuring compliance with regulatory standards, fostering consumer trust, and facilitating market access. This not only protects a brand's reputation but also minimizes legal risks. Registered manufacturers adhere to safety regulations, which significantly enhances consumer confidence and allows for smoother entry into various markets. Thus, verifying a manufacturer's registration status is crucial for maintaining brand integrity. By prioritizing registration, companies can navigate compliance challenges effectively, leading to successful market positioning and a robust reputation.
Introduction
Manufacturer registration represents a critical yet often overlooked facet of business operations, one that can profoundly influence a brand's integrity and market presence. The proper registration of manufacturers not only ensures adherence to legal standards but also cultivates consumer trust and opens avenues to new markets. Yet, many brands face a pressing dilemma: what are the consequences of operating with an unregistered manufacturer? This article explores the significance of manufacturer registration, outlines the steps for verifying it, and examines the potential risks associated with neglecting this essential process. Ultimately, it serves as a guide for brands aiming to protect their reputation and ensure compliance.
Understand the Importance of Manufacturer Registration
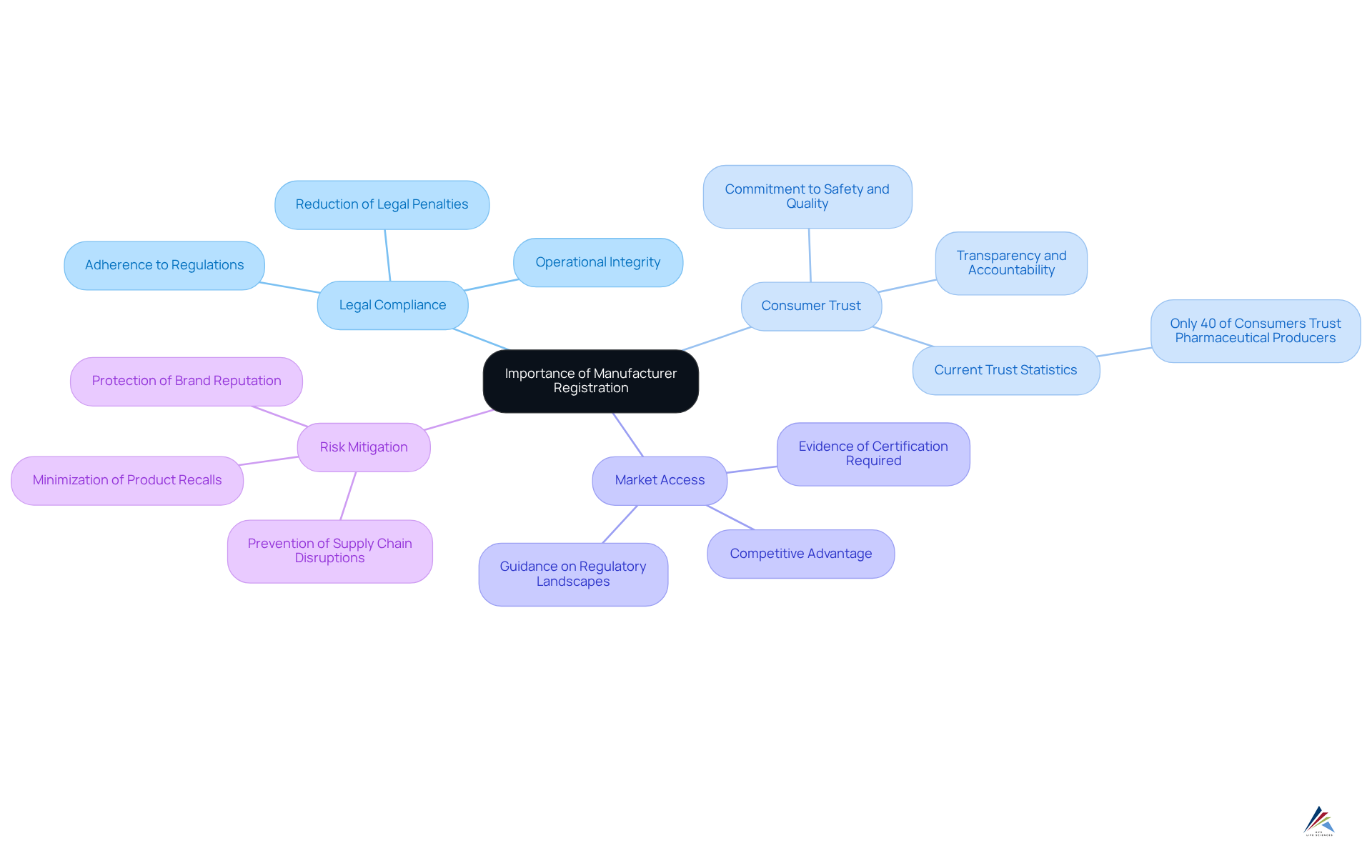
Manufacturer enrollment is essential for ensuring that products comply with regulatory standards and are safe for consumer use. Operating without proper documentation exposes your brand to significant legal and financial risks. Understanding the importance of manufacturer registration is crucial for several key reasons:
- Legal Compliance: Registered manufacturers adhere to local and international regulations, significantly reducing the risk of legal penalties. This compliance is vital for maintaining operational integrity and avoiding costly fines. AVS Life Sciences offers comprehensive GXP regulatory services tailored to the biopharmaceuticals, medical devices, and nutraceuticals sectors, ensuring your operations meet these critical standards.
- Consumer Trust: Is your manufacturer registered? Why it matters for your brand is that registration fosters consumer confidence in your products, demonstrating a commitment to safety and quality. In 2025, statistics suggest that only 40% of consumers view pharmaceutical producers as reliable, highlighting the necessity for transparency and accountability in the industry. By partnering with AVS Life Sciences, you can leverage expert quality solutions that enhance your brand's reputation.
- Market Access: Numerous markets require evidence of manufacturer certification for product entry, which raises the question, is your manufacturer registered? Why it matters for your brand in terms of business expansion. Without this enrollment, companies may struggle to in key markets. AVS Life Sciences provides guidance on navigating these regulatory landscapes, ensuring your products can reach their intended audiences.
- Risk Mitigation: Ensuring that your suppliers are registered minimizes the risk of product recalls and the associated costs. A proactive strategy for enrollment is essential; is your manufacturer registered? Why it matters for your brand is that it can prevent potential disruptions in the supply chain and safeguard your brand's reputation. With AVS Life Sciences' deep industry experience, you can implement effective quality compliance and validation solutions that protect your operations.
In conclusion, producer enrollment is not merely a bureaucratic necessity; it is a crucial element of responsible business conduct in the life sciences sector. As industry leaders emphasize, "Compliance is not just about avoiding penalties; it's about building trust and ensuring the safety of our products." By emphasizing enrollment and partnering with AVS Life Sciences, companies can enhance their credibility and cultivate enduring relationships with consumers.
Steps to Verify Manufacturer Registration
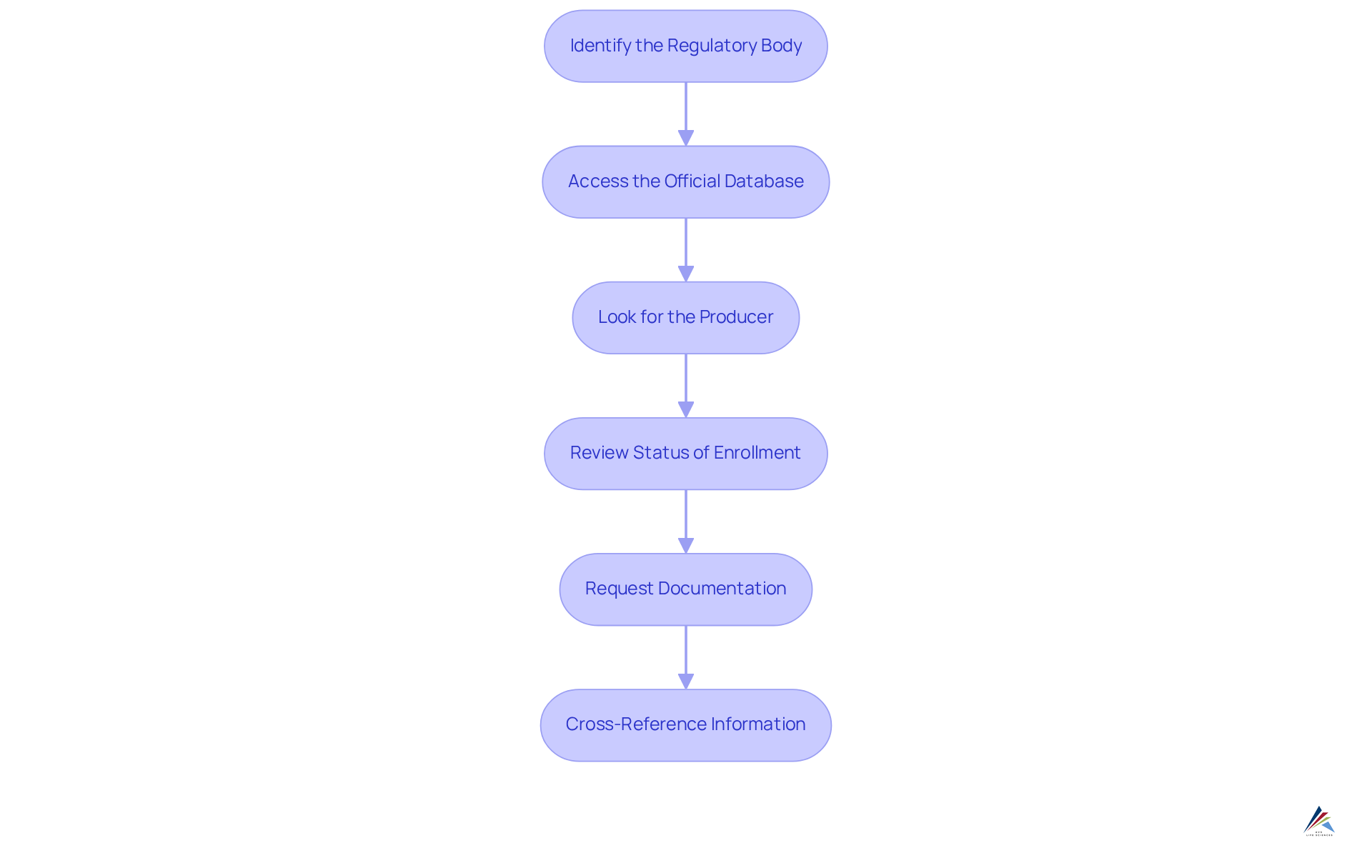
To verify a manufacturer's registration, follow these systematic steps:
- Identify the Regulatory Body: Begin by determining the appropriate regulatory authority overseeing the manufacturer, such as the FDA for pharmaceuticals in the U.S.
- Access the Official Database: Navigate to the official website of the regulatory authority and locate their licensing section.
- Look for the Producer: Enter the producer’s name or identification number into the database, ensuring correct spelling and details.
- Review Status of Enrollment: Confirm that the enrollment is active and not expired, and check for any compliance issues or warnings associated with the producer.
- Request Documentation: If necessary, acquire copies of the producer’s certification or other pertinent documents to validate their assertions.
- Cross-Reference Information: Validate the information against additional sources, such as industry reports or third-party audits, to ensure thorough due diligence.
By following these steps, you can confidently ascertain if your producer is your manufacturer registered? why it matters for your brand and .
Troubleshoot Common Verification Issues
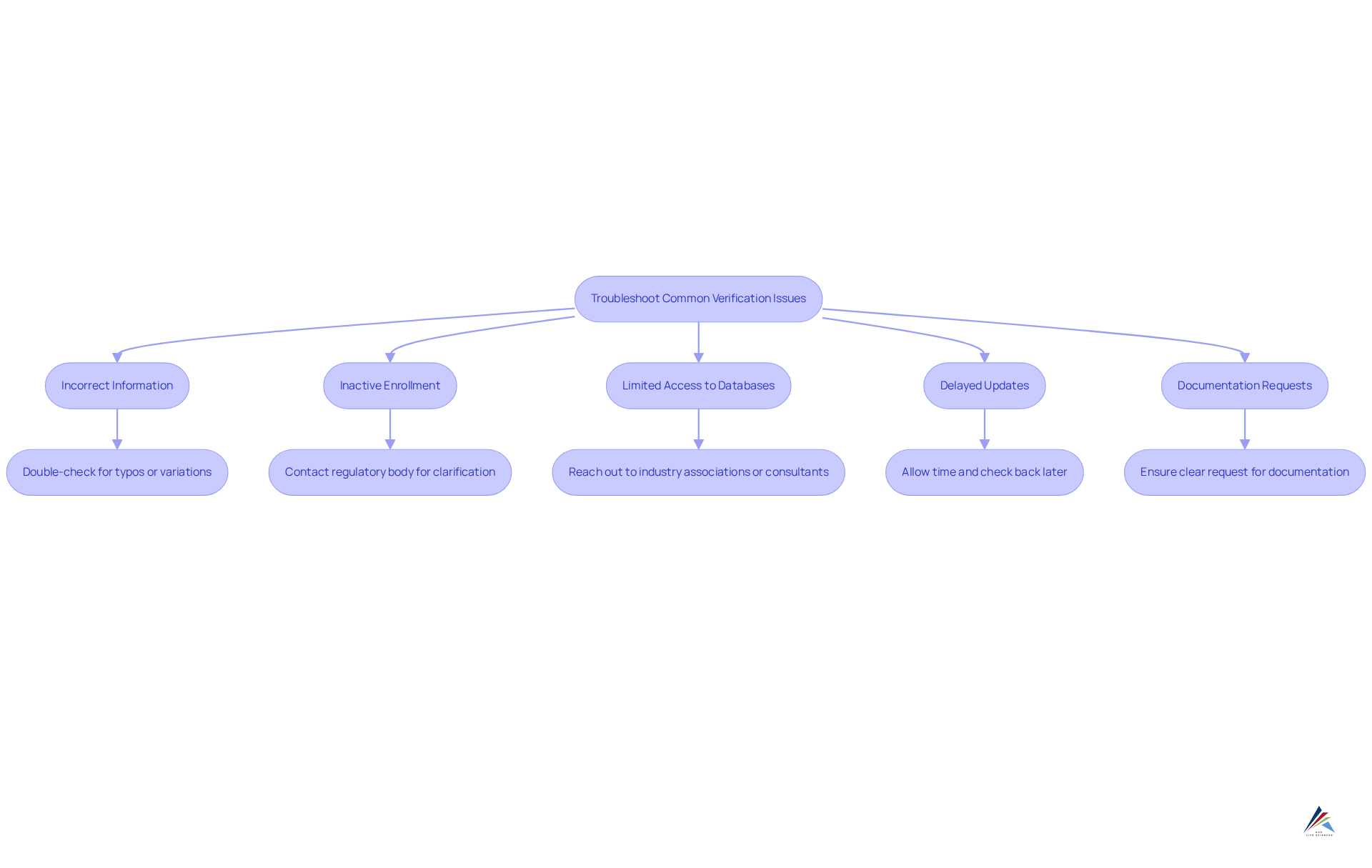
When checking producer enrollment, several typical problems may arise. Here’s how to troubleshoot them effectively:
- Incorrect Information: If the manufacturer’s name or registration number yields no results, double-check for typos or variations. Manufacturers often operate under different names or aliases, which can lead to confusion.
- Inactive Enrollment: If the enrollment appears inactive, it’s essential to contact the regulatory body for clarification. There may be pending renewals or compliance issues that need to be addressed. Remarkably, a considerable portion of producers has dormant enrollments, which can impact your supply chain. Industry statistics indicate that approximately 20% of manufacturers have inactive registrations, underscoring the importance of verifying registration status.
- Limited Access to Databases: Some compliance databases require specific credentials or subscriptions for access. If you encounter obstacles, consider reaching out to industry associations or compliance consultants who may possess the necessary access to assist you.
- Delayed Updates: Regulatory databases may not reflect real-time updates. If you suspect a producer is registered but cannot find confirmation, allow some time and check back later. This delay can often be attributed to the time it takes for regulatory bodies to process updates.
- Documentation Requests: If a producer is reluctant to provide documentation, this may signal potential issues. Ensure you have a clear request and emphasize the importance of transparency in your business relationship. A proactive approach can help mitigate risks associated with non-compliance. As highlighted by regulatory specialists, maintaining precise manufacturer registrations is essential for guaranteeing adherence and raises the question, is your manufacturer registered? why it matters for your brand.
To further support your verification process, consider utilizing the user manuals and offered by AVS Life Sciences. Our team of top industry experts is dedicated to delivering customized consulting solutions that enhance your regulatory efforts and ensure the integrity of your supply chain. By being aware of these common issues and knowing how to address them, you can navigate the verification process more effectively, ensuring that your brand maintains compliance and quality standards.
Conclusion
Ensuring that a manufacturer is properly registered transcends regulatory formality; it stands as a cornerstone of a brand's integrity and success. The significance of manufacturer registration extends beyond mere compliance; it plays a vital role in fostering consumer trust, ensuring market access, and mitigating risks associated with product safety and quality. By prioritizing this essential aspect of business operations, brands can enhance their credibility and cultivate lasting relationships with their customers.
Key insights from this article underscore the critical reasons for manufacturer registration, including:
- Legal compliance
- Consumer confidence
- Effective risk management
The steps outlined for verifying a manufacturer's registration status provide a practical framework for businesses to ensure their partners meet industry standards. Moreover, addressing common verification issues equips brands with the necessary tools to navigate potential challenges in maintaining compliance.
Ultimately, the message is unequivocal: the registration of manufacturers is crucial for safeguarding both brand reputation and consumer safety. Companies are urged to take proactive measures in verifying their manufacturers' registrations and to seek expert guidance when necessary. By doing so, brands not only protect their interests but also contribute to a more transparent and trustworthy marketplace.
Frequently Asked Questions
Why is manufacturer registration important?
Manufacturer registration is essential for ensuring compliance with regulatory standards, which helps protect consumer safety and reduces legal and financial risks for brands.
How does manufacturer registration affect legal compliance?
Registered manufacturers adhere to local and international regulations, significantly lowering the risk of legal penalties, maintaining operational integrity, and avoiding costly fines.
What role does manufacturer registration play in consumer trust?
Registration fosters consumer confidence by demonstrating a commitment to safety and quality, which is crucial as only 40% of consumers are expected to view pharmaceutical producers as reliable by 2025.
How does manufacturer registration impact market access?
Many markets require evidence of manufacturer certification for product entry. Without registration, companies may struggle to compete effectively in key markets.
What are the risks of not having registered manufacturers?
Not ensuring that suppliers are registered increases the risk of product recalls and associated costs, which can disrupt the supply chain and damage a brand's reputation.
How can AVS Life Sciences assist with manufacturer registration?
AVS Life Sciences offers comprehensive GXP regulatory services that help companies navigate regulatory landscapes, ensure compliance, and enhance product quality, thereby supporting manufacturer registration efforts.
