Implementing EQMS / EDMS for Validation: Key Practices for Compliance

Overview
The article addresses the fundamental practices essential for the successful implementation of Enterprise Quality Management Systems (EQMS) and Electronic Document Management Systems (EDMS) aimed at ensuring regulatory compliance. It underscores the necessity of:
- Establishing clear objectives
- Actively engaging stakeholders
- Conducting comprehensive risk assessments
- Maintaining meticulous documentation
These elements are vital for effective validation processes and adherence to regulations such as FDA 21 CFR Part 11. By systematically addressing these compliance challenges, organizations can enhance their validation efforts and foster a culture of accountability and excellence.
Introduction
Implementing effective quality management systems is no longer merely a regulatory requirement; it has evolved into a fundamental necessity for organizations striving for excellence in the pharmaceutical and life sciences sectors.
The integration of Enterprise Quality Management Systems (EQMS) and Electronic Document Management Systems (EDMS) for validation presents a powerful opportunity to streamline compliance processes and enhance operational efficiency.
However, navigating the complexities of these systems raises critical questions:
- How can organizations ensure they are leveraging EQMS and EDMS effectively to meet stringent standards?
- What best practices can be adopted to foster a culture of continuous improvement while maintaining compliance?
This article delves into key practices for implementing EQMS and EDMS, providing insights that can empower organizations to thrive in a challenging regulatory landscape.
Define EQMS and EDMS: Core Concepts and Differences
An Enterprise Quality Management System (EQMS) serves as a digital platform designed to centralize, automate, and streamline management processes related to organizational standards, including EQMS / EDMS for validation. It focuses on overseeing quality-related tasks, including audits, corrective measures, and adherence monitoring. In contrast, an Electronic Document Management System (EDMS) primarily manages documents, encompassing their creation, storage, retrieval, and sharing. While an EDMS aids in document control, the use of EQMS / EDMS for validation encompasses extensive management processes, making it essential for organizations aiming for . Recognizing these distinctions is vital for organizations to select the appropriate system for their needs and ensure that both systems operate in tandem to enhance overall quality management.
For example, an organization might utilize an EDMS to manage standard operating procedures (SOPs) while employing an EQMS to track deviations and corrective actions related to those SOPs. This integration is crucial for maintaining adherence to regulatory standards such as FDA 21 CFR Part 11 and ISO 9001.
The pharmaceutical quality management systems market, valued at USD 1.4 billion in 2022, is projected to grow at a compound annual growth rate (CAGR) of 13.1% by 2032, driven by the rising demand for high-quality drugs and stringent regulatory requirements. This underscores the importance of implementing robust quality management systems and EQMS / EDMS for validation to effectively navigate the complexities of compliance.
Implement Best Practices for EQMS/EDMS in Validation Processes
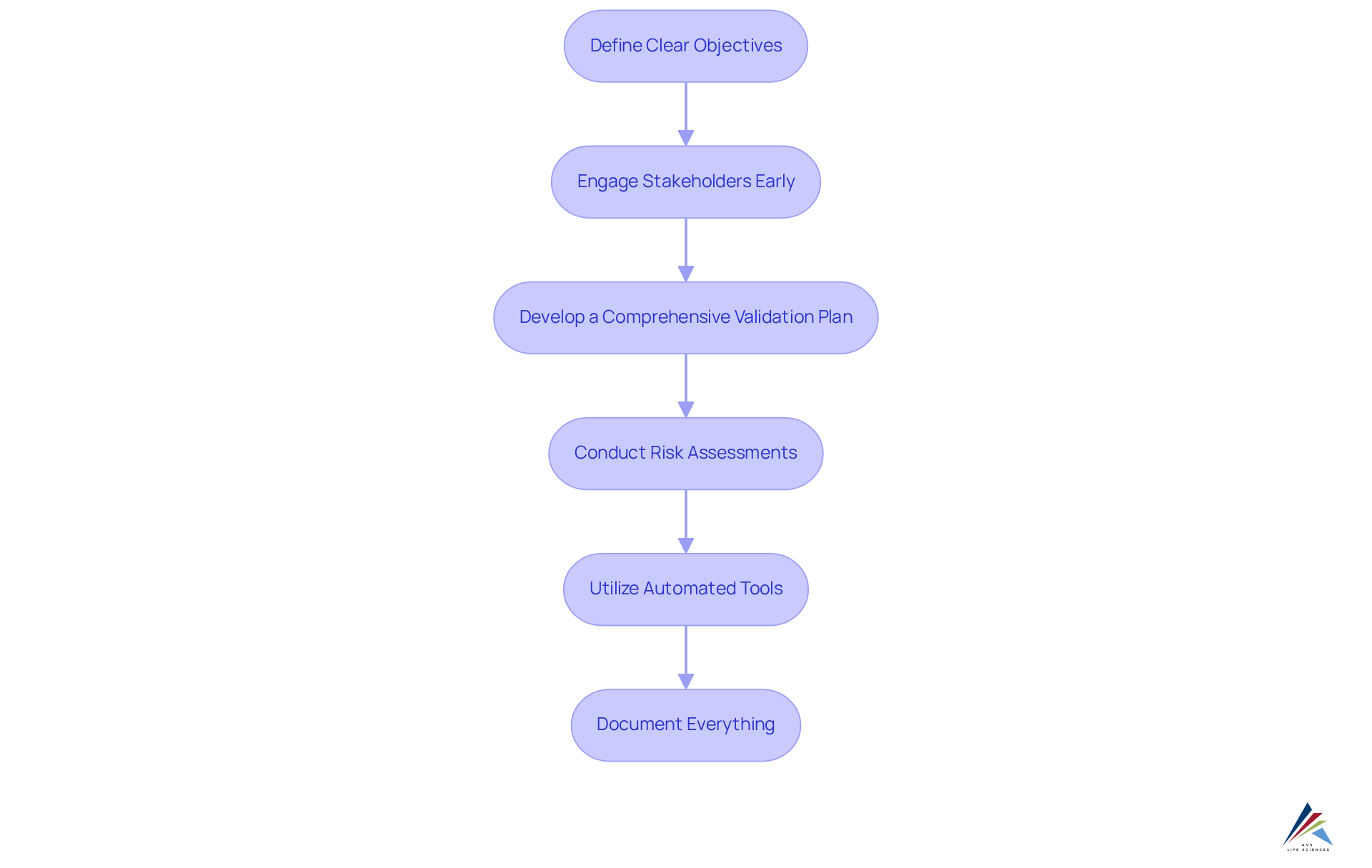
To effectively implement best practices for EQMS and EDMS in validation processes, organizations should adhere to the following steps:
- Define Clear Objectives: Establish specific goals for the validation process, ensuring alignment with regulatory requirements and internal standards. Highlighting the Cost of Poor Quality (COPQ), which can range from 10% to 20% of a company’s annual revenue, underscores the necessity of effective EQMS / EDMS for validation to mitigate these costs.
- Engage Stakeholders Early: Involve key stakeholders—such as quality assurance, IT, and end-users—right from the beginning. This engagement is crucial for tailoring the systems to meet their needs and expectations, ultimately enhancing adoption rates.
- Develop a Comprehensive Validation Plan: Create a detailed plan that outlines the scope, resources, timelines, and responsibilities associated with the validation process. This structured approach helps in . The plan should also incorporate the stages of Computer System Validation (CSV), including defining User Requirement Specifications (URS) and Design Specifications, to ensure thorough preparation.
- Conduct Risk Assessments: Identify potential risks linked to the implementation of EQMS / EDMS for validation. Developing mitigation strategies for these risks is essential to ensure a smooth transition and compliance. This aligns with the CSV process, where risk assessments are integral to the use of EQMS / EDMS for validation to ensure that systems operate as intended.
- Utilize Automated Tools: Leverage automation features within EQMS / EDMS for validation to streamline your tasks. Notably, over 65% of manufacturers still rely on manual inspection methods, contributing to high recall rates of 40%. Automation not only minimizes manual errors but also improves data integrity, which is essential in upholding standards, particularly during the Installation Qualification (IQ) and Operational Qualification (OQ) testing phases of CSV.
- Document Everything: Maintain thorough documentation throughout the validation process. This documentation serves as evidence of compliance and is vital for facilitating audits. The reporting stage of CSV emphasizes the importance of organizing validation results as tangible evidence that the system is ready for release.
For instance, a pharmaceutical firm adopting a quality management system can automate the monitoring of validation tasks, ensuring that all required documentation is created and kept in line with regulatory standards. This proactive approach not only improves efficiency but also significantly reduces the risk of non-compliance, as evidenced by organizations that have successfully implemented EQMS / EDMS for validation in their processes, achieving higher success rates and improved stakeholder satisfaction. As W. Edwards Deming stated, "Excellence should be integrated, not checked," emphasizing the importance of proactive management of standards.
Ensure Compliance with Regulatory Standards and Quality Management
To ensure compliance with regulatory standards and quality management, organizations must take decisive actions:
- Understand Relevant Regulations: It is imperative to familiarize yourself with applicable regulations, such as , ISO 9001, and GxP guidelines. Ensuring that your eqms / edms for validation are compliant is crucial. Particularly, compliance with 21 CFR Part 11 is essential, as it mandates the integrity and authenticity of electronic records and signatures, which are fundamental for maintaining data reliability.
- Implement Data Integrity Controls: Establish robust controls to ensure data integrity, including audit trails, access controls, and data encryption. These measures are vital for safeguarding sensitive information and ensuring that all alterations, modifications, and removals are meticulously documented in the database, thereby protecting against data breaches and unauthorized access.
- Regularly review and update your systems, focusing on eqms / edms for validation, to ensure compliance with evolving regulations and industry standards. Frequent updates are essential to adapt to changes in regulatory requirements, such as those specified in ISO 9001:2025, which emphasizes effective monitoring and measurement of quality management systems.
- Train staff on compliance requirements by providing comprehensive training for employees on regulatory requirements and the proper use of EQMS / EDMS for validation. This training fosters a culture of adherence and equips staff to manage data integrity issues effectively, which is vital for upholding high standards in the life sciences sector.
- Conduct Internal Audits: Regularly perform internal audits to evaluate adherence to established procedures and identify areas for enhancement. These audits are crucial for demonstrating compliance with regulatory standards and sustaining an effective management system.
For instance, AVS Life Sciences successfully assisted a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This partnership not only ensured adherence to regulatory standards but also allowed the client to focus on developing medications that enhance patient well-being. By implementing a robust training program for its staff, the firm improved compliance and built trust with stakeholders, illustrating a commitment to data integrity and quality management. By adhering to 21 CFR Part 11, organizations can achieve enhanced data integrity and operational efficiency.

Foster Continuous Improvement and Training for Effective System Use
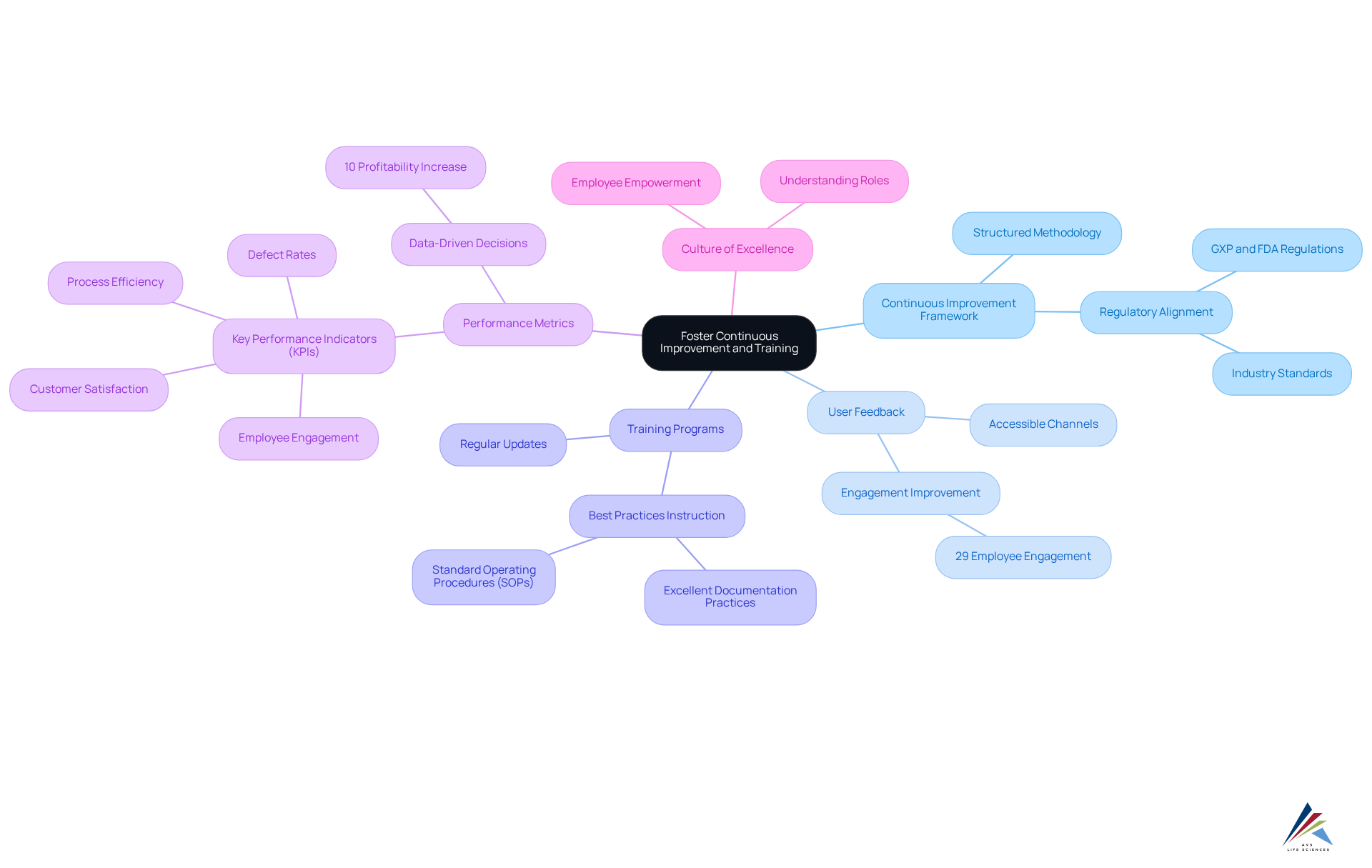
To foster continuous improvement and enhance the effectiveness of EQMS and EDMS, organizations should adopt the following key practices:
- Establish a Continuous Improvement Framework: Implement a structured methodology to identify, analyze, and enhance processes associated with EQMS / EDMS for validation, ensuring alignment with regulatory requirements such as GXP and FDA regulations, as well as industry standards.
- Encourage Feedback from Users: Create accessible channels for users to share insights on performance and usability. This feedback is essential for guiding future improvements and ensuring the frameworks meet user requirements effectively. Significantly, merely 29% of employees are completely involved in their tasks, emphasizing the necessity of efficient feedback systems to improve engagement and adherence.
- Regularly Update Training Programs: Continuously refresh training initiatives to incorporate the latest regulations, technological advancements, and best practices, ensuring that all users are well-equipped to utilize the platforms effectively. This encompasses instruction on Excellent Documentation Practices and Standard Operating Procedures (SOPs) that are crucial for upholding regulations.
- Utilize Performance Metrics: Monitor related to system usage and adherence. This data-driven approach helps identify areas needing improvement and supports informed decision-making. Companies that implement continuous improvement programs see an average 10% increase in profitability, underscoring the value of tracking performance metrics.
- Encourage a Culture of Excellence: Nurture an organizational environment where every employee comprehends their role in management processes and feels empowered to assist in upholding standards. This is especially crucial in the context of managing standards in a virtual company and ensuring adherence to CFR Part 11 regulations.
For instance, a medical device producer might establish a quarterly assessment procedure to gauge the effectiveness of its processes, specifically focusing on EQMS / EDMS for validation, while directly utilizing AVS Life Sciences' knowledge in management. This review would not only assess system performance but also provide targeted training sessions based on user feedback and performance metrics, thereby enhancing overall compliance and operational efficiency. As Micky J., a CEO, noted, "With its easy-to-use interface, Minitab is really one of the best applications out there … I use Minitab to analyze process data for overall product and quality improvement purposes.
Conclusion
Implementing an Enterprise Quality Management System (EQMS) and an Electronic Document Management System (EDMS) for validation is essential for organizations striving to achieve compliance and enhance quality management. Understanding the distinct roles these systems play enables businesses to effectively integrate them, streamlining processes and upholding regulatory standards. This integration not only facilitates compliance with critical regulations such as FDA 21 CFR Part 11 and ISO 9001 but also significantly mitigates the costs associated with poor quality.
Key practices for implementing EQMS and EDMS include:
- Setting clear objectives
- Engaging stakeholders
- Developing comprehensive validation plans
- Conducting risk assessments
- Utilizing automation
- Maintaining thorough documentation
Collectively, these steps ensure that organizations can navigate the complexities of compliance while fostering a culture of continuous improvement. Furthermore, regular training and updates are vital for keeping staff informed about evolving regulations and best practices, ultimately enhancing operational efficiency and data integrity.
In conclusion, the successful implementation of EQMS and EDMS is not merely a regulatory obligation but a strategic advantage that can lead to improved quality outcomes and stakeholder satisfaction. Organizations must embrace these systems as integral components of their quality management strategy, continuously seeking ways to refine processes and engage users. By doing so, they not only comply with regulatory standards but also position themselves for sustainable success in an increasingly competitive landscape.
Frequently Asked Questions
What is an Enterprise Quality Management System (EQMS)?
An EQMS is a digital platform designed to centralize, automate, and streamline management processes related to organizational standards, focusing on quality-related tasks such as audits, corrective measures, and adherence monitoring.
What is an Electronic Document Management System (EDMS)?
An EDMS primarily manages documents, including their creation, storage, retrieval, and sharing. It aids in document control within an organization.
How do EQMS and EDMS differ from each other?
While an EQMS oversees extensive management processes related to quality, an EDMS focuses on document management. EQMS is essential for comprehensive compliance, whereas EDMS is more about document control.
How can organizations utilize both EQMS and EDMS?
Organizations can use an EDMS to manage standard operating procedures (SOPs) while employing an EQMS to track deviations and corrective actions related to those SOPs, ensuring regulatory compliance.
Why is it important to integrate EQMS and EDMS?
Integration is crucial for maintaining adherence to regulatory standards, such as FDA 21 CFR Part 11 and ISO 9001, enhancing overall quality management within the organization.
What is the current market trend for pharmaceutical quality management systems?
The pharmaceutical quality management systems market was valued at USD 1.4 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 13.1% by 2032, driven by the demand for high-quality drugs and stringent regulatory requirements.
