How AVS Helps Brands Stay MoCRA-Compliant: A Step-by-Step Approach

Overview
AVS empowers brands to achieve MoCRA compliance through customized consulting services and resources designed to navigate the regulatory landscape of the Modernization of Cosmetics Regulation Act. This article delineates a systematic approach to compliance, encompassing:
- Facility registration
- Item listing
- The adoption of Good Manufacturing Practices
It underscores how AVS's specialized knowledge aids brands in effectively managing these complexities. By engaging with AVS Life Sciences, brands can confidently address compliance challenges and implement robust solutions that ensure adherence to industry standards.
Introduction
The Modernization of Cosmetics Regulation Act (MoCRA) marks a pivotal shift in the landscape of cosmetic regulation, ushering in a new era of accountability and safety for brands. As companies navigate the complex maze of compliance requirements—from mandatory facility registrations to stringent Good Manufacturing Practices (GMP)—the stakes have never been higher.
Brands must ask themselves: how can they effectively align their operations with these evolving regulations while ensuring they meet consumer safety standards? This guide delves into how AVS Life Sciences empowers brands to understand MoCRA and implement a strategic, step-by-step approach to achieve and maintain compliance.
Ultimately, this not only safeguards their reputation but also fortifies consumer trust.
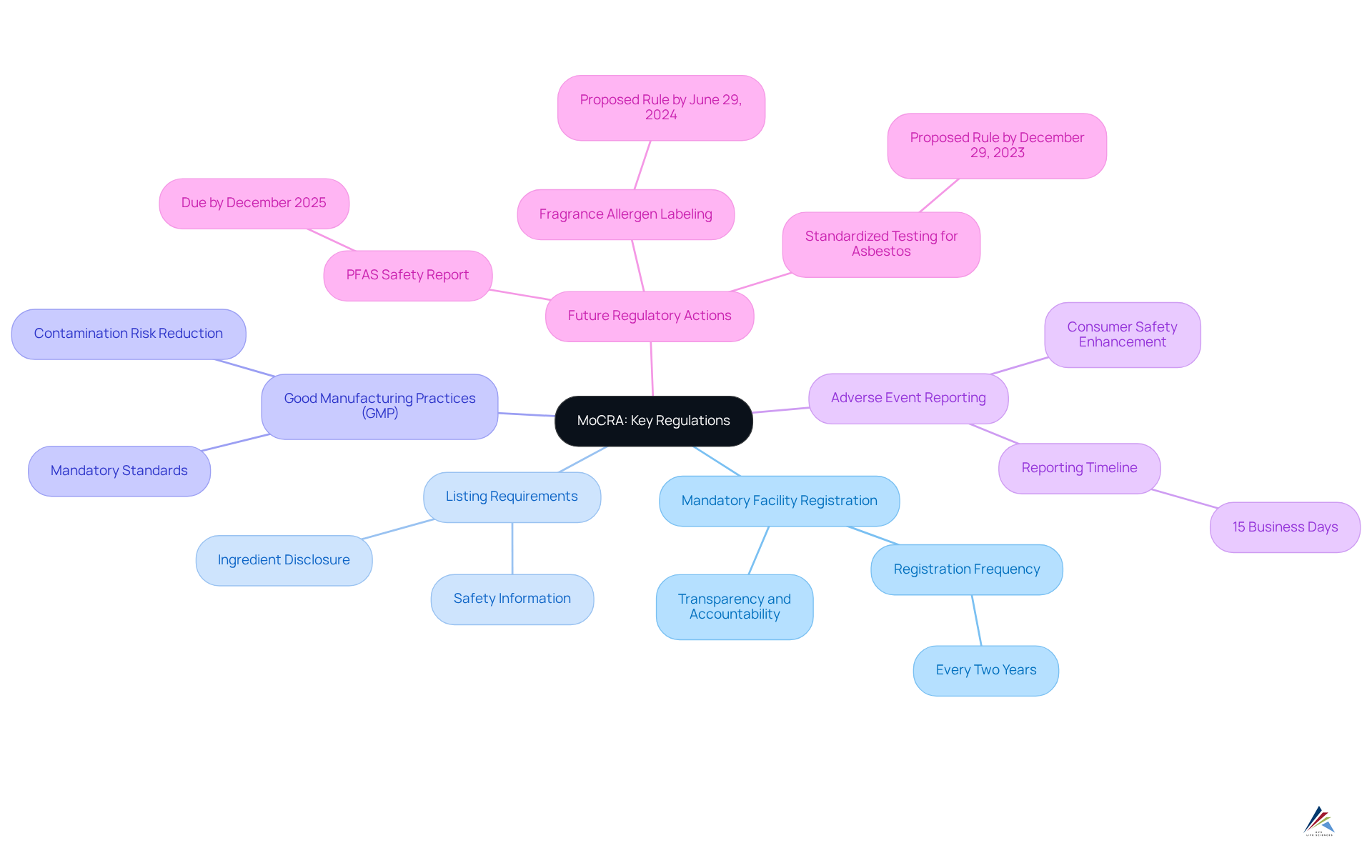
Understand MoCRA: Key Regulations and Implications
The Modernization of Cosmetics Regulation Act represents a significant evolution in the regulation of cosmetics within the United States, marking the most substantial expansion of the FDA’s authority over cosmetic products since the Federal Food, Drug, and Cosmetic (FD&C) Act of 1938. Key aspects include:
- Mandatory Facility Registration: All cosmetic manufacturers must register their facilities with the FDA, ensuring transparency and accountability.
- Listing Requirements: Brands are required to list their items with the FDA, providing essential information about ingredients and safety.
- Good Manufacturing Practices (GMP): MoCRA enforces GMP standards, which are crucial for ensuring safety and quality. This transition from voluntary to mandatory GMP is expected to significantly reduce contamination risks and enhance overall integrity.
- Adverse Event Reporting: Companies must report any serious adverse events linked to their offerings within 15 business days, thereby enhancing consumer safety.
Moreover, by December 2025, the FDA is mandated to release a report evaluating the safety of PFAS in cosmetic products, underscoring the importance of compliance for producers. The evolving nature of regulatory requirements is further highlighted by the FDA's directive to propose a rule for fragrance allergen labeling by June 29, 2024.
Understanding these regulations is imperative for brands to maintain standards and avoid penalties. Familiarizing yourself with the requirements of the legislation will aid in aligning your operations with the new standards set forth by the FDA. As the industry adapts to these changes, AVS Life Sciences stands ready to assist brands in navigating the complexities of regulations and demonstrates how AVS helps brands stay mocra-compliant through its extensive expertise and tailored consulting services.
Implement Compliance Strategies: Step-by-Step Guidance
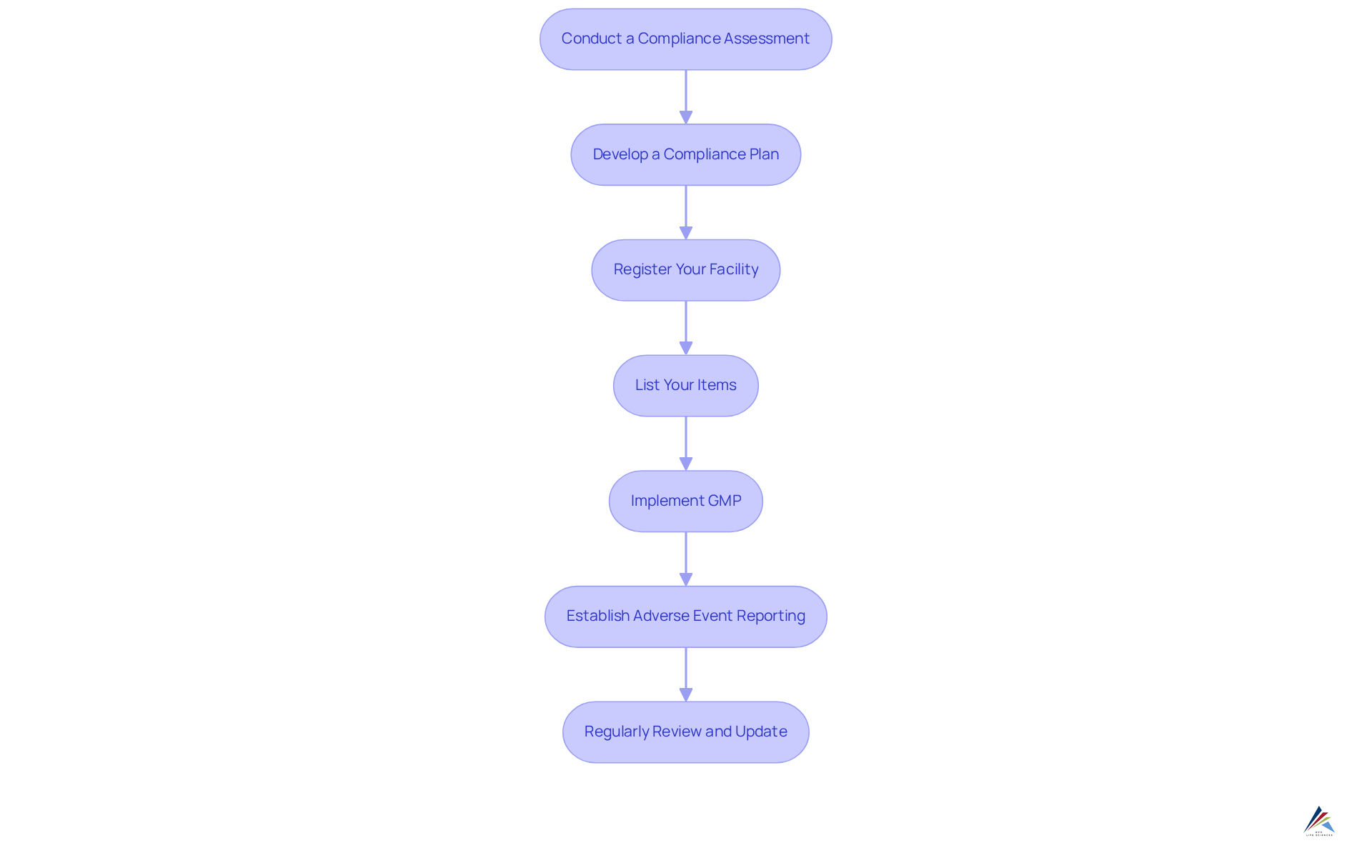
To implement effective compliance strategies under MoCRA, follow these steps:
- Conduct a Compliance Assessment: Begin by evaluating your current practices against the relevant regulations. Identify gaps in your processes, documentation, and training. This step is vital, as 42% of regulation experts highlight educating employees on policies as a major challenge.
- [Develop a Compliance Plan](https://drata.com/blog/compliance-statistics): Create a detailed plan outlining how your organization will meet MoCRA's requirements. Include timelines, responsibilities, and necessary resources.
- Register Your Facility: Ensure that your manufacturing facility is registered with the FDA. This essential initial step in compliance is particularly crucial, as items promoted after July 1, 2024, must be registered/listed within 120 days of entering the market.
- List Your Items: Submit item listings to the FDA, including all necessary information about ingredients and safety data.
- Implement GMP: Establish and document [Good Manufacturing Practices](https://avslifesciences.com/biopharma) within your operations. This includes training staff, maintaining equipment, and ensuring quality control measures are in place.
- Establish Adverse Event Reporting: Create a system for monitoring and documenting adverse events related to your items. Ensure that all employees are trained on this process.
- Regularly Review and Update: Compliance is an ongoing process. Frequently assess your practices and revise your adherence plan as necessary to incorporate any alterations in regulations or company operations. Remember to refresh item listings and registrations within 60 days of any modifications to ensure adherence.
Utilize Resources: Tools and Support for Continuous Compliance
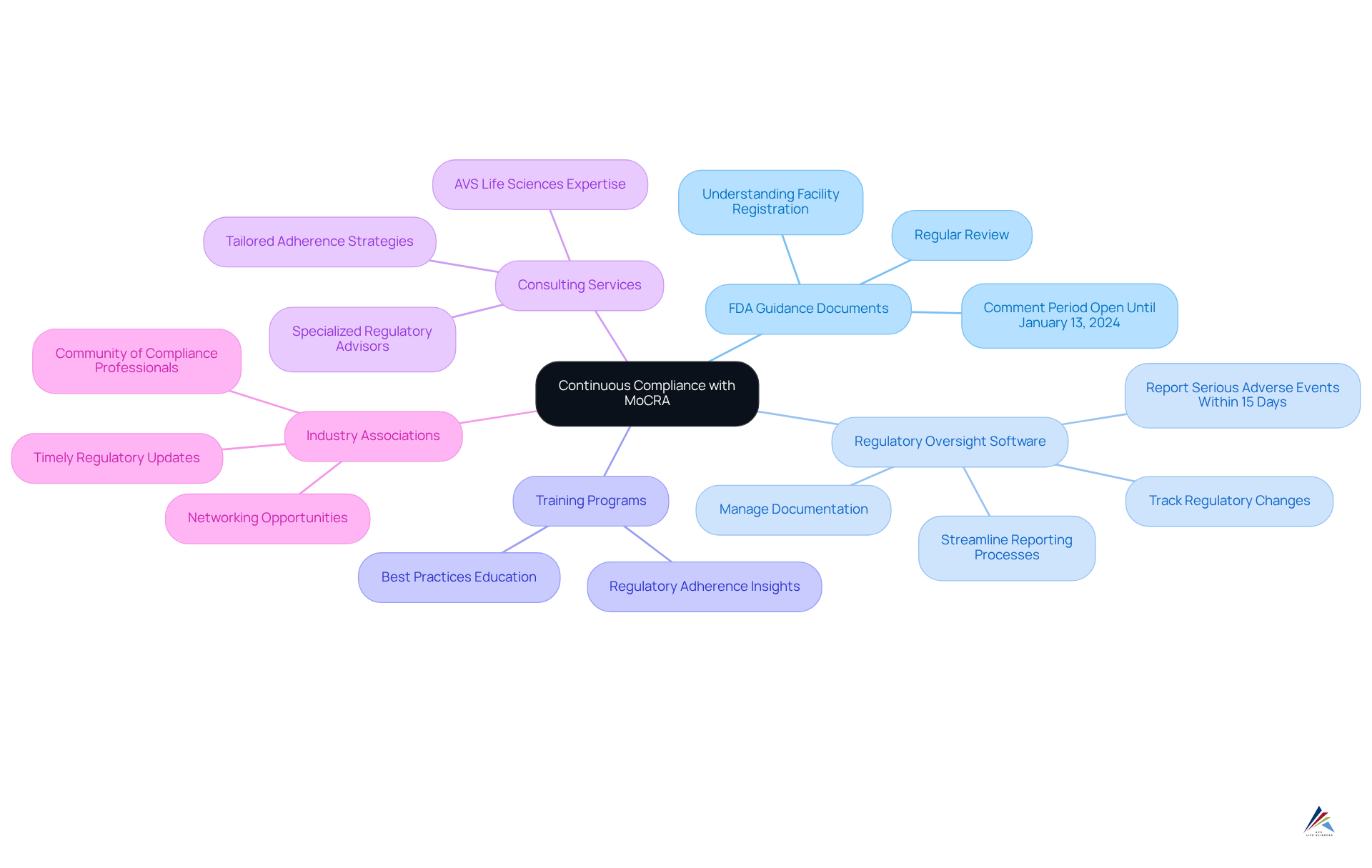
To ensure continuous compliance with the Modernization of Cosmetics Regulation Act (MoCRA), brands must strategically leverage essential resources:
- FDA Guidance Documents: Regularly reviewing the FDA's guidance documents related to MoCRA is crucial for staying updated on compliance requirements and clarifications. The FDA's recent updates, including FAQs and legal requirements, are vital for understanding the intricacies of facility registration and listing. Importantly, the comment period for the FDA's updated guidance on cosmetic product registration remains open until January 13, 2024.
- Regulatory Oversight Software: Implementing regulatory oversight software specifically designed for the cosmetics sector can effectively track regulatory changes, manage documentation, and streamline reporting processes. This ensures that all necessary information is readily accessible and current. The urgency of reporting serious adverse events to the FDA within 15 business days underscores the necessity of having efficient systems in place.
- Training Programs: Engaging in training initiatives focused on regulatory adherence is essential. These programs provide critical insights and keep your team informed about best practices, thereby enhancing overall readiness.
- Consulting Services: Collaborating with regulatory advisors who specialize in the Act can greatly assist in navigating complex regulations. Their expertise can facilitate the implementation of effective adherence strategies tailored to your organization's needs. AVS Life Sciences, with its extensive experience and customized consulting expertise, stands as a valuable partner in overcoming these challenges.
- Industry Associations: Joining industry associations dedicated to cosmetics regulation offers invaluable resources, networking opportunities, and timely updates on regulatory changes. This fosters a community of compliance-focused professionals.
By utilizing these resources, brands can significantly bolster their adherence efforts, illustrating how AVS helps brands stay MoCRA-compliant while ensuring alignment with MoCRA's evolving requirements and upholding high standards of product safety and quality. As Bruna Marzarotto aptly stated, "Proven steps for Quality Control Medical Devices and how a robust QMS aligned with ISO 13485:2016 can help you meet FDA compliance.
Conclusion
Navigating the complexities of the Modernization of Cosmetics Regulation Act (MoCRA) is essential for brands aiming to ensure compliance and uphold consumer safety. This article outlines how AVS Life Sciences equips brands with the necessary tools and strategies to meet the evolving regulatory landscape. Understanding MoCRA's key provisions—such as mandatory facility registration, GMP standards, and adverse event reporting—is crucial.
Key insights shared include a comprehensive step-by-step approach to implementing compliance strategies. This process ranges from conducting thorough assessments to engaging in continuous education and utilizing specialized resources. By following these guidelines, brands can effectively align their operations with MoCRA requirements, thereby reducing the risk of penalties and enhancing product integrity.
Ultimately, the proactive measures outlined in this article underscore the significance of staying informed and prepared in the face of regulatory changes. Brands are encouraged to leverage the expertise of AVS Life Sciences and utilize available resources to cultivate a culture of compliance. By doing so, they not only safeguard their operations but also contribute to the broader goal of ensuring safety and quality in the cosmetics industry.
Frequently Asked Questions
What is MoCRA?
The Modernization of Cosmetics Regulation Act (MoCRA) is a significant update in the regulation of cosmetics in the United States, expanding the FDA's authority over cosmetic products since the FD&C Act of 1938.
What are the key requirements introduced by MoCRA?
Key requirements include mandatory facility registration with the FDA, listing of cosmetic items, enforcement of Good Manufacturing Practices (GMP), and mandatory reporting of serious adverse events within 15 business days.
Why is facility registration important under MoCRA?
Mandatory facility registration ensures transparency and accountability among cosmetic manufacturers, allowing the FDA to oversee compliance with regulations.
What are Good Manufacturing Practices (GMP) and why are they significant?
GMP are standards that ensure the safety and quality of cosmetic products. MoCRA makes GMP compliance mandatory, which is expected to reduce contamination risks and enhance product integrity.
What is the requirement for adverse event reporting under MoCRA?
Companies must report any serious adverse events related to their products to the FDA within 15 business days to improve consumer safety.
What is the deadline for the FDA to evaluate the safety of PFAS in cosmetics?
The FDA is required to release a report evaluating the safety of PFAS in cosmetic products by December 2025.
What is the FDA's directive regarding fragrance allergen labeling?
The FDA is expected to propose a rule for fragrance allergen labeling by June 29, 2024, highlighting the evolving nature of cosmetic regulations.
How can brands ensure compliance with MoCRA?
Brands should familiarize themselves with the new regulations to align their operations with FDA standards, and consider consulting services for guidance on compliance. AVS Life Sciences offers expertise and tailored consulting to assist brands in navigating these regulations.
