FDA Cleared vs FDA Approved: Key Differences for Compliance Officers

Overview
The key difference between FDA cleared and FDA approved is rooted in their regulatory processes.
- FDA clearance, achieved through the 510(k) pathway, enables medical devices to enter the market based on substantial equivalence to existing products.
- In contrast, FDA approval necessitates rigorous clinical trials to demonstrate safety and effectiveness.
This distinction is crucial for compliance officers, as it significantly impacts compliance strategies, product development timelines, and overall regulatory adherence.
Understanding these pathways is essential to ensure patient safety and uphold quality standards. By grasping the nuances of FDA regulations, compliance professionals can better navigate the complexities of the medical device landscape.
Introduction
Navigating the complex landscape of medical device regulation presents significant challenges, particularly in differentiating between FDA clearance and approval. Both pathways are crucial for market entry; however, they involve markedly different processes and compliance implications for officers. Grasping these distinctions transcends mere regulatory adherence; it profoundly influences product development timelines and compliance strategies.
How can compliance officers harness this understanding to bolster patient safety and ensure stringent adherence to regulatory standards?
Differentiate Between FDA Clearance and Approval
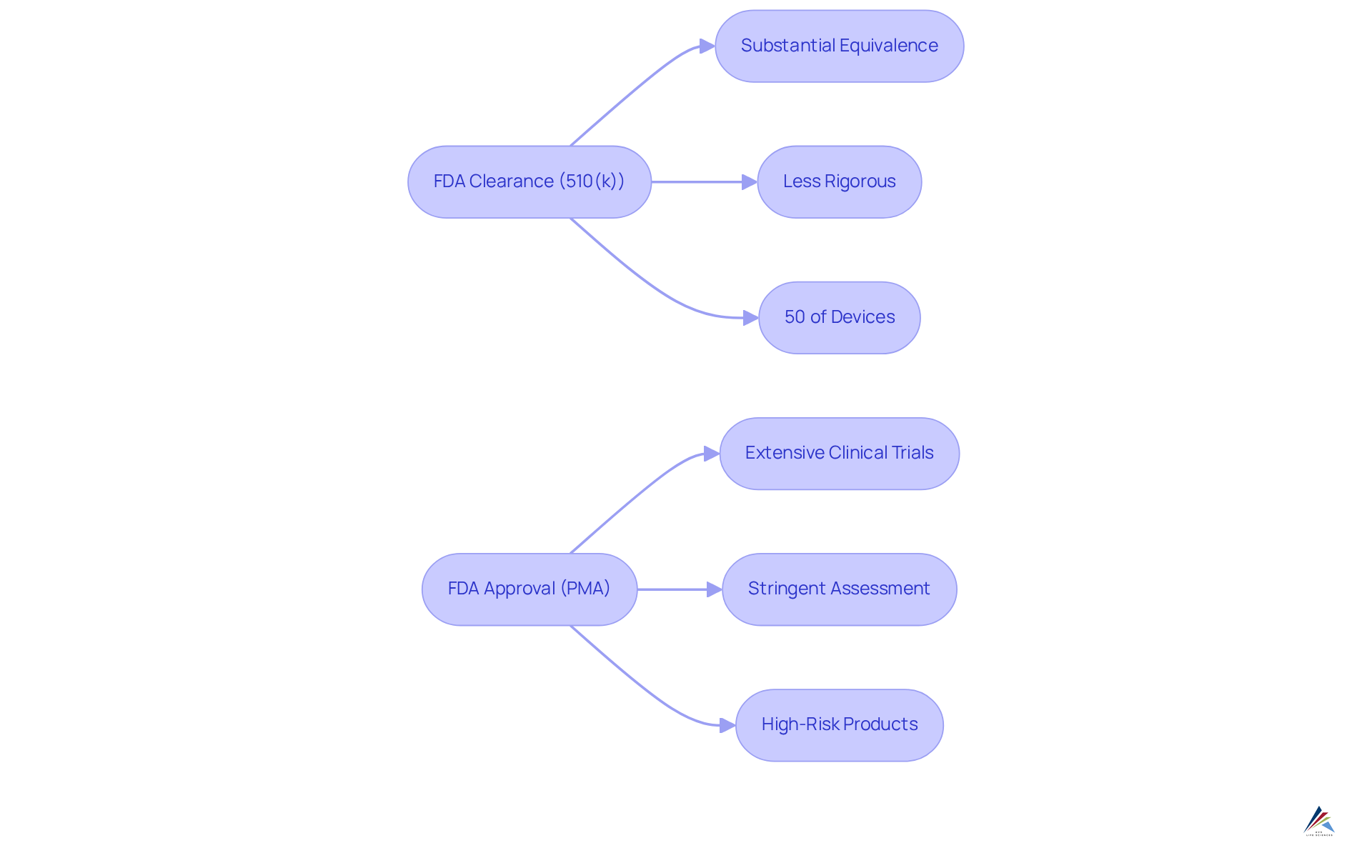
represents the pathway through which the FDA permits a medical instrument to enter the market, based on its substantial equivalence to an existing, legally marketed item, commonly referred to as a predicate. This pathway, known as the , is generally less rigorous than the FDA approval process. Conversely, FDA approval demands a more stringent assessment, requiring extensive clinical trials and significant data to demonstrate safety and effectiveness, particularly for high-risk medical products and pharmaceuticals.
For regulatory officers, grasping these distinctions is imperative for navigating the and ensuring compliance with the appropriate standards. Notably, around 50% of all medical devices in the U.S. are cleared via the , underscoring its critical role within the industry.
Regulatory specialists emphasize that between vs can significantly impact and product development timelines. As Mike Drues articulates, 'When discussing FDA cleared vs FDA approved, a 510(k) is never approved; it is cleared, while a PMA is approved and a de novo is granted.' This distinction is vital for to effectively manage regulatory requirements and mitigate risks associated with product liability.
Explore Regulatory Pathways: 510(k) vs. PMA
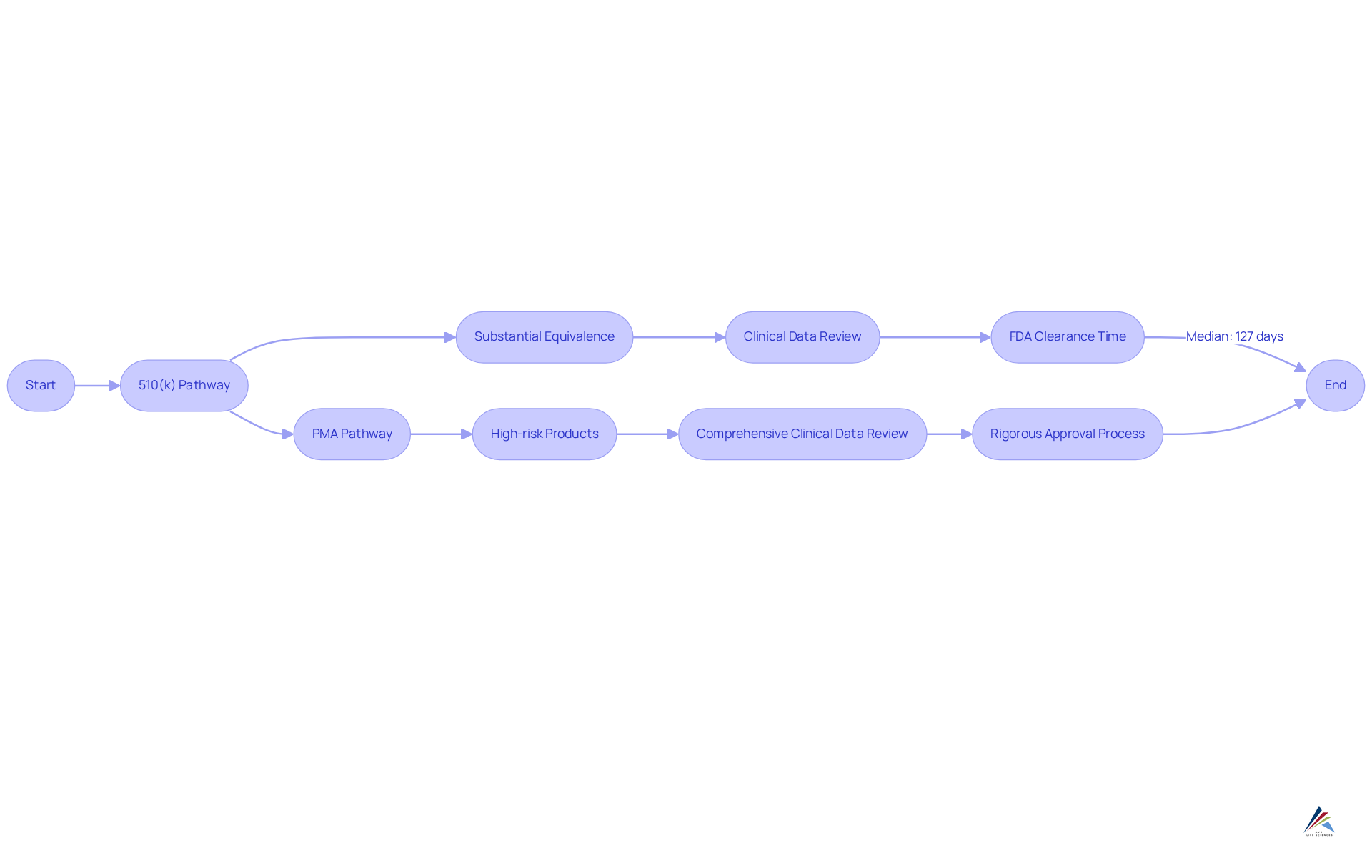
The serves as a streamlined route for products deemed substantially equivalent to existing ones, enabling quicker and more cost-effective market entry. In contrast, the is mandated for high-risk products, necessitating a comprehensive review of clinical data to validate safety and efficacy.
Compliance officers must meticulously assess the , as this critical evaluation dictates the oversight pathway and significantly influences the related requirements and timelines for market entry.
Industry experts assert that transcends mere compliance; it represents a strategic opportunity to enhance . For example, the average time for FDA 510(k) clearance has fluctuated, with recent data revealing a median decision time of 127 days, while decision times have spanned from 1 to 634 days. Moreover, 255 products were approved via the in January 2025, underscoring its relevance in the current .
A thorough understanding of these oversight pathways is essential for professionals aiming to navigate the intricate terrain of efficiently. As a leading regulatory expert remarked, ' is not just a hurdle to be cleared; it’s an ongoing commitment to patient safety and product quality.
Assess the Impact of FDA Decisions on Compliance Practices
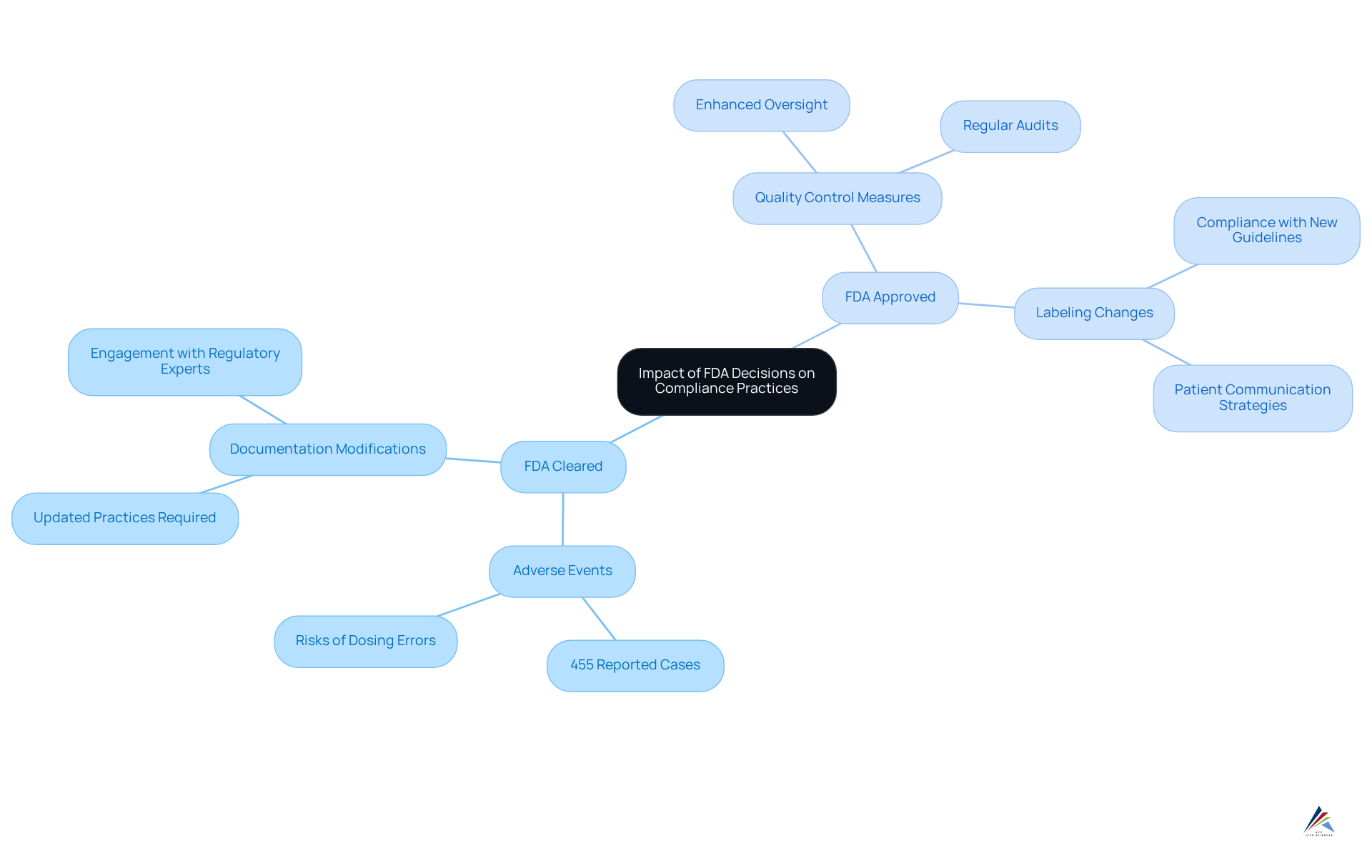
, which can be categorized as , wield significant influence over adherence practices within organizations. Notably, the FDA has documented over 455 adverse events linked to , underscoring the critical importance of adherence and the inherent dangers associated with non-adherence. Recent FDA guidelines may require modifications in documentation practices, , or even product labeling. Therefore, oversight personnel must remain vigilant and updated on FDA announcements, adjusting their strategies accordingly to mitigate risks and ensure continuous alignment with legal obligations.
With pivotal deadlines approaching—such as the by state-licensed pharmacies (503A) after April 22, 2025—organizations must act decisively. Consistent can empower organizations to remain agile in their adherence efforts. , such as Leslie Devos, who emphasizes the necessity of proactive approaches to , can further enhance understanding of these evolving changes. Entities that proactively adapt to new guidelines frequently experience smoother interactions with regulatory authorities and improved adherence outcomes.
Implement Best Practices for FDA Compliance Management
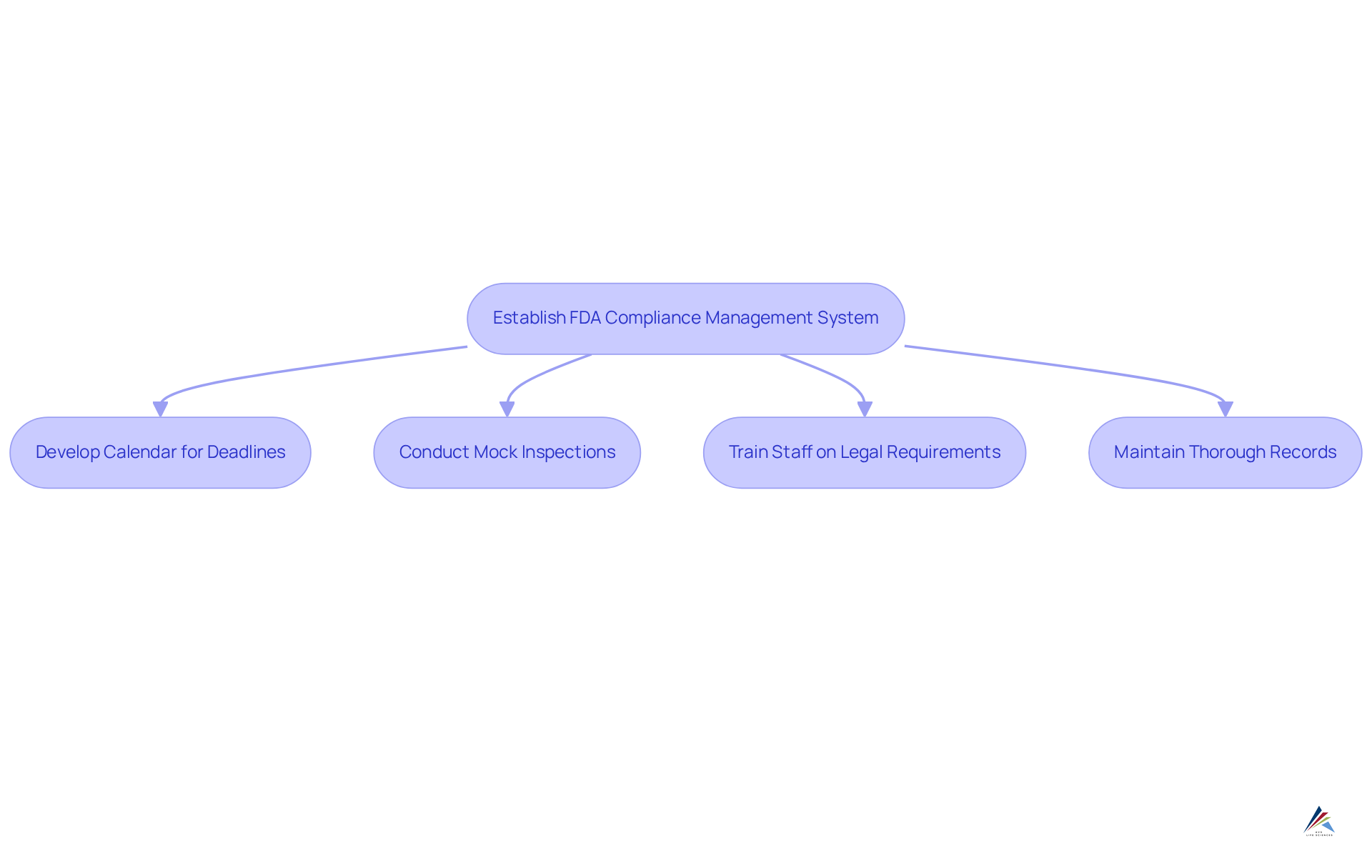
To effectively navigate FDA regulations, organizations must establish a robust management system that encompasses regular , , and meticulous documentation practices. Consider these key best practices:
- Develop a calendar to monitor critical deadlines and submissions, ensuring .
- Conduct , significantly enhancing preparedness and identifying potential gaps.
- Train all staff on legal requirements and foster an environment where reporting issues is encouraged, as 40% of organizations struggle with on updated policies.
- Maintain thorough records of all activities related to regulations, essential for demonstrating adherence during audits, especially since is the top challenge for 47% of organizations.
By cultivating a , organizations can improve their readiness for and ensure alignment with . This proactive approach not only enhances operational integrity but also positions organizations to respond effectively to .
Conclusion
Understanding the distinctions between FDA clearance and approval is paramount for compliance officers navigating the intricate regulatory landscape of medical devices and pharmaceuticals. FDA clearance facilitates a more streamlined market entry via the 510(k) pathway, whereas FDA approval demands rigorous clinical evaluations and extensive data to ensure safety and efficacy. Recognizing these differences not only aids in compliance but also enhances strategic planning for product development.
Key insights highlighted throughout the article include:
- The critical role of the 510(k) pathway, which accounts for approximately 50% of medical devices entering the U.S. market.
- The necessity for compliance officers to assess risk categorization, as it directly influences the regulatory pathway and the corresponding requirements.
- The impact of FDA decisions on compliance practices, underscoring the importance of remaining informed about evolving guidelines and the potential consequences of non-adherence.
The significance of proactive compliance management cannot be overstated. Organizations that implement best practices—such as conducting mock inspections and maintaining thorough documentation—position themselves to navigate regulatory challenges effectively. By fostering a culture of compliance and engaging with industry experts, organizations can enhance their operational integrity while ensuring alignment with FDA standards. This commitment not only safeguards patient safety but also cultivates a sustainable framework for future product development and market entry.
Frequently Asked Questions
What is FDA clearance?
FDA clearance is the process through which the FDA allows a medical instrument to enter the market based on its substantial equivalence to an existing, legally marketed item, known as a predicate. This process is typically less rigorous than FDA approval.
What is FDA approval?
FDA approval is a more stringent process that requires extensive clinical trials and significant data to demonstrate the safety and effectiveness of high-risk medical products and pharmaceuticals.
What is the 510(k) pathway?
The 510(k) pathway is the regulatory route through which many medical devices are cleared by the FDA, based on their substantial equivalence to already marketed devices. Approximately 50% of all medical devices in the U.S. are cleared via this pathway.
Why is it important for regulatory officers to understand the differences between FDA clearance and approval?
Understanding these distinctions is crucial for regulatory officers to navigate the regulatory landscape, ensure compliance with appropriate standards, and effectively manage product development timelines and compliance strategies.
What does Mike Drues say about the distinction between FDA cleared and FDA approved?
Mike Drues emphasizes that a 510(k) is never approved; it is cleared, while a Premarket Approval (PMA) is approved, and a de novo is granted. This distinction is essential for compliance officers to manage regulatory requirements and mitigate product liability risks.
