Facility Registration and Adverse Event Reporting Under MoCRA: A Step-by-Step Guide

Overview
This article serves as a comprehensive guide on facility registration and adverse event reporting under the Modernization of Cosmetics Regulation Act (MoCRA), highlighting the critical need for compliance among cosmetic manufacturing facilities. It begins by addressing the compliance challenges faced by these facilities, followed by essential steps for registration and reporting. Key actions include:
- Gathering required information
- Submitting reports within specified timelines
- Maintaining thorough records
These measures are vital for ensuring consumer safety and regulatory accountability in the cosmetics industry. By adhering to these guidelines, facilities not only meet regulatory obligations but also foster trust and integrity within the marketplace.
Introduction
Navigating the complexities of cosmetic regulation has become increasingly critical for manufacturers in the United States, particularly with the introduction of the Modernization of Cosmetics Regulation Act (MoCRA). This pivotal legislation mandates facility registration and adherence to good manufacturing practices, while also emphasizing the necessity of reporting adverse events to ensure consumer safety.
As the compliance deadline of July 1, 2024, approaches, organizations are confronted with the urgent challenge of aligning their operations with these new requirements.
How can companies effectively manage this transition while safeguarding their reputation and ensuring product safety? This question is not merely rhetorical; it demands strategic solutions that balance compliance with operational integrity.
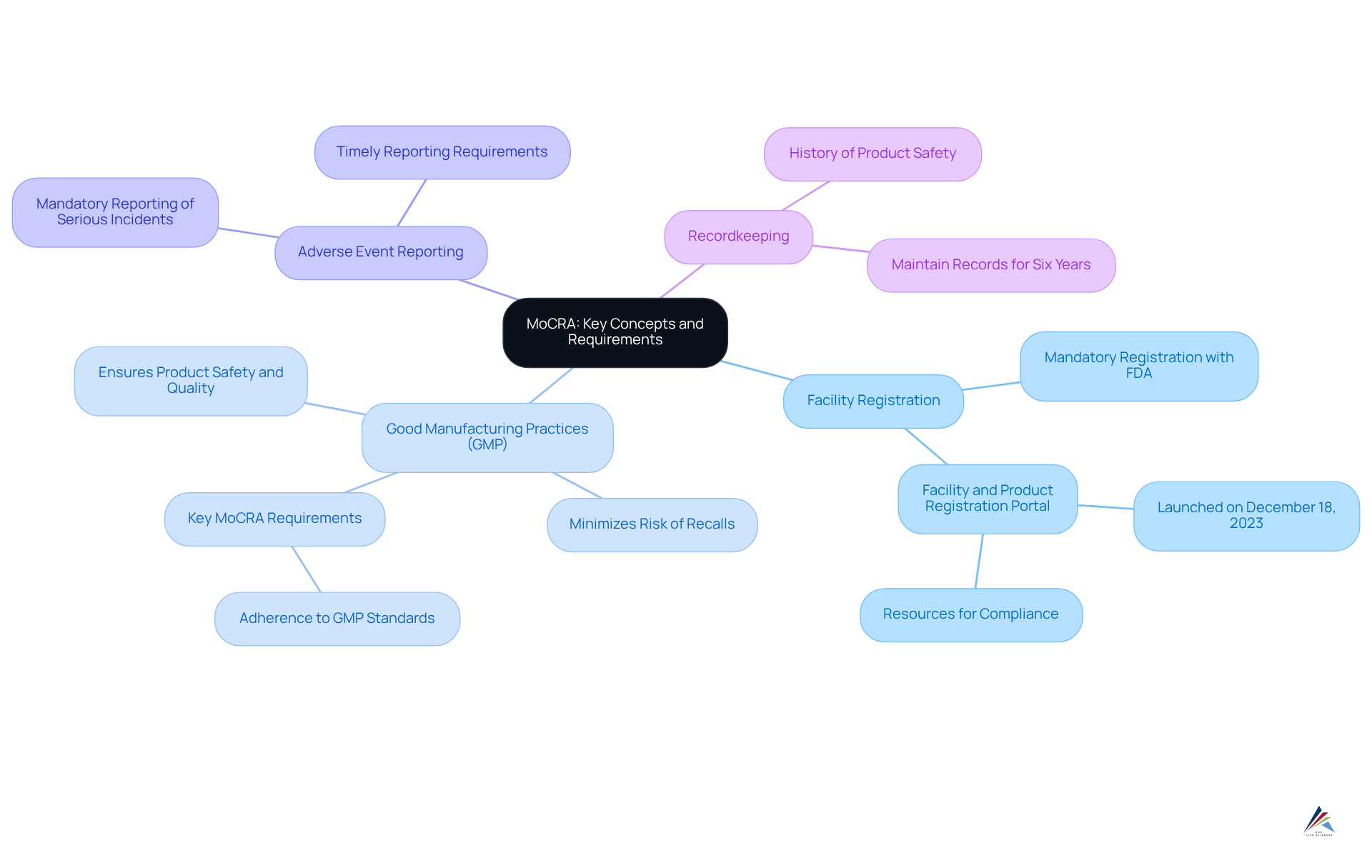
Understand MoCRA: Key Concepts and Requirements
The Modernization of Cosmetics Regulation Act was enacted to significantly enhance the safety and regulation of cosmetic products in the United States. This legislation introduces several key components that organizations must navigate to ensure compliance:
- Facility Registration: All cosmetic manufacturing facilities are mandated to register with the FDA, supplying crucial operational information. This requirement is essential for maintaining oversight and ensuring adherence across the industry. The Facility and Product Registration Portal, launched on December 18, 2023, offers vital resources for compliance.
- Good Manufacturing Practices (GMP): Adherence to GMP standards is imperative for all facilities, as these practices are designed to guarantee product safety and quality. Compliance with GMP not only protects consumers but also minimizes the risk of costly recalls and legal repercussions. As noted by Jennifer Diaz, key MoCRA requirements include facility registration and adverse event reporting under MoCRA, along with adherence to GMPs.
- Facility registration and adverse event reporting under MoCRA: Companies are mandated to report serious negative incidents related to their products within a specified timeframe. This proactive approach is vital for consumer safety and regulatory accountability.
- Recordkeeping: Responsible parties must maintain records of adverse events for at least six years, ensuring a reliable history of product safety and adherence.
Understanding the and adverse event reporting under MoCRA is crucial for organizations aiming to navigate the complexities of compliance effectively. The typical American shopper utilizes six to 12 cosmetic items each day, underscoring the significance of regulations in safeguarding consumer safety. Non-compliance can lead to significant penalties, including product recalls, which can severely affect a company's reputation and financial standing. Furthermore, critical deadlines under the new regulations take effect on July 1, 2024, highlighting the urgency of adherence. As the FDA continues to strengthen its regulatory framework, staying informed about these requirements is essential for maintaining operational integrity and consumer trust.
Complete Facility Registration: Step-by-Step Process
To successfully complete facility registration under the Modernization of Cosmetics Regulation Act (MoCRA), it is essential to adhere to the following steps:
- Determine Eligibility: Verify that your facility meets the criteria for a cosmetic manufacturing site as outlined by the regulations.
- Gather Required Information: Compile essential details, including the facility's name, address, and contact information.
- Access the FDA Registration Portal: Visit the FDA's official registration portal designated for cosmetic facilities.
- Complete the Registration Form: Accurately fill out the registration form, ensuring all mandatory fields are completed.
- Designate a U.S. Agent: If your facility operates outside the U.S., appoint a U.S. Agent to facilitate communication with the FDA.
- Submit the Registration: Review your information for accuracy before submitting the registration form electronically.
- Receive Confirmation: Upon submission, you will receive a confirmation from the FDA. Retain this confirmation for your records.
It is important to note that the for the legislation is July 1, 2024. As of January 1, 2025, there are 9,528 unique, active facility registrations. Additionally, cosmetic products, including free samples or promotional gifts, generally require a product listing submission under section 607(c) of the FD&C Act. By following these steps, your facility will ensure facility registration and adverse event reporting under MoCRA, which will help maintain compliance with relevant regulations and support the overall integrity of the cosmetics industry.

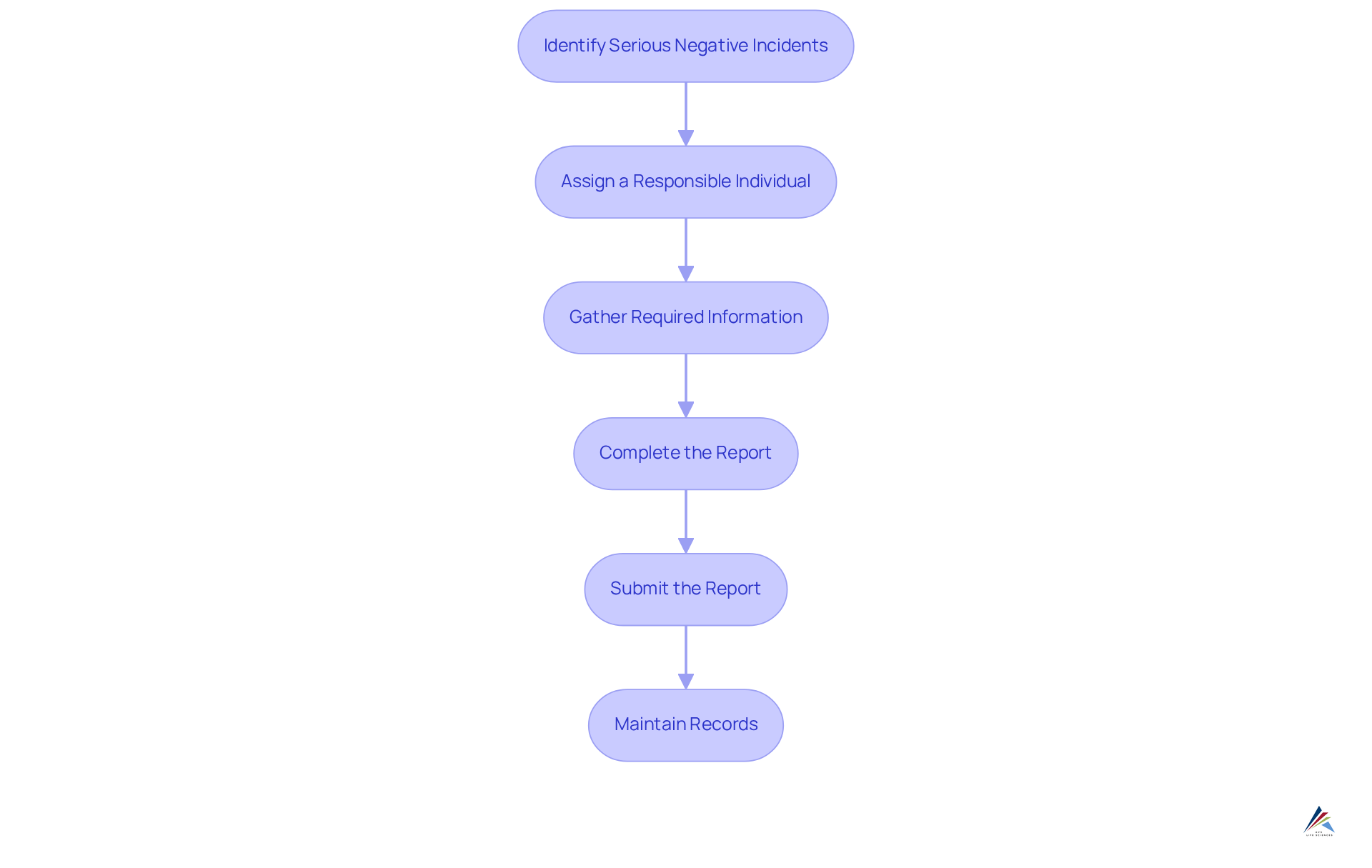
Implement Adverse Event Reporting: Essential Steps
To effectively implement adverse event reporting under the Modernization of Cosmetics Regulation Act (MoCRA), organizations must adhere to essential steps that ensure compliance and enhance consumer safety:
- Identify Serious Negative Incidents: Recognize occurrences that qualify as serious negative incidents, which may include hospitalization, significant injury, disability, congenital anomalies, or death. Notably, there was a 300% rise in negative incident reports in 2016 compared to earlier years, underscoring the critical need for prompt reporting.
- Assign a Responsible Individual: Appoint a designated person within your organization to manage the negative occurrence reporting process. This individual should be well-versed in regulatory requirements and best practices to ensure accountability and compliance.
- Gather Required Information: Collect comprehensive details regarding the negative occurrence, including product specifics, consumer information, and any relevant circumstances surrounding the incident. Thorough documentation is crucial to avoid regulatory issues.
- Complete the Report: Utilize the FDA's MedWatch Form to precisely document the negative occurrence, ensuring that all mandatory fields are thoroughly filled out to facilitate effective review.
- Submit the Report: Ensure that the finished report is submitted to the FDA within 15 business days of receiving information about the negative incident, as prompt reporting is essential for consumer safety.
- Maintain Records: Keep records of all negative occurrences for at least six years, in accordance with MoCRA regulations, to aid in ongoing safety monitoring and regulatory oversight.
By diligently following these steps, organizations can significantly enhance their adverse event reporting processes, thereby contributing to improved consumer safety and rigorous adherence to regulations. It is also prudent to stay informed about evolving regulations and best practices to ensure continual compliance.
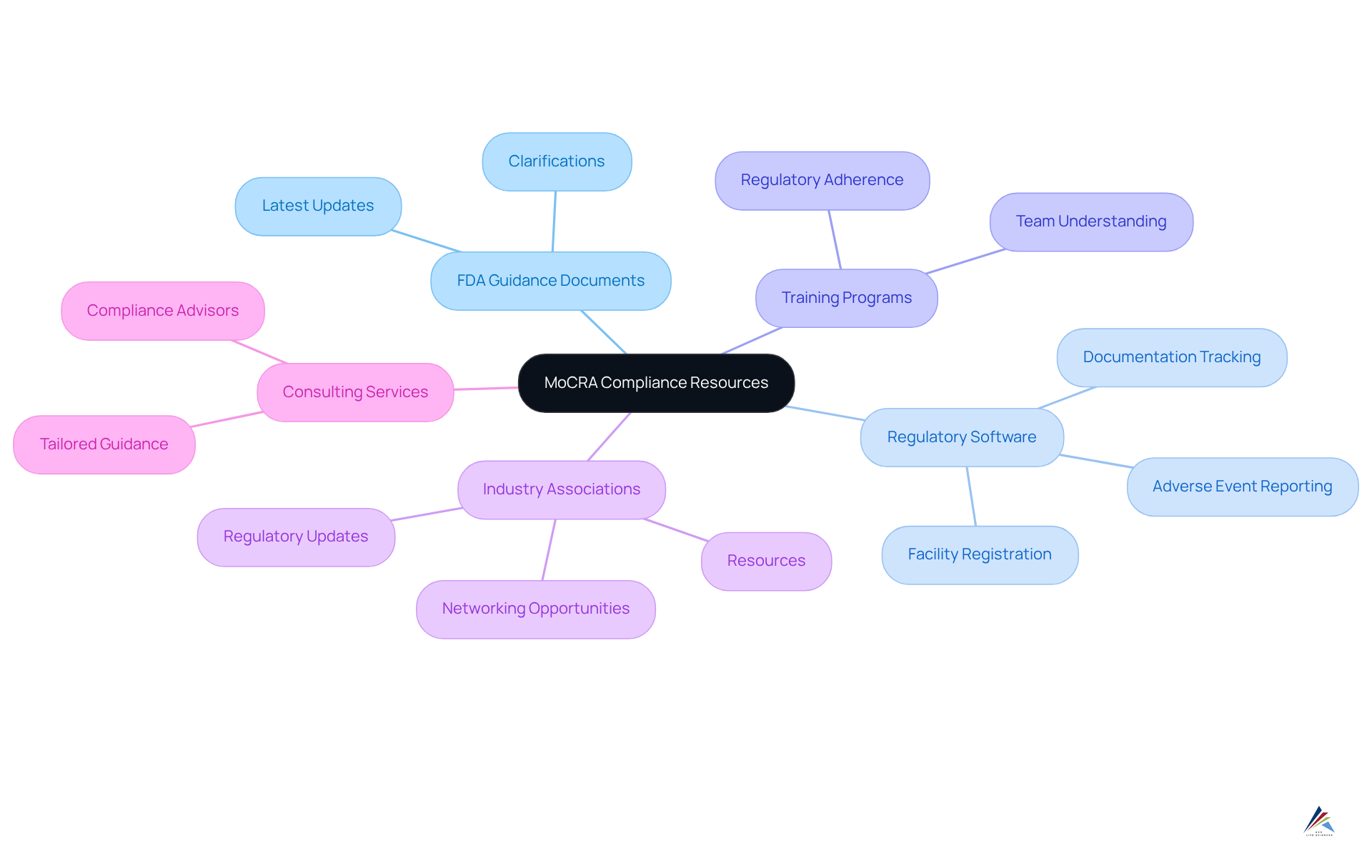
Utilize Resources and Tools for MoCRA Compliance
To ensure compliance with MoCRA, it is essential to leverage a variety of resources and tools:
- FDA Guidance Documents: Regularly review the FDA's official guidance documents related to MoCRA for the latest updates and clarifications.
- Regulatory Software: Invest in management software designed for facility registration and adverse event reporting under MoCRA, as well as for tracking documentation effectively.
- Training Programs: Engage in training programs focused on regulatory adherence to enhance your team's understanding of the guidelines.
- Industry Associations: Join industry associations that offer resources, networking opportunities, and timely updates on regulatory changes.
- Consulting Services: Collaborate with compliance advisors specializing in specific regulations to receive tailored guidance and support.
Utilizing these resources empowers organizations to remain informed and compliant with MoCRA regulations, ultimately fostering a culture of .
Conclusion
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a transformative moment in cosmetic safety and regulation, compelling organizations to adapt to evolving compliance standards. By grasping the essential elements of facility registration and adverse event reporting, companies can not only fulfill regulatory obligations but also bolster consumer trust and safety. This proactive stance is critical for navigating the intricacies of the cosmetics industry.
In this article, we have delineated the vital steps for facility registration and adverse event reporting. From verifying eligibility and compiling necessary information to ensuring accurate record-keeping, each step is paramount for compliance. By underscoring the significance of good manufacturing practices and the timely reporting of serious incidents, organizations can prioritize consumer safety while mitigating potential penalties and reputational harm.
As the compliance deadline looms on July 1, 2024, organizations must act swiftly to align with MoCRA requirements. Utilizing available resources, such as FDA guidance documents and regulatory software, can greatly facilitate the transition and cultivate a culture of regulatory excellence. Ultimately, prioritizing compliance under MoCRA not only safeguards consumers but also fortifies the integrity of the cosmetics industry as a whole.
Frequently Asked Questions
What is the Modernization of Cosmetics Regulation Act (MoCRA)?
MoCRA is legislation enacted to enhance the safety and regulation of cosmetic products in the United States, introducing key components that organizations must follow to ensure compliance.
What is required for facility registration under MoCRA?
All cosmetic manufacturing facilities must register with the FDA, providing essential operational information to maintain oversight and ensure compliance across the industry.
What are Good Manufacturing Practices (GMP)?
GMP standards are practices that facilities must adhere to in order to guarantee product safety and quality, protecting consumers and minimizing the risk of recalls and legal issues.
What is the importance of adverse event reporting under MoCRA?
Companies are required to report serious negative incidents related to their products within a specified timeframe, which is essential for consumer safety and regulatory accountability.
How long must records of adverse events be maintained?
Responsible parties must keep records of adverse events for at least six years to ensure a reliable history of product safety and compliance.
What are the consequences of non-compliance with MoCRA?
Non-compliance can result in significant penalties, including product recalls, which can adversely affect a company's reputation and financial standing.
When do the critical deadlines under the new regulations take effect?
The critical deadlines for compliance with the new regulations take effect on July 1, 2024.
Why is it important for organizations to stay informed about MoCRA requirements?
Staying informed is crucial for maintaining operational integrity and consumer trust as the FDA strengthens its regulatory framework.
