Enhance GxP Compliance & Digital Validation with Proven Strategies

Overview
The article underscores the critical need to enhance GxP compliance and digital validation through the strategic integration of digital tools, robust validation strategies, and continuous monitoring practices. By leveraging technologies such as Electronic Quality Management Systems (eQMS) and establishing comprehensive validation plans, organizations can markedly improve operational efficiency and mitigate risks associated with non-compliance. This proactive approach not only safeguards the safety and quality of life sciences products but also instills confidence in compliance solutions, prompting organizations to engage with AVS Life Sciences for further support.
Introduction
In the highly regulated world of life sciences, GxP compliance stands as a cornerstone for ensuring the safety and efficacy of pharmaceuticals and medical devices. Organizations face the dual challenge of navigating evolving regulations and the increasing complexity of compliance. This landscape presents a vital opportunity: the integration of digital tools and robust validation strategies can significantly enhance operational efficiency and mitigate risks. Yet, the question persists: how can companies effectively navigate this intricate terrain to not only meet regulatory standards but also cultivate a culture of continuous improvement and accountability? Addressing these challenges is not merely a regulatory obligation; it is a pathway to fostering innovation and excellence in the industry.
Understand GxP Compliance: Definitions and Importance
GxP, or Good 'x' Practice, encompasses a series of regulations and guidelines designed to ensure the quality and safety of items within the life sciences sector. These practices are crucial for guaranteeing that pharmaceuticals, medical devices, and related items meet stringent safety and efficacy standards. The significance of GxP adherence is profound; it not only protects consumers but also bolsters the credibility of organizations within the industry. By following GxP guidelines, companies can effectively mitigate risks associated with failures and regulatory penalties, thereby cultivating a culture of quality and safety throughout the lifecycle of their offerings.
Recent updates to [Good Manufacturing Practices (GMP)](https://avslifesciences.com/about-us) regulations have further underscored the necessity for rigorous compliance measures. For example, the FDA and EMA have introduced enhanced guidelines reflecting advancements in science and technology, ensuring that manufacturing processes uphold the highest quality standards. Adhering to GxP not only safeguards public health but also enhances operational efficiency, as organizations prioritizing these practices frequently experience fewer recalls and regulatory challenges.
Statistics reveal that adherence to GxP guidelines significantly reduces the likelihood of product failures. Organizations that establish robust GxP compliance & digital validation frameworks report a marked decrease in adverse events and regulatory penalties, underscoring the critical role of these practices in ensuring safety and effectiveness. Real-world examples illustrate the essential function of GxP in life sciences; entities that have implemented comprehensive GxP strategies have successfully navigated complex regulatory environments, ensuring their offerings meet the required safety and quality benchmarks.
Understanding the is vital for organizations seeking to adeptly navigate this intricate regulatory landscape. By fostering a thorough understanding of these guidelines, companies can enhance their adherence efforts, ultimately leading to improved product safety and increased consumer trust.

Leverage Digital Tools for Enhanced GxP Compliance
Integrating digital tools into GxP compliance & digital validation strategies significantly enhances both efficiency and accuracy. Technologies such as Electronic Quality Management Systems (eQMS), data analytics, and automation software streamline documentation processes, minimize human error, and bolster traceability. For instance, eQMS can automate workflows, ensuring that compliance-related tasks are executed promptly and in accordance with regulatory standards. This automation has been shown to reduce manual investigation timelines from months to hours, demonstrating a remarkable increase in operational efficiency.
Moreover, leveraging data analytics enables organizations to proactively identify trends and potential regulatory risks. Companies investing in quality management practices report a 40% reduction in customer complaints, underscoring the effectiveness of these digital solutions. By implementing eQMS, organizations not only achieve GxP compliance & digital validation of regulatory requirements more efficiently but also foster a culture of continuous improvement and innovation in their practices. The integration of predictive analytics further enhances this strategy, allowing for the extraction of valuable insights from data to anticipate quality issues before they manifest.
Examples of successful eQMS implementation in the life sciences sector vividly illustrate these benefits. Organizations with mature quality management systems achieve higher on-time delivery rates, and the adoption of eQMS has been correlated with significant cost savings, with some companies realizing over $1 million in savings over three years. However, it is crucial to recognize common pitfalls in implementing eQMS, such as inadequate training and resistance to change, which can impede the effectiveness of these systems. This positions companies to adeptly navigate the complexities of regulatory environments while ensuring GxP compliance & digital validation and upholding high standards of quality.

Implement Robust Validation Strategies for Compliance Success
To achieve regulatory success, organizations must implement robust validation strategies that encompass all operational aspects, focusing on & digital validation. A comprehensive validation plan is essential for GxP compliance & digital validation, detailing the scope, objectives, and methodologies for validating processes and systems. Key elements of an effective validation strategy for GxP compliance & digital validation include:
For instance, performing regular re-validation of essential systems is crucial to ensure ongoing GxP compliance & digital validation. Statistics indicate that 91% of companies intend to establish ongoing adherence in the next five years, reflecting a shift towards proactive validation strategies. Furthermore, educating staff on validation processes and the significance of adherence fosters a culture of accountability and excellence within the organization. By prioritizing GxP compliance & digital validation, companies can significantly mitigate risks associated with non-compliance, enhance operational efficiency, and maintain high-quality standards throughout their processes.

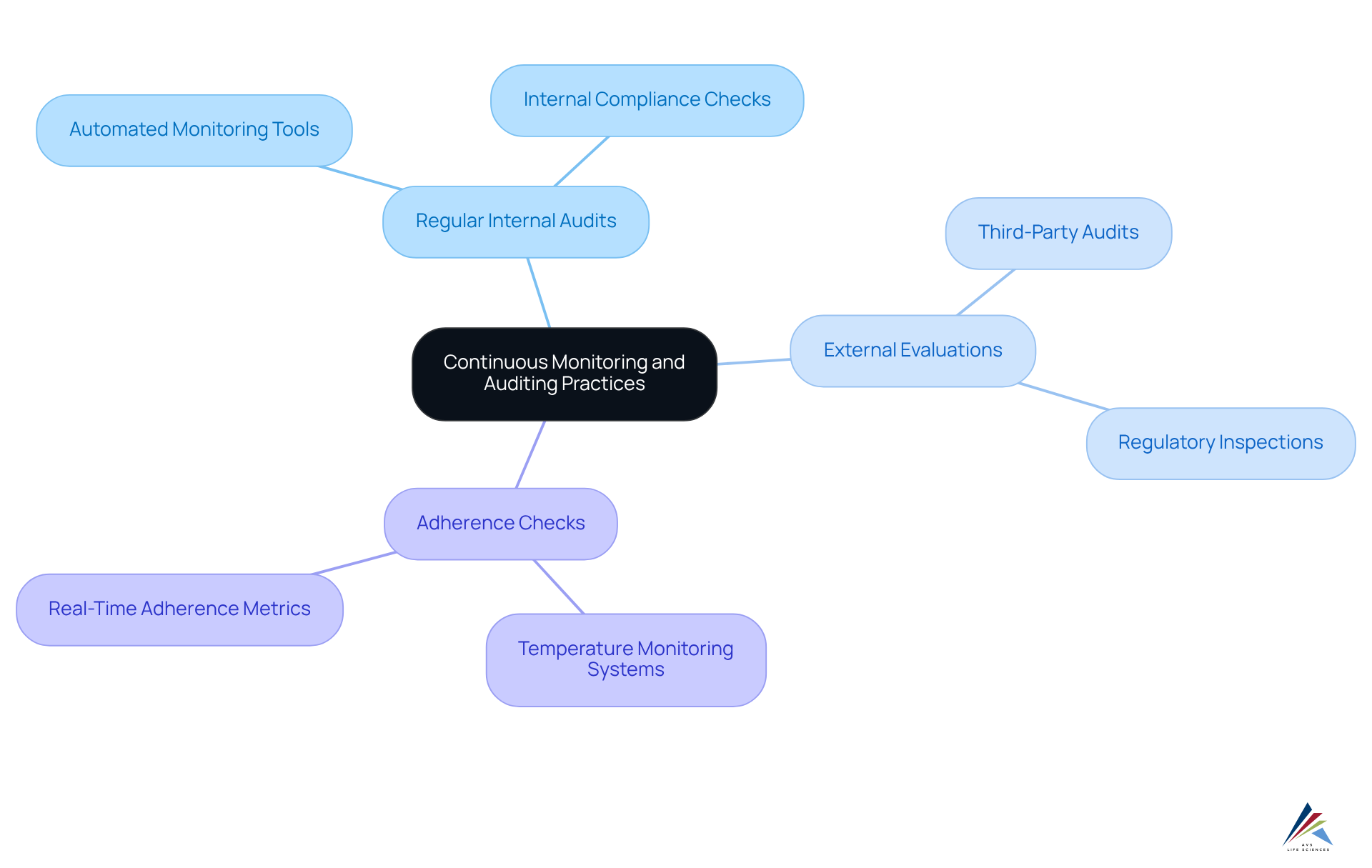
Establish Continuous Monitoring and Auditing Practices
Implementing ongoing monitoring and auditing practices is essential for upholding GxP standards. Organizations must establish a structured audit program that encompasses:
- Regular internal audits
- External evaluations
- Adherence checks
These audits not only verify compliance with GxP regulations but also identify areas for improvement. For example, employing automated monitoring tools facilitates real-time tracking of adherence metrics, enabling companies to swiftly address any deviations from established protocols. A practical application of this is the deployment of continuous temperature monitoring systems in storage facilities, which ensures that products remain within required conditions, thereby preserving product integrity.
By fostering a culture of openness and responsibility through these practices, organizations can significantly enhance their regulatory adherence and build trust with regulators and stakeholders. Furthermore, the importance of internal audits cannot be overstated; they serve as a proactive measure to detect potential regulatory gaps before they escalate into major issues.
Current trends in auditing practices emphasize the integration of technology and data analytics, which streamline verification processes and enhance efficiency. Ultimately, a steadfast commitment to rigorous auditing practices is vital for achieving & digital validation and ensuring the safety and efficacy of products within the life sciences sector.
Conclusion
Emphasizing the importance of GxP compliance and digital validation is essential for organizations operating within the life sciences sector. By adhering to Good 'x' Practice guidelines, companies not only safeguard public health but also enhance their operational efficiency and credibility. The integration of robust compliance frameworks cultivates a culture of quality and safety, ultimately benefiting both the organization and its consumers.
The article highlights several key strategies for achieving effective GxP compliance:
- Understanding the nuances of GMP, GLP, and GCP is crucial.
- Leveraging digital tools like Electronic Quality Management Systems (eQMS) that streamline processes and reduce human error.
- Implementing comprehensive validation strategies.
- Establishing continuous monitoring and auditing practices are vital steps in maintaining compliance and ensuring product integrity.
As the landscape of regulatory compliance continues to evolve, organizations must remain proactive in their approach. Embracing these best practices and investing in the right digital solutions will not only mitigate risks associated with non-compliance but also pave the way for innovation and excellence within the industry. By prioritizing GxP compliance, stakeholders can contribute to a safer and more reliable life sciences sector, ultimately fostering greater trust and confidence in their products.
Frequently Asked Questions
What does GxP stand for and what does it encompass?
GxP stands for Good 'x' Practice, which includes a series of regulations and guidelines aimed at ensuring the quality and safety of products in the life sciences sector, particularly pharmaceuticals and medical devices.
Why is GxP compliance important?
GxP compliance is crucial for protecting consumers, enhancing the credibility of organizations, mitigating risks associated with failures and regulatory penalties, and fostering a culture of quality and safety throughout the lifecycle of products.
What recent updates have been made to Good Manufacturing Practices (GMP)?
Recent updates to GMP regulations by the FDA and EMA have introduced enhanced guidelines that reflect advancements in science and technology to ensure that manufacturing processes meet the highest quality standards.
How does GxP compliance impact operational efficiency?
Organizations that prioritize GxP compliance often experience fewer product recalls and regulatory challenges, leading to enhanced operational efficiency.
What are the benefits of adhering to GxP guidelines?
Adhering to GxP guidelines significantly reduces the likelihood of product failures and adverse events, and organizations with robust GxP compliance frameworks report fewer regulatory penalties.
What specific GxP guidelines should organizations understand?
Organizations should understand the specific requirements of Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP) to navigate the regulatory landscape effectively.
How can understanding GxP guidelines improve product safety and consumer trust?
By fostering a thorough understanding of GxP guidelines, companies can enhance their adherence efforts, which ultimately leads to improved product safety and increased consumer trust.
