CRO Definition: Key Insights for Pharmaceutical Compliance Officers

Overview
The article defines a Contract Research Organization (CRO) as a specialized entity that provides outsourced research services essential for the pharmaceutical, biotechnology, and medical device industries. It focuses on critical aspects such as study design, patient recruitment, and regulatory compliance. The significance of CROs in streamlining the drug development process and enhancing clinical research efficiency cannot be overstated. Their high success rates in completing studies on time, coupled with their strategic role in ensuring adherence to regulatory standards, exemplify their value in the industry.
Introduction
In the rapidly evolving landscape of pharmaceuticals, the role of Contract Research Organizations (CROs) has become increasingly vital. These specialized entities not only streamline the drug development process but also ensure compliance with stringent regulatory standards, allowing pharmaceutical companies to focus on their core competencies. As the demand for efficient and effective clinical trials grows, questions arise about how CROs can navigate complex regulations while enhancing the quality and speed of research.
What are the key characteristics that define a successful CRO, and how do they contribute to the overall success of drug development? Understanding these dynamics is crucial for stakeholders aiming to optimize their compliance strategies and improve research outcomes.
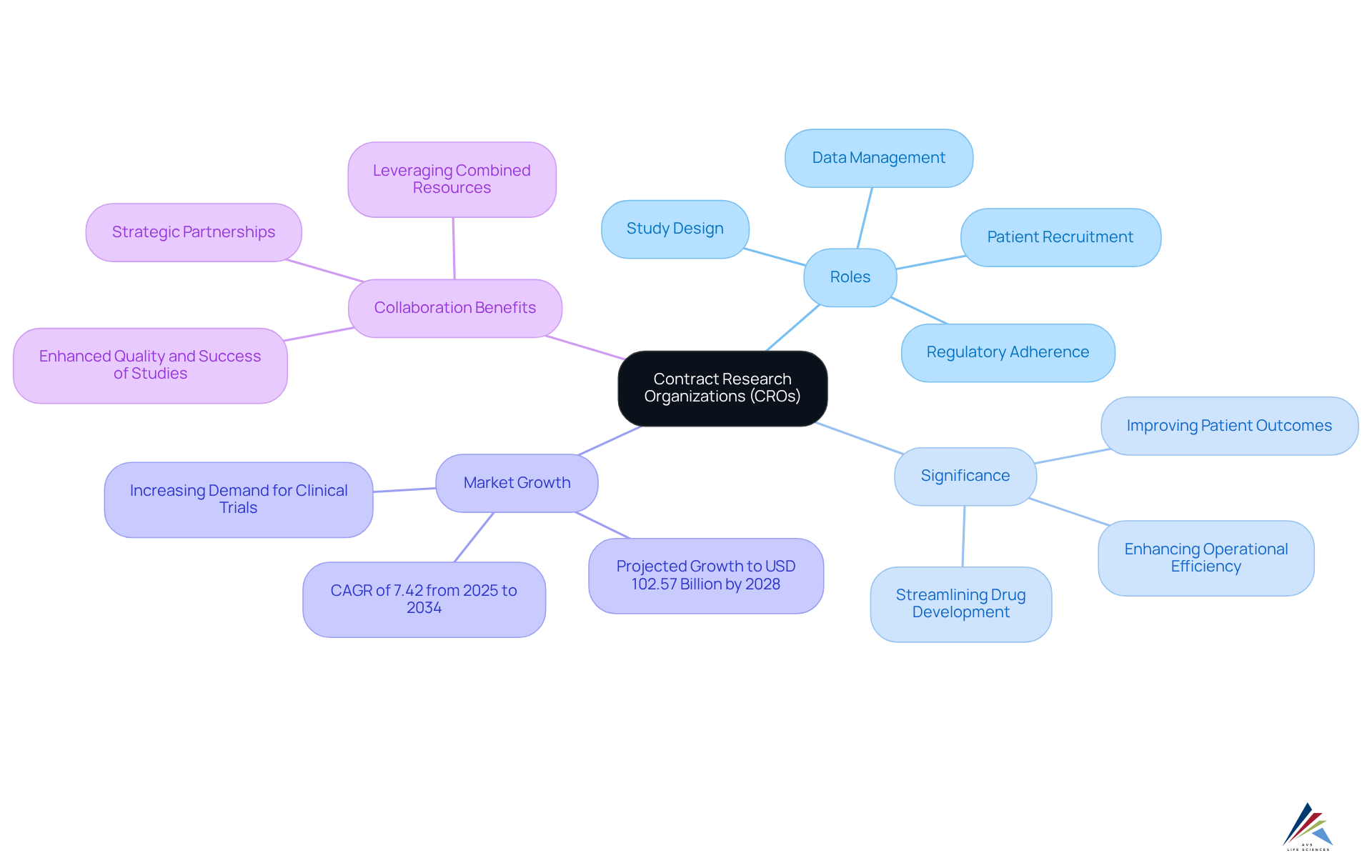
Define CRO: Understanding the Acronym and Its Significance
According to the CRO definition, a Contract Research Organization (CRO) serves as a specialized entity providing outsourced research services to the pharmaceutical, biotechnology, and medical device industries. These organizations are pivotal in overseeing various elements of research studies, including:
- Study design
- Patient recruitment
- Data management
- Adherence to regulations
Their significance stems from the ability to streamline the drug development process, allowing sponsors to concentrate on their core competencies while ensuring studies are conducted efficiently and in compliance with established standards. This collaboration is vital for accelerating the introduction of new therapies to the market, ultimately benefiting patients and healthcare systems worldwide.
The global contract research organization market is projected to grow from approximately USD 64.42 billion in 2023 to over USD 102.57 billion by 2028, reflecting an increasing reliance on these entities for managing research studies. As pharmaceutical companies face rising costs and complex regulatory landscapes, outsourcing to contract research organizations has emerged as a strategic choice to enhance operational efficiency and reduce time-to-market for new medications. Moreover, successful collaborations between contract research organizations and pharmaceutical firms have demonstrated significant improvements in clinical research efficiency, with reports indicating that 93% of studies are completed on time or ahead of schedule.
Expert insights underscore the critical role of contract research organizations, as detailed in the CRO definition, in the drug development process. By leveraging the specialized expertise of CROs, pharmaceutical firms gain valuable insights into regulatory expectations and study designs, transforming the organization from a mere service provider into a strategic partner. This evolution fosters a collaborative environment that enhances the overall quality and success of medical studies, ultimately leading to .
Contextualize CRO: Its Role in Regulatory Compliance and Quality Assurance
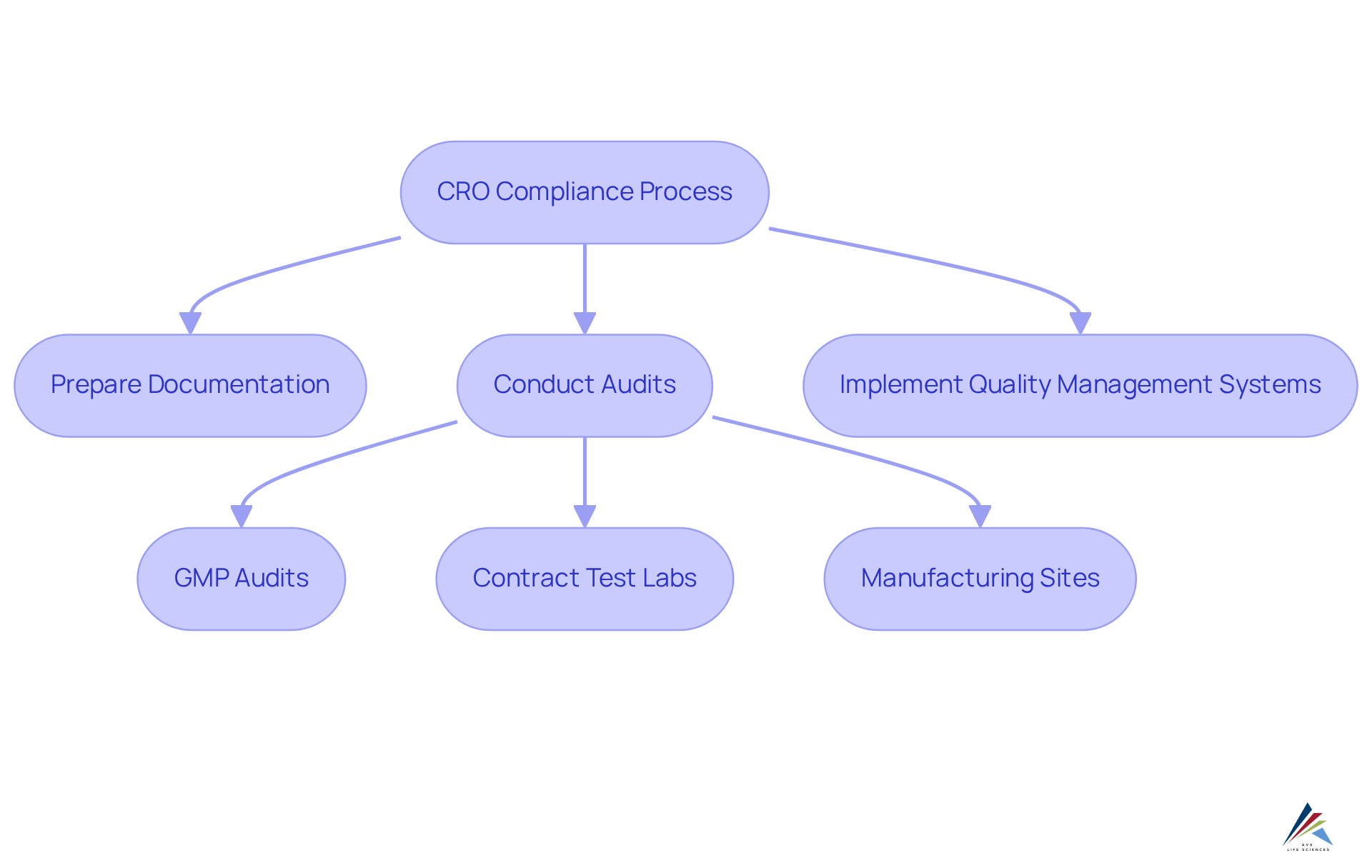
According to the cro definition, Contract Research Organizations (CROs) are integral to ensuring that clinical trials comply with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). AVS Life Sciences, a leading provider of comprehensive GXP compliance services, plays a crucial role in this process. They meticulously prepare and submit essential documentation to governing entities, conduct thorough audits—including GMP audits for API and drug product CMOs, contract test labs, and manufacturing sites—and implement robust quality management systems.
By leveraging their specialized knowledge, AVS Life Sciences assists sponsors in navigating the complex compliance landscape, ensuring that every aspect of the study adheres to relevant laws and guidelines. This proactive strategy not only but also significantly enhances the credibility of results, which is vital for securing approval and facilitating market access.
Recent data demonstrates that contract research organizations have achieved a success rate of over 93% in managing phase II/III trials, underscoring their effectiveness in regulatory submissions. Furthermore, the application of machine learning models trained on extensive medical data has yielded a 20-40% reduction in deviation closure time, showcasing the efficiency improvements that AVS Life Sciences can deliver.
By ensuring adherence to GMP, ISO, and QSR, AVS Life Sciences and other research organizations play a pivotal role in fostering a compliant and trustworthy environment for trials, aligning with the cro definition.
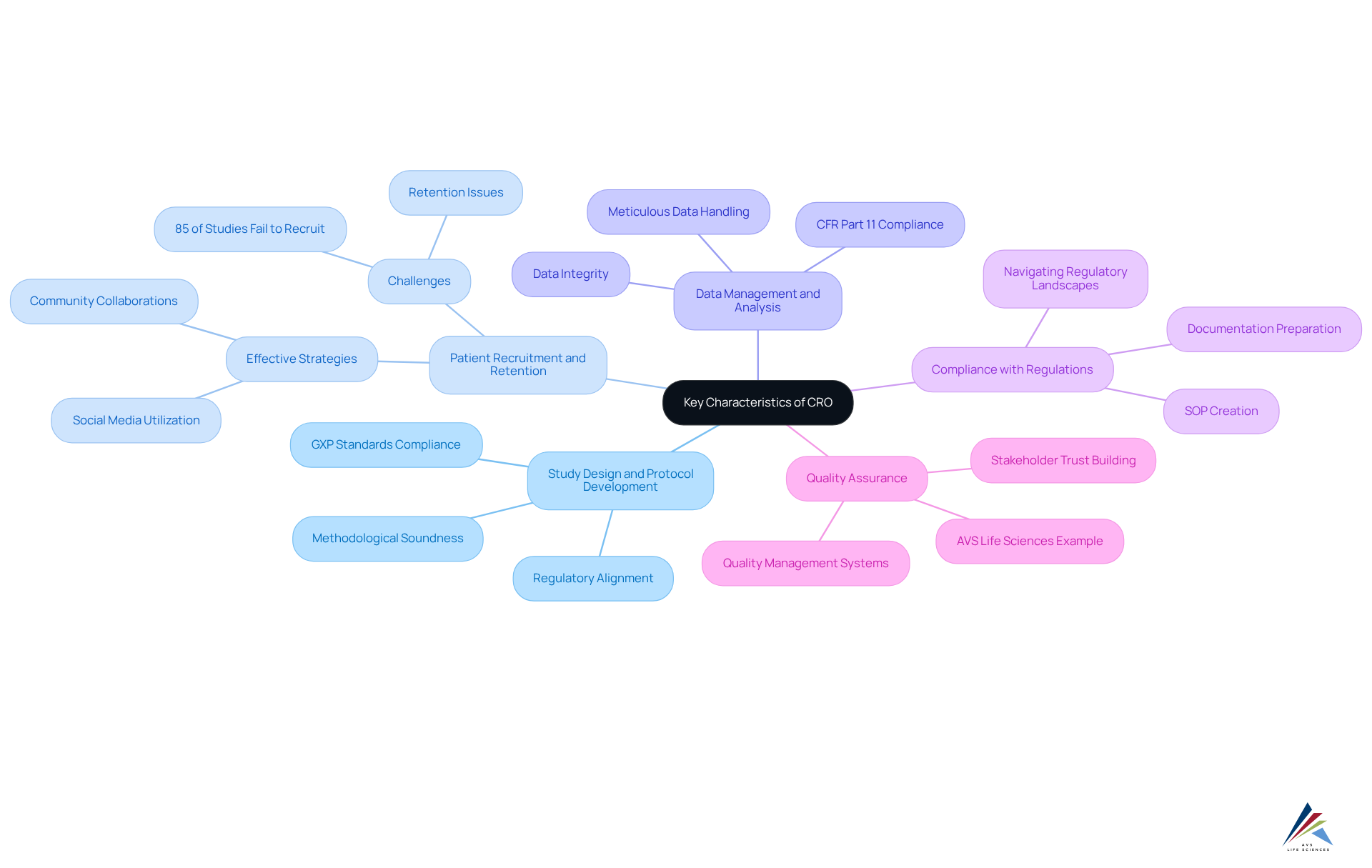
Explore Key Characteristics of CRO: Functions and Responsibilities
CROs are defined by their diverse and essential functions in the clinical trial landscape, which include:
- Study Design and Protocol Development: CROs play a pivotal role in crafting clinical trial designs that align with regulatory requirements and scientific objectives. Their expertise ensures that studies are methodologically sound and compliant from the outset, adhering to GXP standards and FDA regulations.
- Patient Recruitment and Retention: Effective patient recruitment strategies are crucial, as studies show that up to 85% of clinical studies fail to recruit or retain a sufficient sample size. Contract research organizations employ creative strategies, including utilizing social media and community collaborations, to improve participant involvement and retention during the study period. For instance, a rapid recruitment rate of 55 participants in less than one month using Facebook demonstrates the effectiveness of these strategies.
- Data Management and Analysis: Contract Research Organizations are responsible for the meticulous management of study data, encompassing collection, storage, and analysis. They guarantee that data integrity is upheld and that all procedures adhere to compliance standards, including CFR Part 11 adherence, which is essential for the credibility of study outcomes.
- Compliance with Regulations: Preparing and submitting necessary documentation to governing bodies is a core function of CROs. They navigate complex regulatory landscapes, ensuring that all research activities adhere to applicable laws and guidelines, thereby facilitating smoother approval processes. This encompasses creating Standard Operating Procedures (SOPs) that represent best practices in documentation and compliance, along with addressing Data Integrity Deviations, Investigations, and CAPA to maintain the integrity of the study.
- Quality Assurance: Implementing robust quality management systems is essential for CROs to monitor compliance and uphold high standards throughout the study process. This dedication to quality not only improves the integrity of the process but also . AVS Life Sciences exemplifies this by providing comprehensive quality management and compliance solutions tailored for the life sciences sector.
These traits emphasize the essential function that the CRO definition fulfills in enabling effective and compliant clinical studies, ultimately aiding in the successful creation of new therapies. Their involvement is crucial in addressing the persistent challenges of patient recruitment and regulatory adherence, ensuring that trials are conducted effectively and ethically.
Conclusion
The role of Contract Research Organizations (CROs) in the pharmaceutical industry is indispensable, providing essential services that streamline the drug development process. By outsourcing research functions to CROs, pharmaceutical companies can concentrate on their core operations while ensuring compliance with regulatory standards and enhancing the efficiency of clinical trials. This strategic partnership not only accelerates the introduction of new therapies but also significantly improves patient outcomes.
Key insights regarding the multifaceted responsibilities of CROs include:
- Study design
- Patient recruitment
- Data management
- Adherence to regulatory compliance
The data highlight the impressive success rates achieved by CROs in managing clinical trials and the effectiveness of their quality assurance measures. Moreover, collaboration between CROs and pharmaceutical firms has proven to enhance the overall quality of medical studies, ensuring they are conducted ethically and efficiently.
In conclusion, the significance of CROs in the pharmaceutical landscape cannot be overstated. As the industry continues to evolve, the reliance on these specialized organizations will only grow, emphasizing the need for pharmaceutical compliance officers to understand their functions and benefits. By embracing the expertise of CROs, the pharmaceutical sector can navigate the complexities of drug development, ultimately leading to improved health outcomes for patients worldwide.
Frequently Asked Questions
What does CRO stand for?
CRO stands for Contract Research Organization.
What services do Contract Research Organizations provide?
CROs provide outsourced research services including study design, patient recruitment, data management, and adherence to regulations for the pharmaceutical, biotechnology, and medical device industries.
Why are CROs significant in the drug development process?
CROs streamline the drug development process, allowing sponsors to focus on their core competencies while ensuring that studies are conducted efficiently and in compliance with established standards, ultimately benefiting patients and healthcare systems.
What is the projected growth of the global CRO market?
The global CRO market is projected to grow from approximately USD 64.42 billion in 2023 to over USD 102.57 billion by 2028.
Why are pharmaceutical companies outsourcing to CROs?
Pharmaceutical companies are outsourcing to CROs to enhance operational efficiency, reduce time-to-market for new medications, and manage rising costs and complex regulatory landscapes.
What impact do CROs have on clinical research efficiency?
Collaborations between CROs and pharmaceutical firms have led to significant improvements in clinical research efficiency, with reports indicating that 93% of studies are completed on time or ahead of schedule.
How do CROs transform their role in the drug development process?
By leveraging the specialized expertise of CROs, pharmaceutical firms gain insights into regulatory expectations and study designs, transforming CROs from mere service providers into strategic partners, which enhances the quality and success of medical studies.
