Create a CAPA Form: A Step-by-Step Guide for Compliance Officers

Overview
The primary focus of this article is to deliver a comprehensive, step-by-step guide for compliance officers on the creation of a Corrective and Preventive Action (CAPA) form. It begins by identifying the challenges faced in compliance, then elaborates on essential steps such as:
- Pinpointing issues
- Conducting thorough root cause analyses
- Implementing effective corrective and preventive measures
The article underscores the critical importance of meticulous documentation and review processes, which are vital for ensuring regulatory compliance and achieving operational excellence. By following these guidelines, compliance officers will enhance their capabilities and foster a culture of continuous improvement.
Introduction
Creating a robust Corrective and Preventive Action (CAPA) form is essential for compliance officers navigating the intricate landscape of pharmaceutical and biotechnology regulations. This systematic approach not only addresses existing non-conformities but also establishes a foundation for preventing future issues, thereby fostering a culture of quality and safety.
With the concerning statistic that inadequate CAPA management accounts for nearly 40% of FDA 483 submissions, the challenge persists: how can compliance officers effectively implement and document these critical actions to ensure both regulatory adherence and operational excellence?
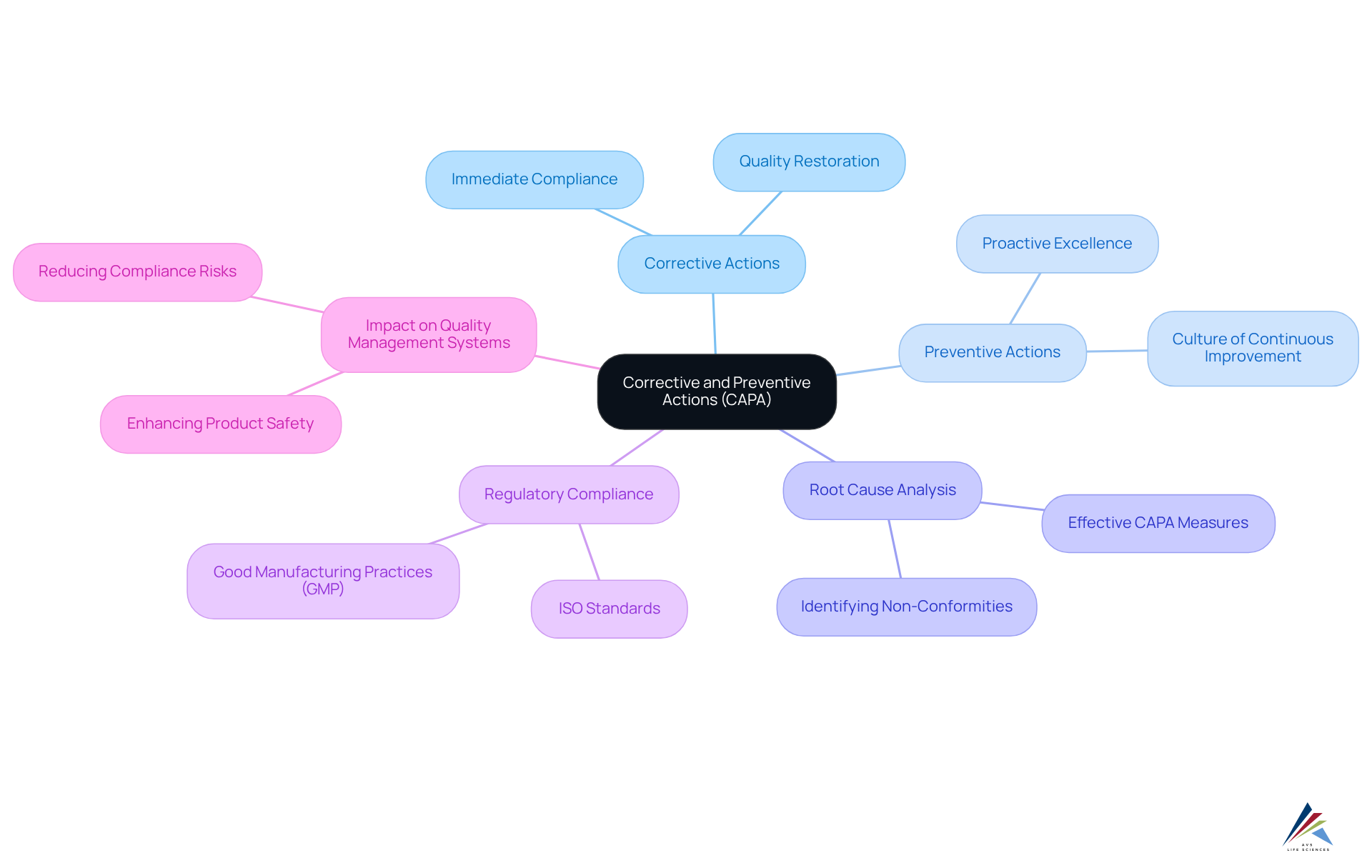
Understand Corrective and Preventive Actions (CAPA)
Corrective and Preventive Actions (capa form) represent vital systematic methods aimed at identifying, addressing, and preventing issues that could jeopardize product standards and compliance. Corrective actions focus on eliminating the root causes of existing non-conformities, while preventive actions seek to avert potential non-conformities from arising in the future. For compliance officers, a comprehensive grasp of these concepts is essential, as they support management systems within the pharmaceutical and biotechnology sectors. Mastery of capa form not only assists in meeting regulatory requirements, such as Good Manufacturing Practices (GMP) and ISO standards, but also significantly enhances overall product excellence and safety.
Key Components of CAPA
- Corrective Actions: Steps taken to rectify identified issues, ensuring immediate compliance and quality restoration.
- Capa Form: Measures implemented to avoid future issues, thus fostering a culture of proactive excellence.
- Root Cause Analysis: A critical process that identifies the underlying causes of non-conformities, enabling effective corrective and preventive measures.
The significance of corrective and preventive actions in management systems is underscored by the fact that constitutes about 40% of FDA 483 submissions. This statistic highlights the necessity for robust corrective and preventive action (CAPA form) processes to mitigate compliance risks. Industry leaders emphasize that quality is not merely a task but must be viewed as a capa form, reinforcing the notion that ongoing enhancement through effective corrective and preventive actions is essential for maintaining high standards.
Real-world examples illustrate the impact of corrective and preventive actions in biotechnology. For instance, a prominent biotech company established a thorough capa form system that resulted in a 20% decrease in non-conformities within six months, demonstrating the efficacy of corrective measures in enhancing operational efficiency. Moreover, understanding the distinction between corrective and preventive measures is crucial; while corrective measures address immediate concerns, preventive measures ensure that similar problems do not recur, thereby strengthening the overall management framework in the capa form.
By mastering these components, compliance officers can effectively manage quality issues and ensure adherence to regulatory standards, ultimately contributing to a culture of excellence within their organizations.
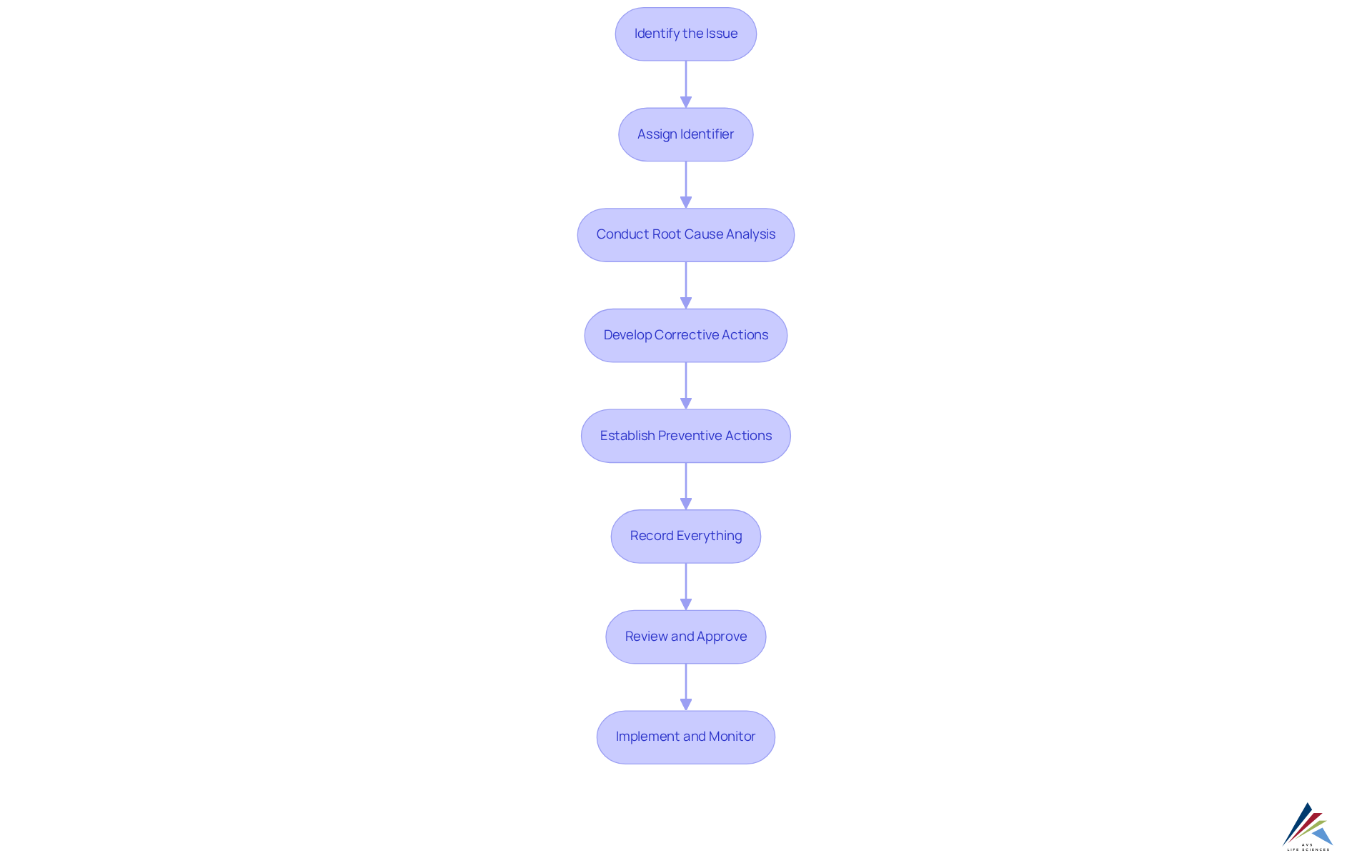
Follow the Step-by-Step Process to Create a CAPA Form
Creating a CAPA form involves several essential steps to ensure effective management of [corrective and preventive actions](https://avslifesciences.com/biopharma):
- Identify the Issue: Clearly define the problem or non-conformity that needs to be addressed. Include specific details such as dates, locations, and affected products to provide a comprehensive overview.
- Assign a unique identifier for tracking purposes. This number will be referenced throughout the CAPA process, facilitating easy retrieval and monitoring.
- Conduct a Root Cause Analysis: Investigate the underlying causes of the problem using effective techniques such as the 5 Whys or Fishbone Diagram. These methods help in systematically identifying root causes, ensuring that the analysis is thorough and actionable.
- Develop Corrective Actions: Outline the steps that will be taken to rectify the identified problem. Identify accountable individuals for each task and establish clear deadlines for completion, ensuring responsibility and prompt resolution.
- Establish Preventive Actions: Identify measures that will be implemented to prevent recurrence of the issue. This may involve changes to processes, additional training, or updates to documentation, all aimed at mitigating future risks.
- Record Everything: Ensure that all discoveries, measures, and decisions are thoroughly documented in the form. This documentation is crucial for compliance with regulatory standards and for facilitating future audits in the CAPA form.
- Review and Approve: Submit the by relevant stakeholders. Obtain necessary approvals before executing the steps to ensure alignment and support from all involved parties.
- Implement and Monitor: Execute the corrective and preventive actions as outlined. Continuously assess their effectiveness over time to ensure the problem is resolved and does not reappear, thereby improving the overall quality management system.
By following these steps, compliance officers can create a strong CAPA form that not only addresses urgent concerns but also fosters a culture of ongoing enhancement and regulatory adherence.
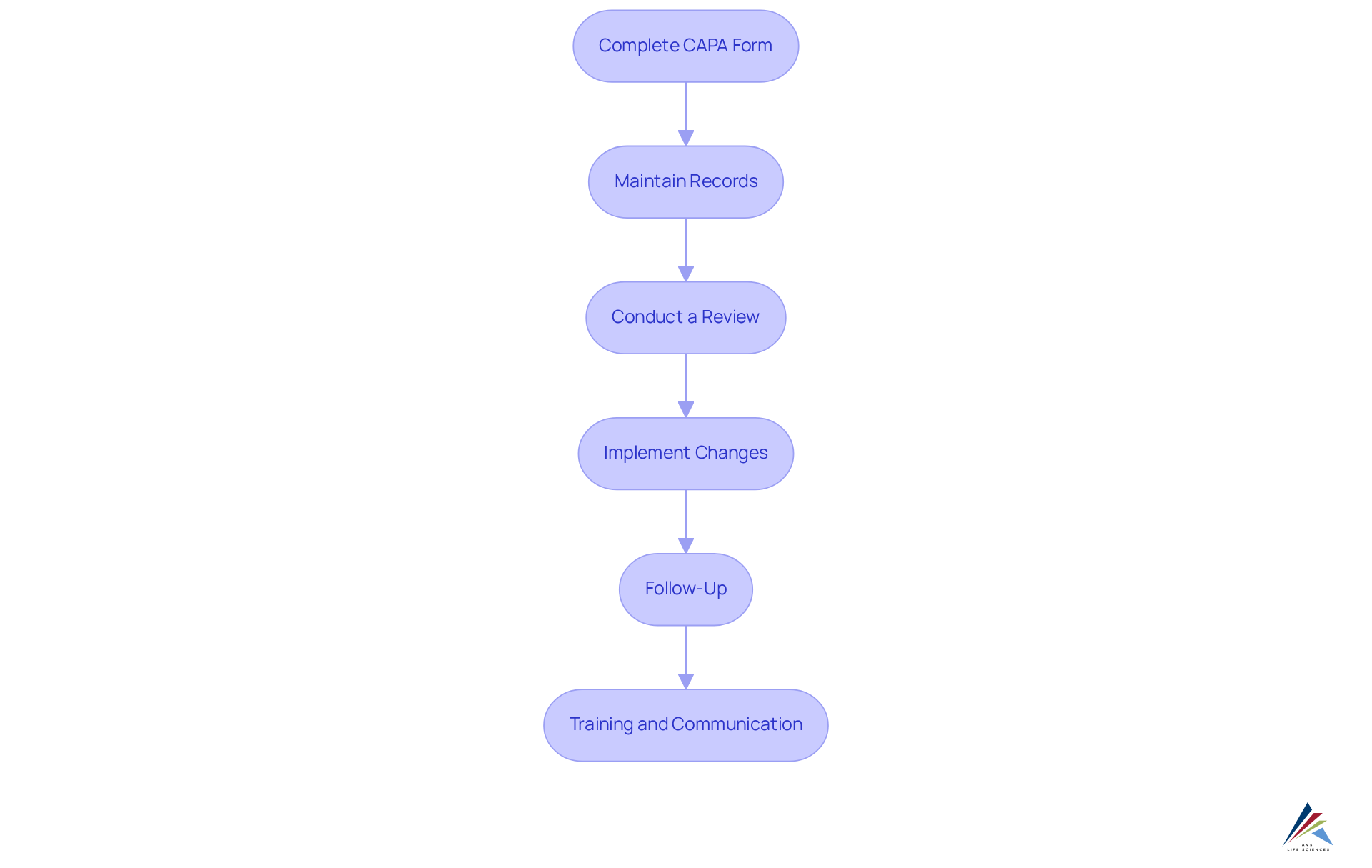
Document and Review Your CAPA Form for Compliance
Completing the capa form is the initial step; however, meticulous documentation and thorough review are paramount for compliance and effectiveness.
- Ensure the completeness of the capa form by confirming that every section is fully completed. This includes identifying the problem, conducting root cause analysis, outlining corrective and preventive measures, and designating responsible parties. A clear problem statement is crucial for effective corrective and preventive actions, facilitating later verification of effectiveness in resolving the issue.
- Maintain Records: All documentation related to the corrective and preventive action process must be meticulously maintained. This includes initial reports, analysis results, and follow-up actions. Such documentation should be readily available for audits and inspections, as are frequently noted in regulatory assessments. AVS Life Sciences underscores the importance of comprehensive records to ensure compliance with evolving regulations.
- Conduct a Review: Organize a review meeting with relevant stakeholders to evaluate the corrective action plan form. This discussion should focus on the effectiveness of the proposed actions and any additional measures that may be required. Involving leadership in the corrective and preventive action process demonstrates a commitment to excellence and adherence, which is a foundational principle of AVS Life Sciences.
- Implement Changes: Following the review, make necessary adjustments to the capa form based on the feedback received. Document all changes meticulously to ensure a clear audit trail. AVS Life Sciences' expertise in standards management guarantees that these adjustments align with industry regulations.
- Follow-Up: Develop a follow-up plan to monitor the effectiveness of the corrective and preventive actions. This may involve regular check-ins or additional audits to ensure compliance and effectiveness. Regular audits and a culture of quality are vital for sustaining a robust corrective and preventive action system, as emphasized by AVS Life Sciences' extensive GXP regulatory services.
- Training and Communication: Effectively communicate the results of the corrective action process to all relevant personnel. Provide training as necessary to ensure that everyone understands the adjustments made and their responsibilities in preventing future issues. A strong reporting culture encourages employees to identify problems promptly, which is essential for efficient corrective and preventive action management.
By adhering to these steps, compliance officers can ensure that their CAPA processes are not only compliant but also effective in upholding quality standards and preventing future issues.
Conclusion
Creating an effective CAPA form is essential for compliance officers dedicated to upholding product standards and meeting regulatory requirements. Implementing Corrective and Preventive Actions not only addresses immediate issues but also cultivates a proactive culture of quality within organizations. By grasping the nuances of CAPA, compliance officers can efficiently manage both current and future non-conformities, ultimately enhancing overall product excellence.
The article delineates a comprehensive step-by-step process that encompasses:
- Identifying issues
- Conducting root cause analyses
- Developing corrective and preventive actions
- Ensuring meticulous documentation and review
Each step is vital for maintaining compliance with industry standards while simultaneously promoting a culture of continuous improvement. Real-world examples underscore the tangible benefits of a well-structured CAPA process, showcasing its significant role in reducing non-conformities and enhancing operational efficiency.
In conclusion, the importance of a robust CAPA system cannot be overstated. Compliance officers are urged to adopt the outlined best practices and sustain a commitment to quality through rigorous documentation and regular reviews. By prioritizing corrective and preventive actions, organizations can not only meet regulatory expectations but also establish a sustainable framework for excellence that ultimately benefits their operations and stakeholders.
Frequently Asked Questions
What are Corrective and Preventive Actions (CAPA)?
Corrective and Preventive Actions (CAPA) are systematic methods aimed at identifying, addressing, and preventing issues that could jeopardize product standards and compliance. Corrective actions focus on eliminating the root causes of existing non-conformities, while preventive actions aim to avert potential non-conformities from arising in the future.
Why is CAPA important for compliance officers?
A comprehensive understanding of CAPA is essential for compliance officers as it supports management systems within the pharmaceutical and biotechnology sectors, helps meet regulatory requirements such as Good Manufacturing Practices (GMP) and ISO standards, and enhances overall product excellence and safety.
What are the key components of CAPA?
The key components of CAPA include: - Corrective Actions: Steps taken to rectify identified issues for immediate compliance and quality restoration. - Preventive Actions: Measures implemented to avoid future issues, fostering a culture of proactive excellence. - Root Cause Analysis: A process that identifies the underlying causes of non-conformities to enable effective corrective and preventive measures.
How significant is CAPA management in the pharmaceutical industry?
Inadequate CAPA management constitutes about 40% of FDA 483 submissions, underscoring the necessity for robust CAPA processes to mitigate compliance risks and maintain high standards.
Can you provide an example of CAPA effectiveness in biotechnology?
A prominent biotech company established a thorough CAPA system that resulted in a 20% decrease in non-conformities within six months, demonstrating the efficacy of corrective measures in enhancing operational efficiency.
What is the difference between corrective and preventive actions?
Corrective actions address immediate concerns by rectifying existing issues, while preventive actions ensure that similar problems do not recur, thereby strengthening the overall management framework.
How can compliance officers contribute to a culture of excellence through CAPA?
By mastering the components of CAPA, compliance officers can effectively manage quality issues and ensure adherence to regulatory standards, ultimately contributing to a culture of excellence within their organizations.
