Biosimilar vs Generic: Key Differences in Efficacy and Safety

Overview
The article delineates the distinctions between biosimilars and generics, underscoring their critical differences in efficacy and safety. It is emphasized that biosimilars necessitate comprehensive clinical trials to establish their similarity to reference biologics, whereas generics are required to demonstrate bioequivalence to small-molecule drugs. This differentiation is further supported by a discussion on the intricate regulatory frameworks and safety assessments governing biosimilars, which demand rigorous monitoring and substantiation of clinical outcomes. Such requirements reflect the inherent complexities of biologics when contrasted with traditional medications.
Introduction
The landscape of pharmaceuticals is evolving, with biosimilars and generics emerging as pivotal players in enhancing access to essential medications. Both serve as cost-effective alternatives to branded drugs; however, their fundamental differences in structure, regulatory pathways, and clinical evidence requirements can significantly impact patient care.
As healthcare professionals navigate these complexities, a pressing question arises: how do the efficacy and safety profiles of biosimilars compare to those of generics? What implications does this have for treatment decisions? These inquiries are crucial for informed decision-making in a rapidly changing healthcare environment.
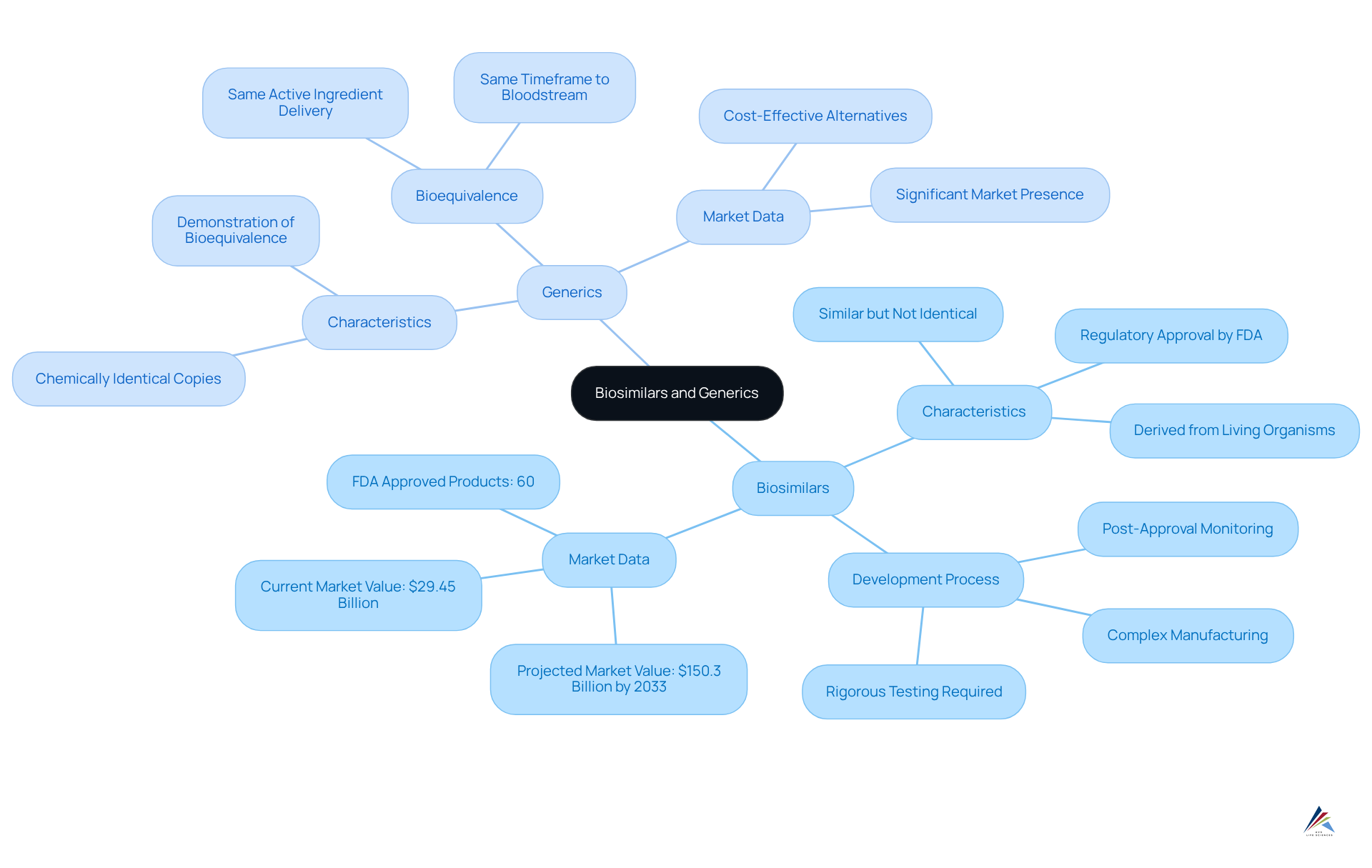
Define Biosimilars and Generics: Key Characteristics
Biosimilars represent a category of biologic medical products that closely resemble an already approved reference biologic, demonstrating no clinically significant differences in safety, purity, or potency. These products are derived from living organisms, resulting in a more intricate structure compared to conventional medications.
In contrast, non-branded versions are chemically identical copies of small-molecule drugs, necessitating the demonstration of bioequivalence to the original product. This requirement indicates that non-branded products must deliver the same quantity of active ingredient into the bloodstream within the same timeframe as their branded counterparts.
While both biosimilars and generics serve as cost-effective alternatives to branded medications, their development processes and regulatory requirements diverge significantly, highlighting the due to the inherent complexities associated with biologics.
The FDA defines a biosimilar as 'a type of biological item that is licensed (approved) by the FDA because it is highly similar to an already FDA-approved biological item (the reference item) and has been shown to have no clinical differences from the reference item.'
As of September 2024, the FDA has approved 60 biosimilar products, reflecting the growing acceptance and integration of these alternatives in the market. The global market for similar biological products was valued at $29.45 billion in 2023 and is projected to reach $150.3 billion by 2033, with a CAGR of 17.7%.
Understanding these distinctions is crucial for healthcare professionals to make informed decisions regarding patient care, especially given the necessity for robust post-approval monitoring programs to ensure the safety and effectiveness of similar biological products.
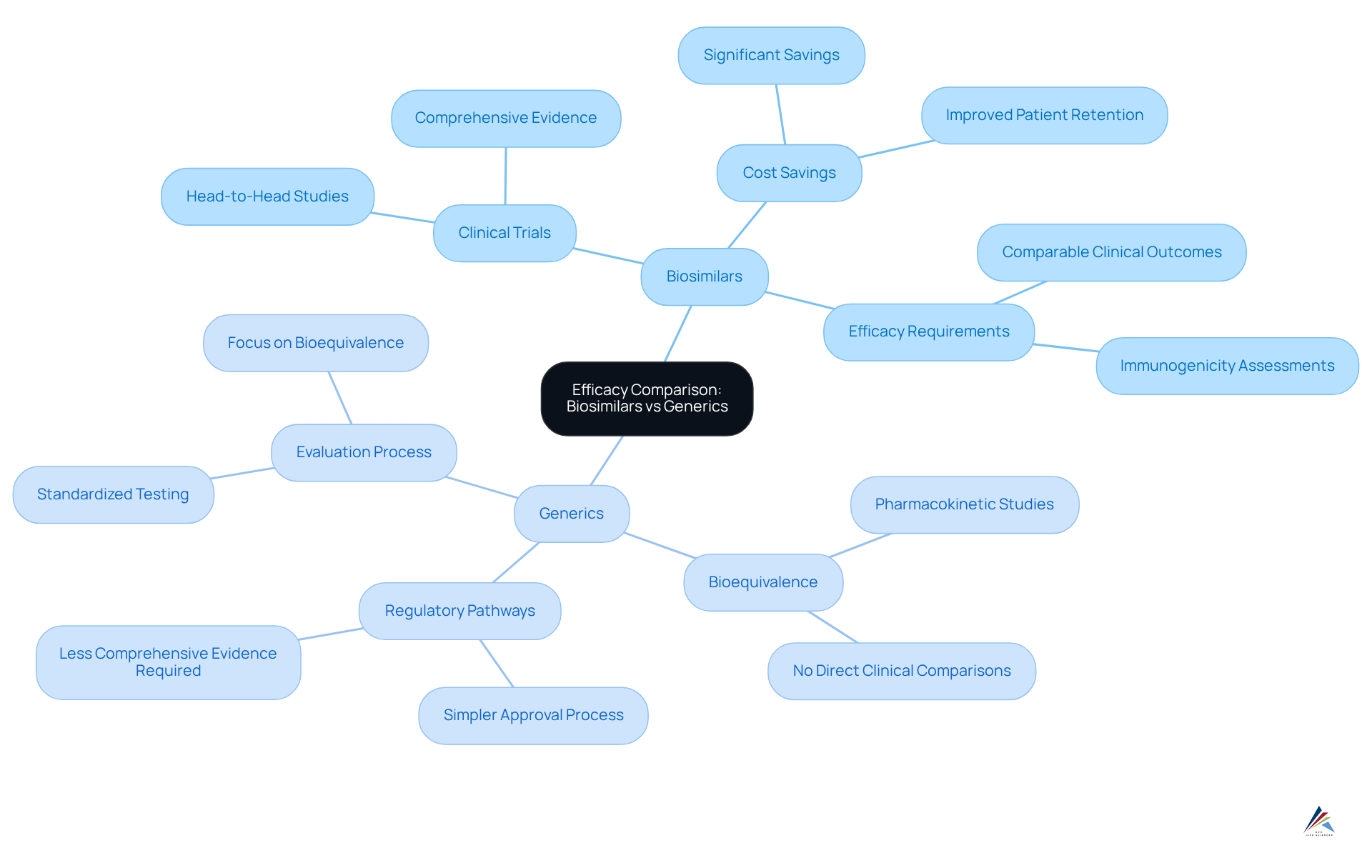
Compare Efficacy: Biosimilars vs Generics
In the realm of therapeutic equivalence, similar biological products must demonstrate comparable clinical outcomes to their reference products through comprehensive clinical trials, often involving head-to-head studies to establish efficacy equivalence. Recent studies illustrate that biosimilar IBI303 offers comparable effectiveness to Humira for ankylosing spondylitis, highlighting significant cost savings while maintaining efficacy.
In contrast, non-branded medications primarily need to demonstrate bioequivalence to their branded counterparts, typically through pharmacokinetic studies rather than direct clinical comparisons. This fundamental difference arises from the , which can exhibit variations that may influence efficacy across different patient populations.
Consequently, while standard medications typically offer a clear efficacy profile, similar biological products necessitate more comprehensive clinical evidence to confirm their application in particular indications. A literature search identified eleven studies centered on similar therapies for multiple sclerosis (MS), underscoring the thorough evaluation process these items undergo.
Overall, the subtle distinctions in regulatory pathways and clinical evidence requirements highlight the significance of comprehending the unique landscape of products in the context of biosimilar vs generic.
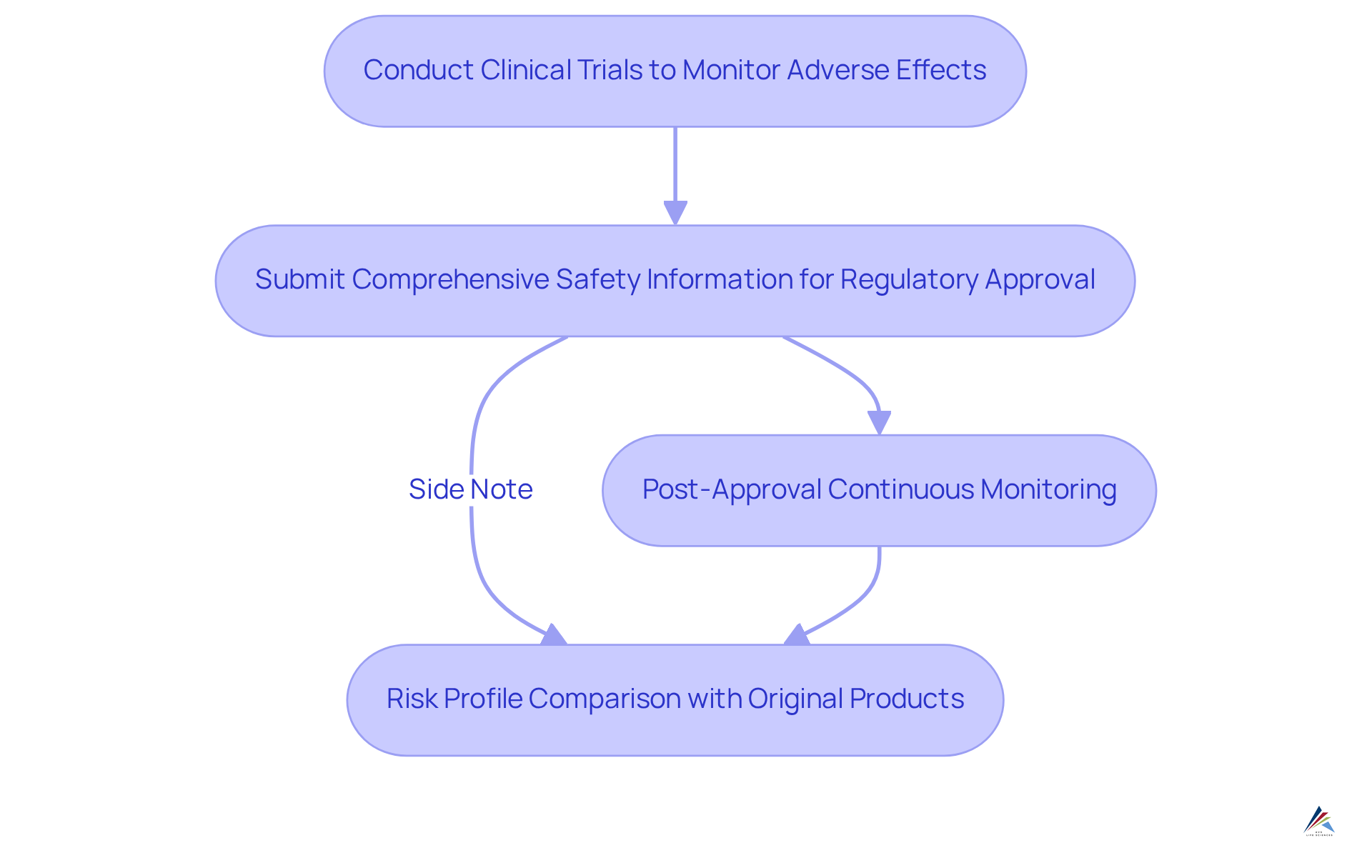
Assess Safety: Evaluating Risks and Benefits
Safety assessments for biosimilars demand extensive clinical trials to monitor adverse effects and confirm that they do not significantly differ from their reference products. Regulatory agencies require comprehensive information on safety prior to approval, with continuous monitoring being essential post-approval.
For biosimilar equivalents, assessments are typically based on the risk profile of the original branded medication, given their chemical similarity. However, variability in excipients or manufacturing processes can impact the reliability of outcomes.
Both biosimilar and generic alternatives must adhere to stringent safety standards, but the inherent complexity of biologics introduces unique considerations for these alternatives that necessitate thorough evaluation by healthcare providers.
A pertinent example is a meta-analysis of risk in transitioning from originator biologics to similar products, which encompassed 44 switch treatment periods from 31 distinct studies involving 5,252 patients. This analysis found no significant differences in serious adverse events, with 436 participants in switch groups and 433 in no-switch groups experiencing serious adverse events. This reinforces the notion that switching is generally safe and underscores the in ensuring patient safety across both categories.
Furthermore, the potential for considerable healthcare expense reductions associated with similar biologics enhances their attractiveness, establishing them as a valuable substitute for standard medications.
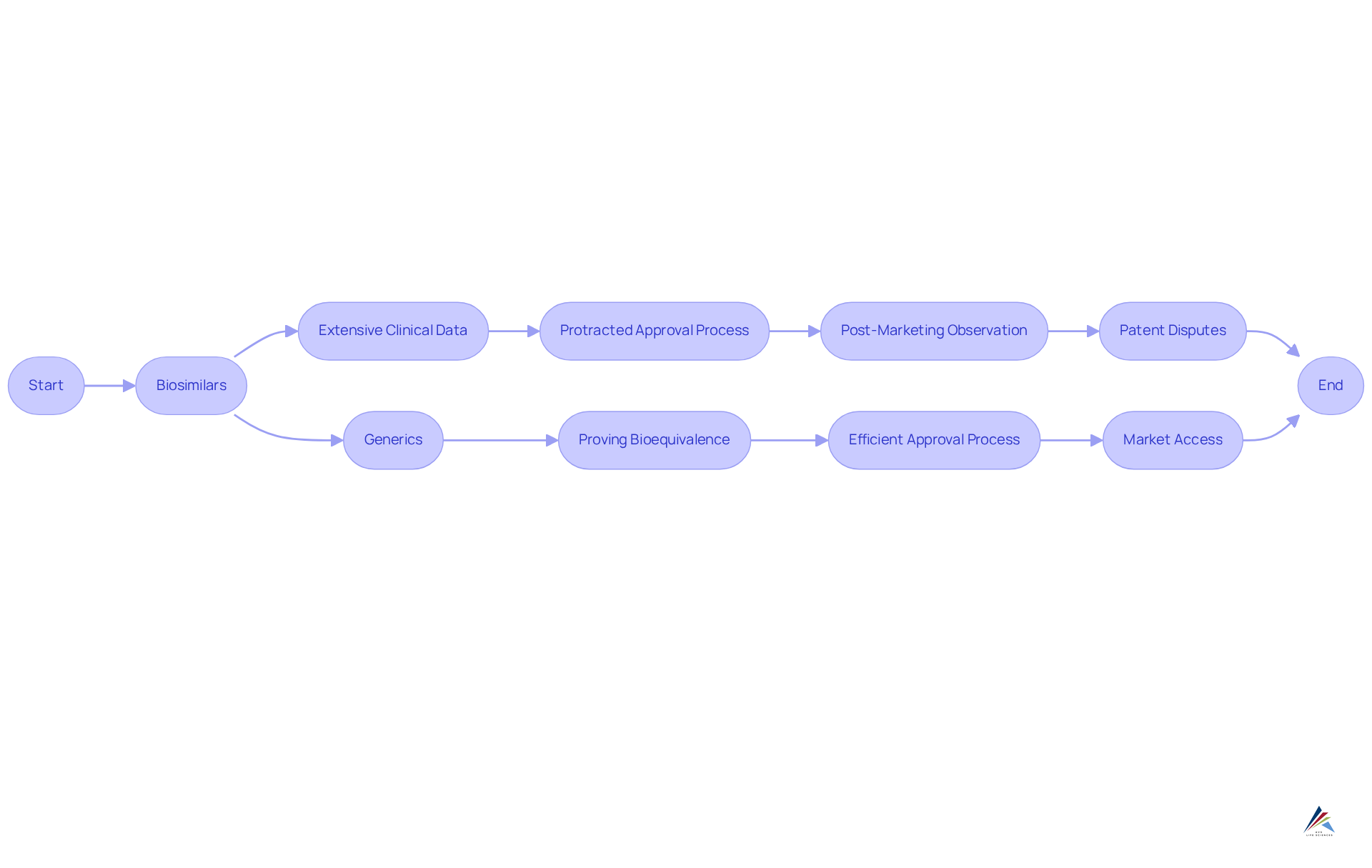
Examine Regulatory Framework: Compliance and Approval Processes
The regulatory framework for biological products presents a complexity that surpasses that of biosimilars vs generics. In the United States, the Biologics Control Act governs the approval of biosimilars, mandating extensive clinical data to demonstrate similarity to the reference item. This stringent requirement often leads to a protracted approval process, as comprehensive studies are essential to . Conversely, non-branded drugs are regulated by the Drug Approval Modernization Act, which facilitates a more efficient process focused on proving bioequivalence to the original product. Consequently, standard medications typically achieve market access more rapidly than their biological counterparts.
Continuous adherence to regulations is paramount for both follow-on biologics and replicas, with post-marketing observation necessary to evaluate long-term effectiveness and well-being. For example, the FDA's Purple Book enhances transparency by cataloging all FDA-approved biologic drugs, including similar biological products, and delineates the required post-marketing safety trials to ensure therapeutic safety. Moreover, the intricacies of patent disputes and exclusivity claims can considerably postpone the entry of biosimilars, highlighting the compliance challenges that arise in the context of biosimilar vs generic drugs. As the landscape evolves, grasping these regulatory nuances is crucial for effectively navigating the approval processes.
Conclusion
Biosimilars and generics represent two distinct categories of alternative medications, each characterized by unique regulatory pathways and implications for patient care. While both aim to provide cost-effective options, it is essential for healthcare professionals to understand the differences in efficacy, safety, and approval processes to make informed decisions.
Biosimilars necessitate rigorous clinical trials to establish their clinical efficacy in comparison to reference biologics, whereas generics primarily focus on demonstrating bioequivalence through pharmacokinetic studies. Additionally, safety assessments for biosimilars involve extensive post-approval monitoring due to the complexity of biologics, while generics adhere to a more straightforward evaluation process. The regulatory frameworks governing these products differ significantly, with biosimilars facing more stringent requirements that can prolong their market entry.
In light of these insights, it is clear that both biosimilars and generics play a crucial role in enhancing patient access to medications while upholding safety and efficacy standards. As the market for biosimilars continues to expand, driven by increasing demand and advancements in research, healthcare providers must remain informed about these developments. This knowledge not only facilitates better patient outcomes but also encourages the adoption of these alternatives in treatment plans, ultimately contributing to a more sustainable healthcare system.
Frequently Asked Questions
What are biosimilars?
Biosimilars are biologic medical products that closely resemble an already approved reference biologic, showing no clinically significant differences in safety, purity, or potency. They are derived from living organisms and have a more complex structure than conventional medications.
How do biosimilars differ from generics?
Biosimilars are not identical copies but rather highly similar to an approved reference biologic, while generics are chemically identical copies of small-molecule drugs. Generics must demonstrate bioequivalence to the original product, meaning they deliver the same amount of active ingredient into the bloodstream within the same timeframe.
What is the FDA's definition of a biosimilar?
The FDA defines a biosimilar as a type of biological product that is licensed because it is highly similar to an already FDA-approved biological product (the reference product) and has been shown to have no clinical differences from the reference product.
How many biosimilars have been approved by the FDA?
As of September 2024, the FDA has approved 60 biosimilar products.
What is the market value of biosimilars?
The global market for similar biological products was valued at $29.45 billion in 2023 and is projected to reach $150.3 billion by 2033, with a compound annual growth rate (CAGR) of 17.7%.
Why is understanding the differences between biosimilars and generics important for healthcare professionals?
Understanding these distinctions is crucial for healthcare professionals to make informed decisions regarding patient care, particularly due to the need for robust post-approval monitoring programs to ensure the safety and effectiveness of similar biological products.
