Avoid Common Mistakes in Cosmetic Labeling with These Steps

Overview
The article delineates how to sidestep prevalent pitfalls in cosmetic labeling by articulating essential labeling requirements and compliance strategies. It underscores the significance of precise item identity, ingredient declarations, and explicit warnings, bolstered by regulatory guidelines, to avert legal complications and bolster consumer trust in cosmetic products.
Introduction
Navigating the intricate world of cosmetic labeling presents significant challenges for manufacturers, particularly amidst the rapidly changing regulations that govern the industry. Understanding the essential elements of labeling is crucial; it not only ensures compliance with FDA standards but also fosters consumer trust and safety.
However, many brands encounter common pitfalls that can lead to legal repercussions and tarnish their reputation. What are the most frequent mistakes made in cosmetic labeling? How can manufacturers effectively avoid these missteps to maintain credibility and safeguard their customers?
These questions are vital as we delve deeper into the landscape of cosmetic compliance.
Understand Cosmetic Labeling Basics
Cosmetic labeling encompasses several crucial elements that must be accurately displayed on packaging to ensure compliance and safety for consumers. These key elements are pivotal:
- Item Identity: Clearly specify the nature of the item, such as 'moisturizer' or 'shampoo,' to prevent consumer confusion.
- Net Contents: Indicate the amount of product in the container, typically expressed in weight or volume, adhering to FDA guidelines that dictate size requirements based on the Principal Display Panel (PDP) area. For a PDP area of 5-25 sq. in, the type size must be 1/8 inches; for 25-100 sq. in, it must be 3/16 inches.
- Ingredient Declaration: List all ingredients in descending order of predominance by weight, utilizing the International Nomenclature of Cosmetic Ingredients (INCI) names. Ingredients present at less than 1% can be listed in any order after those present at more than 1%. This is essential as the (MoCRA), enacted in 2022, mandates clear [ingredient labeling to enhance transparency](http://avslifesciences.com/blog-post/8-key-insights-on-the-cosmetic-ingredient-disclosure-bill) for purchasers.
- Warnings and Directions: Include essential safety alerts and usage guidelines to inform users about proper use, ensuring that all necessary information is displayed prominently to prevent misbranding. It is also important to ensure that all necessary information is duplicated on multiple PDPs if an item has more than one.
- Manufacturer Information: Provide the name and address of the manufacturer or distributor, which is vital for accountability and traceability.
Additionally, fragrance allergens must be disclosed on labels once the FDA issues its final rule on required allergens.
Adhering to these labeling essentials is not only [critical for compliance with FDA regulations](https://artworkflowhq.com/resources/fda-cosmetic-labeling-regulations) but also essential for understanding common mistakes in cosmetic labeling and how to avoid them, which significantly contributes to building consumer trust and informed decision-making. Precise identification helps avoid legal issues and enhances the overall integrity of cosmetic products in the market by addressing common mistakes in cosmetic labeling and how to avoid them. As Mrignayni Pandey states, "If any suitable wordings, directions, warnings, or other necessary information are not shown according to regulations, or if the tags are false or misleading under FDA regulations, your cosmetic descriptions may be considered as misbranded.

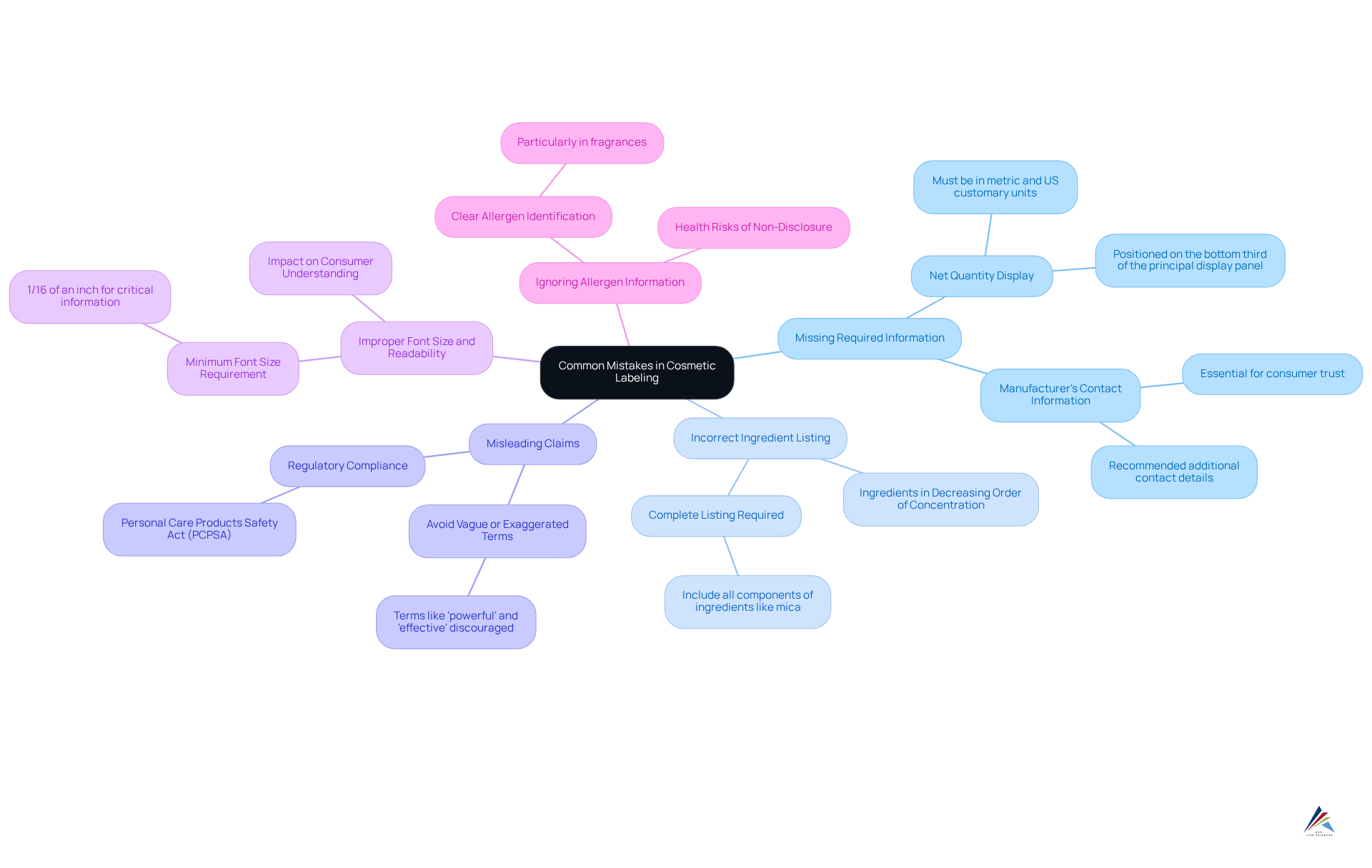
Identify Common Mistakes in Cosmetic Labeling
Common mistakes in cosmetic labeling and how to avoid them can lead to significant compliance issues and legal repercussions. To navigate these pitfalls effectively, manufacturers must be aware of the following key areas to avoid:
- Missing Required Information: Essential details, such as the item's net contents and the manufacturer's contact information, must be clearly displayed. The FDA mandates that the net quantity be shown in both metric and US customary units, positioned on the bottom third of the principal display panel. Non-compliance can result in penalties and product recalls. Furthermore, the Fair Packaging and Labeling Act (FPLA) emphasizes the importance of displaying net quantity to empower buyers in making informed purchasing choices.
- Incorrect Ingredient Listing: Accurate ingredient listings are vital for compliance. Ingredients should be presented in decreasing order of concentration, and any omissions can mislead buyers and violate regulations. For example, listing 'mica' alone as an ingredient is incorrect; it must encompass all components utilized in cosmetic-grade micas. A case study highlighting the absence of manufacturing information underscores the necessity of including critical details such as batch numbers and expiration dates on cosmetic labels, enhancing traceability and safety for users.
- Misleading Claims: Vague or exaggerated assertions can mislead buyers and lead to legal action. Terms like 'powerful' and 'effective' are discouraged, as they may create inflated expectations regarding a product's benefits. The stresses the need for honest advertising to protect consumers. Senator Diane Feinstein's advocacy for the PCPSA further underscores the significance of regulatory compliance in cosmetic labeling.
- Improper Font Size and Readability: Labels must comply with specific font size regulations, with the FDA specifying a minimum height of 1/16 of an inch for critical information. Poor readability can confuse customers and lead to non-compliance, ultimately undermining brand credibility. Ensuring that font sizes meet regulatory standards is crucial for maintaining client trust.
- Ignoring Allergen Information: Neglecting to disclose potential allergens, particularly in fragrances, poses serious health risks. Clear identification of allergens is essential for consumer safety and trust. The absence of allergen disclosure can result in adverse health outcomes, highlighting the necessity for transparency in ingredient listings.
By recognizing and addressing common mistakes in cosmetic labeling and how to avoid them, manufacturers can enhance compliance, avoid costly errors, and ensure their products meet regulatory requirements.
Implement Compliance Strategies for Accurate Labeling
To ensure accurate labeling and compliance, consider the following strategies:
- Conduct Regular Audits: Regular audits are essential for verifying that labels align with current regulations. Approximately 70% of companies report using checklists to streamline this process, ensuring that all required elements are consistently reviewed. The FDA can suspend registration if products present a reasonable likelihood of causing serious harm, emphasizing the critical nature of adherence.
- Utilize Checklists: Create a thorough checklist for tagging that includes all essential elements, assisting in avoiding oversight and ensuring adherence to FDA and MoCRA standards.
- Stay Updated on Regulations: It is crucial to remain informed about changes in cosmetic packaging laws and guidelines. The FDA's upcoming draft guidance on fragrance allergens, expected by late 2024, exemplifies the need for vigilance in regulatory updates.
- Consult Specialists: Consulting with regulatory adherence specialists can provide valuable insights into the accuracy of labels. As Gurudev Mallesha, an ISO Lead Auditor, states, "Compliance becomes manageable when companies start early." Early involvement in the regulatory process can reduce risks and improve product safety.
- Training Programs: Implementing training for staff engaged in tagging is vital. Instructing staff on the significance of regulations and optimal methods promotes a culture of responsibility and accuracy in identification efforts.
By implementing these strategies, manufacturers can greatly diminish the risk of common mistakes in cosmetic labeling and how to avoid them, thereby improving their adherence efforts and ultimately protecting their brand image and consumer confidence. Additionally, referencing case studies such as the "Labelling of Cosmetic Products in the United States" can provide real-world context and reinforce the importance of these strategies.

Utilize Resources for Continuous Learning and Improvement
To foster continuous learning and improvement in cosmetic labeling, manufacturers must consider the following resources:
- FDA Resources: Utilize the FDA's Cosmetics Labeling Guide for current information on marking requirements and regulations, which are essential for adhering to federal standards.
- Industry Webinars: Attend webinars focused on cosmetic regulations and labeling best practices to gain insights from experts. With projected to grow by 77% between 2021 and 2026, staying informed is essential for success in this evolving market.
- Professional Associations: Join organizations such as the Personal Care Products Council for access to industry news, regulatory updates, and networking opportunities that can improve adherence efforts.
- Online Courses: Enroll in courses related to cosmetic science and regulatory standards to enhance your knowledge and skills, ensuring you are well-versed in the latest guidelines.
- Consulting Services: Collaborate with compliance advisors who can offer customized guidance and assistance for your marking requirements. Client testimonials highlight the effectiveness of AVS Life Sciences' consultants in managing compliance-driven projects, demonstrating the value of expert guidance.
As Albert Einstein famously said, "Once you stop learning, you start dying." By leveraging these resources, manufacturers can ensure they remain compliant and informed about the latest developments regarding common mistakes in cosmetic labeling and how to avoid them, ultimately improving their product offerings and market presence.

Conclusion
Accurate cosmetic labeling is essential for ensuring consumer safety and compliance with regulatory standards. Understanding and implementing the key elements of cosmetic labeling allows manufacturers to avoid common pitfalls that may lead to legal issues and undermine consumer trust. Clear identification of products, proper ingredient listings, and adherence to font size regulations are critical components that contribute to effective labeling practices.
This article highlights several common mistakes in cosmetic labeling, such as:
- Missing required information
- Misleading claims
- Neglecting allergen disclosures
By recognizing these errors and employing strategies like:
- Regular audits
- Utilizing checklists
- Consulting with specialists
Manufacturers can significantly enhance their labeling accuracy and compliance. Staying informed about evolving regulations is crucial, as it enables manufacturers to adapt to changes and maintain market integrity.
Ultimately, the significance of precise cosmetic labeling extends beyond mere compliance; it fosters consumer confidence and promotes informed purchasing decisions. Embracing continuous learning through resources like:
- FDA guidelines
- Industry webinars
- Professional associations
Further empowers manufacturers to uphold high standards in their labeling practices. By prioritizing accuracy and transparency, the cosmetic industry can build a stronger foundation of trust with consumers, ensuring a safer and more informed marketplace.
Frequently Asked Questions
What is cosmetic labeling?
Cosmetic labeling refers to the essential elements that must be accurately displayed on product packaging to ensure compliance and safety for consumers.
What are the key elements of cosmetic labeling?
The key elements include Item Identity, Net Contents, Ingredient Declaration, Warnings and Directions, and Manufacturer Information.
What is meant by Item Identity in cosmetic labeling?
Item Identity specifies the nature of the cosmetic product, such as 'moisturizer' or 'shampoo,' to prevent consumer confusion.
How should Net Contents be displayed on cosmetic labels?
Net Contents should indicate the amount of product in the container, expressed in weight or volume, following FDA guidelines based on the Principal Display Panel (PDP) area.
What are the size requirements for displaying Net Contents on the PDP?
For a PDP area of 5-25 square inches, the type size must be 1/8 inches; for 25-100 square inches, it must be 3/16 inches.
How should ingredients be listed on cosmetic labels?
Ingredients must be listed in descending order by weight using the International Nomenclature of Cosmetic Ingredients (INCI) names, with those present at less than 1% listed in any order after those above 1%.
What does the Modernization of Cosmetics Regulation Act (MoCRA) require?
MoCRA mandates clear ingredient labeling to enhance transparency for consumers.
Why are warnings and directions important on cosmetic labels?
Warnings and directions provide essential safety alerts and usage guidelines to inform users about proper use, preventing misbranding.
What manufacturer information is required on cosmetic labels?
The label must include the name and address of the manufacturer or distributor for accountability and traceability.
What new requirement is expected regarding fragrance allergens on cosmetic labels?
Fragrance allergens must be disclosed on labels once the FDA issues its final rule on required allergens.
Why is compliance with cosmetic labeling regulations important?
Compliance is crucial for avoiding legal issues, building consumer trust, and ensuring informed decision-making in the market. Misleading or false labeling can lead to products being considered misbranded.
