Achieve CGMP Compliance: Essential Steps for Success

Overview
This blog outlines the essential steps and common challenges involved in achieving cGMP certification, emphasizing the importance of meeting regulatory standards within the pharmaceutical industry. It presents a structured framework that encompasses:
Additionally, it addresses significant challenges such as:
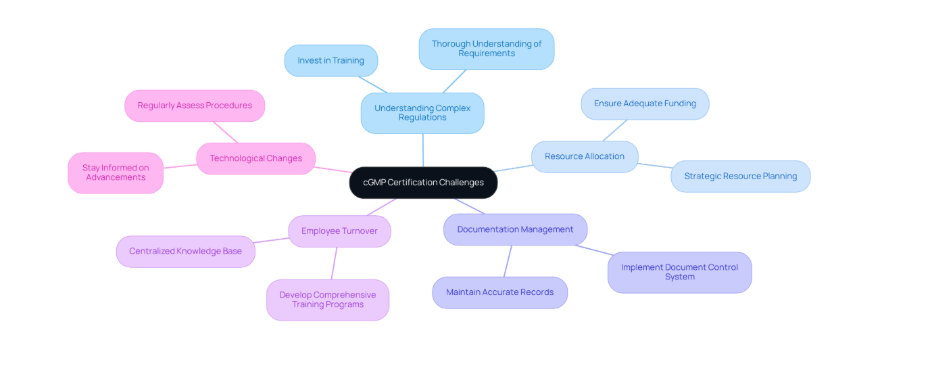
Both of which are vital for maintaining compliance and enhancing product quality.
Introduction
Achieving cGMP certification stands as a pivotal milestone for organizations within the pharmaceutical industry, as it ensures that products adhere to rigorous safety and quality standards. This certification not only enhances a company's reputation but also significantly mitigates the risk of costly product recalls, often resulting from management failures.
Nonetheless, the journey towards certification is laden with challenges, ranging from navigating intricate regulations to effectively managing documentation.
How can organizations streamline this process and surmount the obstacles that impede their progress?
Understand the Importance of CGMP Compliance
Current Good Manufacturing Standards (cGMP) are vital regulations implemented by the FDA to ensure the standard, safety, and effectiveness of pharmaceutical products. These practices encompass various aspects, including facility design, equipment maintenance, and employee training. By implementing cGMP practices, organizations can consistently produce high-quality products and experience fewer recallsthereby protecting patient safety and enhancing operational efficiency.
Essential Steps to Achieve cGMP Certification
To achieve cGMP certification, it is imperative to follow these essential steps:
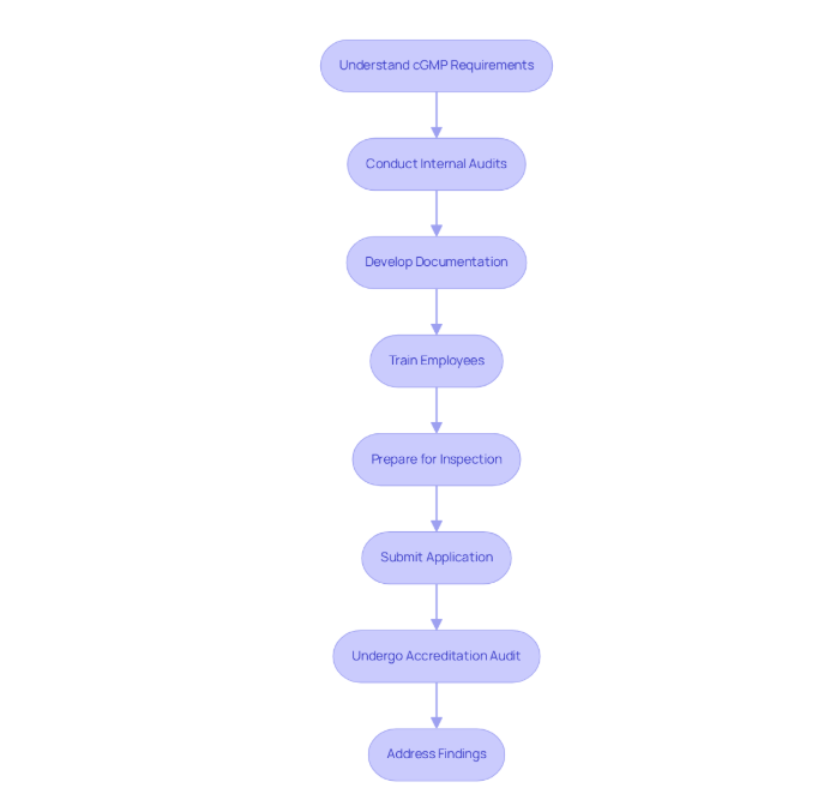
By adhering to these steps, organizations can effectively manage the intricacies of the good manufacturing practices' approval process and enhance their operational standards. Furthermore, leveraging the expertise of AVS Life Sciences can provide tailored consulting solutions that simplify regulatory processes.
Navigating Challenges Throughout the cGMP Certification Process
Achieving cGMP certification involves navigating several significant challenges that require careful consideration and strategic planning:

Leveraging Resources and Tools for Successful cGMP Certification
To support your journey towards cGMP certification, consider utilizing the following resources and tools:
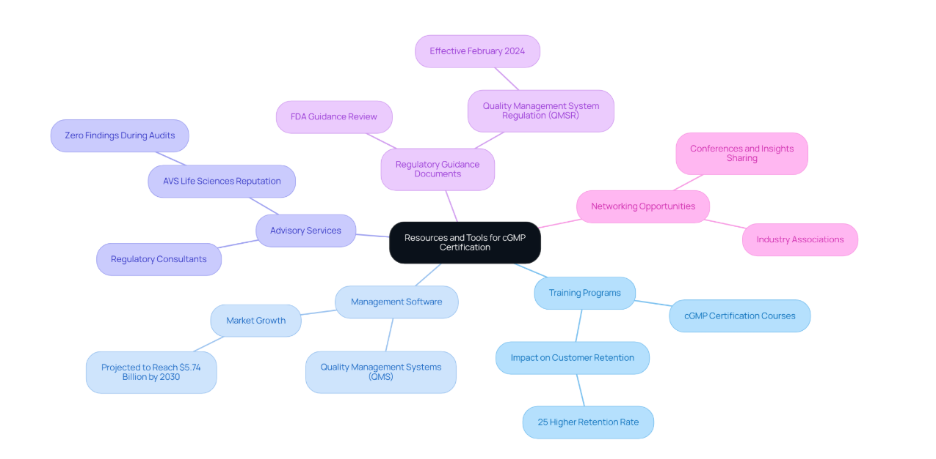
Conclusion
Achieving cGMP certification is a critical endeavor for organizations in the pharmaceutical industry, ensuring compliance with essential regulations that safeguard product quality and patient safety. This certification not only enhances a company's reputation but also significantly reduces the likelihood of costly product recalls, thereby emphasizing the necessity of adhering to good manufacturing practices.
The Essential Steps Toward cGMP Certification:

Addressing Common Challenges in the cGMP Certification Process

By adopting practical strategies to overcome these obstacles, organizations can streamline their certification process and enhance compliance by leveraging available resources, including training programs and advisory services.
Ultimately, the journey to cGMP certification transcends mere regulatory compliance; it fosters a culture of quality and continuous improvement within the organization. Embracing these principles positions companies for success in a competitive landscape and reinforces their commitment to safety and efficacy in pharmaceutical manufacturing. Taking proactive steps today can pave the way for a more compliant and efficient future, ensuring that patient safety remains at the forefront of industry practices.
Frequently Asked Questions
What is cGMP certification?
cGMP certification stands for current Good Manufacturing Practices certification, which ensures that pharmaceutical products are manufactured according to strict regulatory standards set by the FDA. It involves adherence to guidelines related to facility design, equipment maintenance, and employee training.
Why is cGMP certification important?
cGMP certification is important because it guarantees compliance with regulatory standards, enhances a company's reputation, reduces the risk of product recalls, and ensures the consistent production of high-quality products, thereby protecting patient safety and improving operational efficiency.
How does cGMP certification impact product quality and recalls?
Firms that are dedicated to Good Manufacturing Practices and achieve cGMP certification tend to experience fewer product recalls and improved product quality, demonstrating the significance of this accreditation in the competitive pharmaceutical environment.
Are there any updates to FDA regulations regarding cGMP certification?
Yes, as of 2025, updates to FDA regulations continue to emphasize the importance of current Good Manufacturing Practices, making cGMP certification necessary for organizations to stay informed and compliant, thereby mitigating risks associated with product recalls.
