Achieve cGMP Certification: Steps to Overcome Challenges

Overview
The article delineates critical steps and challenges in achieving cGMP certification, underscoring the necessity of adhering to regulatory standards within the pharmaceutical industry. It presents a structured approach that encompasses:
- Understanding certification requirements
- Conducting thorough internal audits
- Effectively training employees
Additionally, it addresses significant challenges such as:
- Navigating complex regulations
- Managing resource allocation
Both of which are vital for maintaining compliance and enhancing product quality.
Introduction
Achieving cGMP certification stands as a pivotal milestone for organizations within the pharmaceutical industry, as it ensures that products adhere to rigorous safety and quality standards. This certification not only enhances a company's reputation but also significantly mitigates the risk of costly product recalls, often resulting from management failures.
Nonetheless, the journey towards certification is laden with challenges, ranging from navigating intricate regulations to effectively managing documentation.
How can organizations streamline this process and surmount the obstacles that impede their progress?
Understand cGMP Certification: Definition and Importance
Present Good Manufacturing Standards are vital regulations implemented by the FDA to ensure the standard, safety, and effectiveness of pharmaceutical products. These practices encompass various aspects, including facility design, equipment maintenance, and employee training. The cgmp certification is of substantial importance; it not only guarantees adherence to regulatory standards but also enhances a company's reputation and reduces the risk of product recalls.
Statistics indicate that almost 30% of FDA recalls arise from management problems, highlighting the critical need for cgmp certification. By implementing these practices, organizations can consistently produce high-quality products, thereby protecting patient safety and enhancing operational efficiency.
Practical instances reveal that firms dedicated to good manufacturing practices and achieving cgmp certification encounter fewer recalls and improved product quality, highlighting the importance of accreditation in today's competitive environment.
As of 2025, updates to FDA regulations continue to emphasize the significance of current good manufacturing practices, highlighting the necessity of cgmp certification for organizations to remain informed and compliant to mitigate risks linked to product recalls.
Follow the Steps to Achieve cGMP Certification
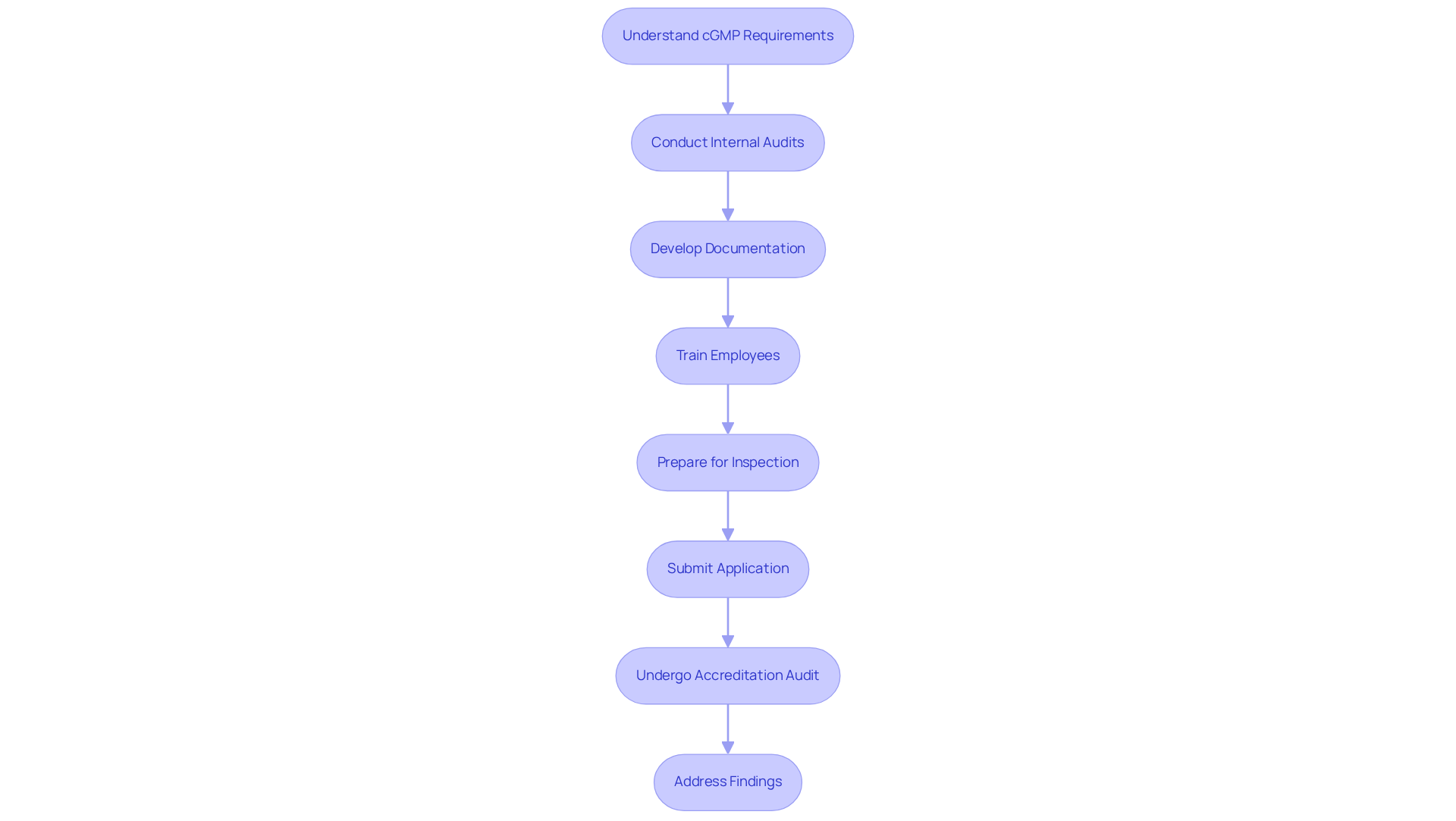
To achieve cGMP certification, it is imperative to follow these essential steps:
- Understand cGMP certification requirements: Familiarize yourself with the specific regulations pertinent to your industry. Review FDA guidelines and industry standards to ensure comprehensive knowledge.
- Conduct Internal Audits: Carry out a comprehensive internal audit to assess your current adherence status. This process is essential, as effective internal audits can greatly improve adherence rates and identify critical gaps.
- Develop Documentation: Create essential documentation, including a Quality Manual, Standard Operating Procedures (SOPs), and Batch Manufacturing Records. Comprehensive documentation is a fundamental element of good manufacturing practices, as it guarantees that all procedures are clearly outlined and adhered to.
- Train Employees: Establish training initiatives to guarantee all staff comprehend good manufacturing practices requirements and their responsibilities in upholding standards. Continuous education fosters a culture of quality and accountability within the organization.
- Prepare for Inspection: Ensure your facility is ready for inspection by the certifying body. This includes maintaining equipment, cleanliness, and proper documentation, which are critical factors in passing inspections.
- Submit your application for cGMP certification: Once you are assured of your adherence, send your application for good manufacturing practices approval to the appropriate authority. Ensure that all required documentation is complete and accurate to avoid delays.
- Undergo Accreditation Audit: Prepare for the accreditation audit, where an external auditor will assess your adherence to cGMP standards. This audit is a crucial step in the approval process, as it evaluates your compliance with established regulations.
- Address Findings: If any non-compliance issues are identified during the audit, resolve them swiftly to obtain approval. Prompt resolution of findings shows a dedication to quality and regulatory standards.
By adhering to these steps, organizations can effectively manage the intricacies of the good manufacturing practices approval process and enhance their operational standards. Furthermore, leveraging the expertise of AVS Life Sciences can provide tailored consulting solutions that simplify regulatory processes.
Overcome Challenges in the cGMP Certification Process
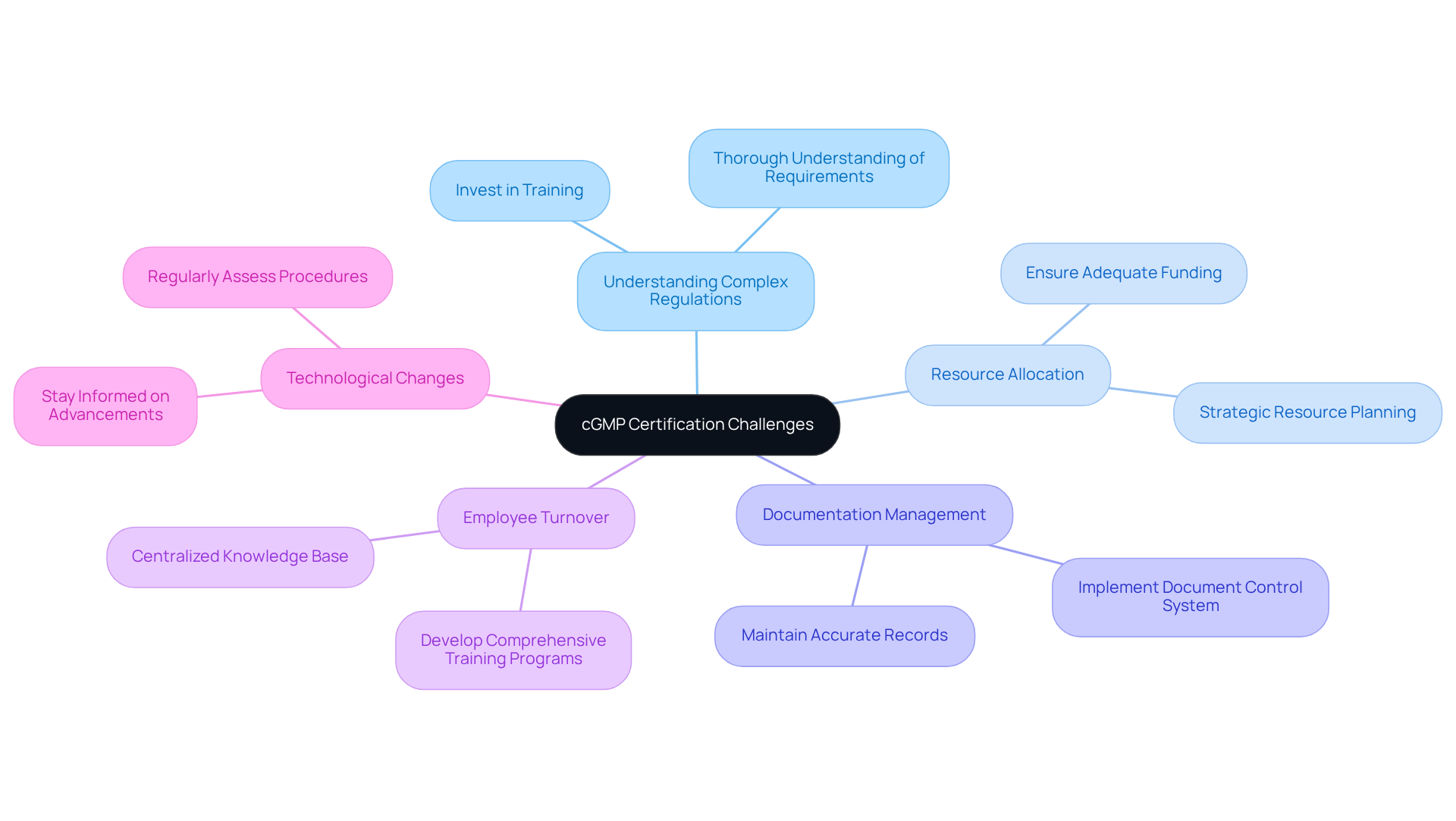
Achieving cGMP certification involves navigating several significant challenges that require careful consideration and strategic planning:
-
Understanding Complex Regulations: The regulatory landscape is often intricate, necessitating a thorough understanding of specific requirements. Investing in training and resources is vital to guarantee adherence to both national and international standards.
-
Resource Allocation: Sufficient resources, including skilled personnel and financial backing, are crucial for implementing necessary changes and sustaining compliance. Statistics reveal that 68% of risk management employers believe their regulatory departments are inadequately funded, highlighting the importance of strategic resource planning.
-
Documentation Management: Accurate and current documentation is essential for compliance. Implementing a robust document control system can streamline record management, ensuring that all documentation meets regulatory standards and is readily accessible during audits.
-
Employee Turnover: Elevated turnover rates can result in a loss of organizational knowledge, detrimental to regulatory efforts. Developing comprehensive training programs and maintaining a centralized knowledge base can mitigate this risk, ensuring that employees are well-versed in cgmp certification principles and practices.
-
Technological Changes: Staying informed about technological advancements is crucial, as these can significantly impact compliance efforts. Regularly assessing and adjusting procedures in response to emerging technologies can enhance operational efficiency and ensure continuous adherence to evolving regulations.
Utilize Resources and Tools for Successful Certification
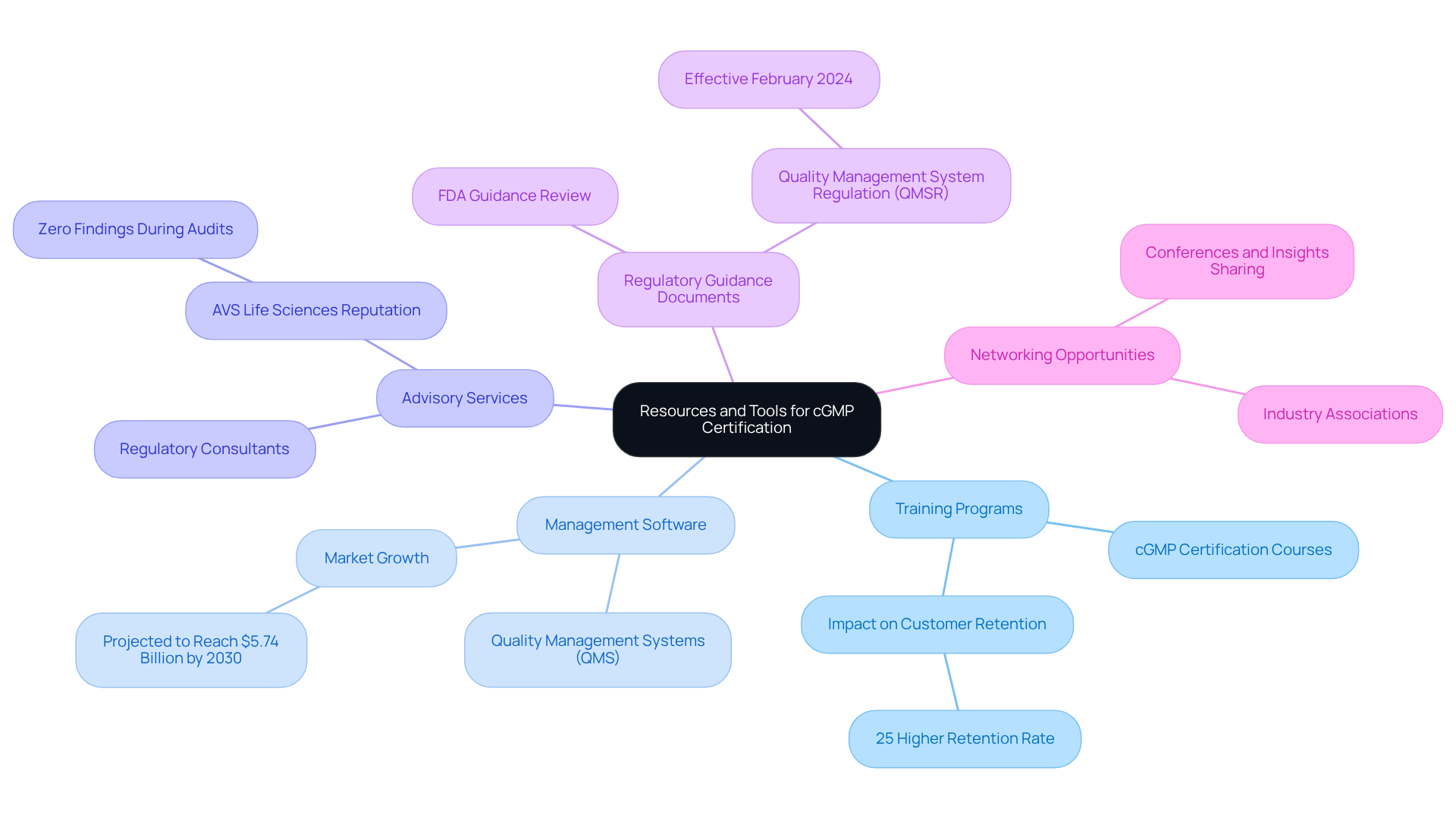
To support your journey towards cGMP certification, consider utilizing the following resources and tools:
-
Training Programs: Enroll in cGMP certification courses provided by respected organizations to enhance your team's awareness of regulatory requirements. These programs are crucial; organizations with efficient management systems report a 25% higher customer retention rate.
-
Management Software: Implement quality management systems (QMS) to streamline documentation, training, and adherence tracking. The worldwide pharmaceutical quality management software market is projected to expand significantly, reaching USD 5.74 billion by 2030, indicating a robust trend towards embracing these systems for improved regulatory adherence.
-
Advisory Services: Collaborate with regulatory consultants specializing in cGMP certification to receive customized assistance and support during the accreditation process. AVS Life Sciences, for example, has built a strong reputation for delivering comprehensive consulting services that align with client expectations, contributing to on-time project delivery and zero findings during audits.
-
Regulatory Guidance Documents: Regularly review FDA guidance documents and industry publications to stay informed about updates and best practices in good manufacturing practices. The recent introduction of the Quality Management System Regulation (QMSR) highlights the necessity for continuous education in this area, and AVS Life Sciences can assist in navigating these regulatory changes.
-
Networking Opportunities: Join industry associations and attend conferences to connect with peers and share insights on overcoming common challenges in achieving cGMP certification. Engaging with the community can provide valuable perspectives and strategies that enhance your compliance efforts.
Conclusion
Achieving cGMP certification is a critical endeavor for organizations in the pharmaceutical industry, ensuring compliance with essential regulations that safeguard product quality and patient safety. This certification not only enhances a company's reputation but also significantly reduces the likelihood of costly product recalls, thereby emphasizing the necessity of adhering to good manufacturing practices.
The article outlines a comprehensive approach to obtaining cGMP certification, detailing crucial steps such as:
- Understanding regulatory requirements
- Conducting internal audits
- Developing thorough documentation
- Training employees
It also addresses common challenges such as:
- Resource allocation
- Documentation management
- The impact of employee turnover
Providing insights into strategies to navigate these obstacles effectively, organizations can streamline their certification process and enhance compliance by leveraging available resources, including training programs and advisory services.
Ultimately, the journey to cGMP certification transcends mere regulatory compliance; it fosters a culture of quality and continuous improvement within the organization. Embracing these principles positions companies for success in a competitive landscape and reinforces their commitment to safety and efficacy in pharmaceutical manufacturing. Taking proactive steps today can pave the way for a more compliant and efficient future, ensuring that patient safety remains at the forefront of industry practices.
Frequently Asked Questions
What is cGMP certification?
cGMP certification stands for current Good Manufacturing Practices certification, which ensures that pharmaceutical products are manufactured according to strict regulatory standards set by the FDA. It involves adherence to guidelines related to facility design, equipment maintenance, and employee training.
Why is cGMP certification important?
cGMP certification is important because it guarantees compliance with regulatory standards, enhances a company's reputation, reduces the risk of product recalls, and ensures the consistent production of high-quality products, thereby protecting patient safety and improving operational efficiency.
What percentage of FDA recalls are attributed to management problems?
Statistics indicate that almost 30% of FDA recalls arise from management problems, highlighting the critical need for cGMP certification.
How does cGMP certification impact product quality and recalls?
Firms that are dedicated to good manufacturing practices and achieve cGMP certification tend to experience fewer product recalls and improved product quality, demonstrating the significance of this accreditation in the competitive pharmaceutical environment.
Are there any updates to FDA regulations regarding cGMP certification?
Yes, as of 2025, updates to FDA regulations continue to emphasize the importance of current Good Manufacturing Practices, making cGMP certification necessary for organizations to stay informed and compliant, thereby mitigating risks associated with product recalls.
