A Guide to FDA Facility Registration Under MoCRA: Key Steps for Compliance

Overview
This article serves as a comprehensive guide to FDA facility registration under the Modernization of Cosmetics Regulation Act (MoCRA). It outlines critical steps for compliance, including:
- Determining eligibility
- Obtaining an FEI number
- Submitting enrollment through the FDA's Cosmetics Direct portal
The significance of adhering to these regulations cannot be overstated; doing so helps avoid penalties, enhances consumer trust, and maintains safety standards. Notably, there has been a significant increase in facility registrations since the act's implementation, underscoring the urgency for compliance.
Introduction
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a pivotal transformation in the regulation of cosmetic products within the United States, affecting not only manufacturers but also the safety of consumers. The new mandates require all cosmetic facilities to register with the FDA, making it imperative for stakeholders to grasp the complexities of this process to ensure compliance and avert penalties. As the deadline for compliance draws near, many facilities are likely to encounter challenges related to:
- Registration
- Product listing
- Adherence to good manufacturing practices
How can these businesses effectively navigate the evolving landscape of cosmetic regulation while ensuring they meet the requisite standards?
Understand MoCRA and Its Impact on FDA Facility Registration
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a pivotal transformation in the regulatory framework governing cosmetic products in the United States. This act, aimed at enhancing consumer safety, mandates that all cosmetic manufacturing facilities enroll with the FDA, establishing this process as a legal requirement rather than a mere formality. Such a requirement empowers the FDA to effectively monitor compliance with established safety standards. Understanding the provisions of MoCRA, as detailed in a , is essential for any facility involved in the production or processing of cosmetics; non-compliance can lead to substantial penalties, including the suspension of certification.
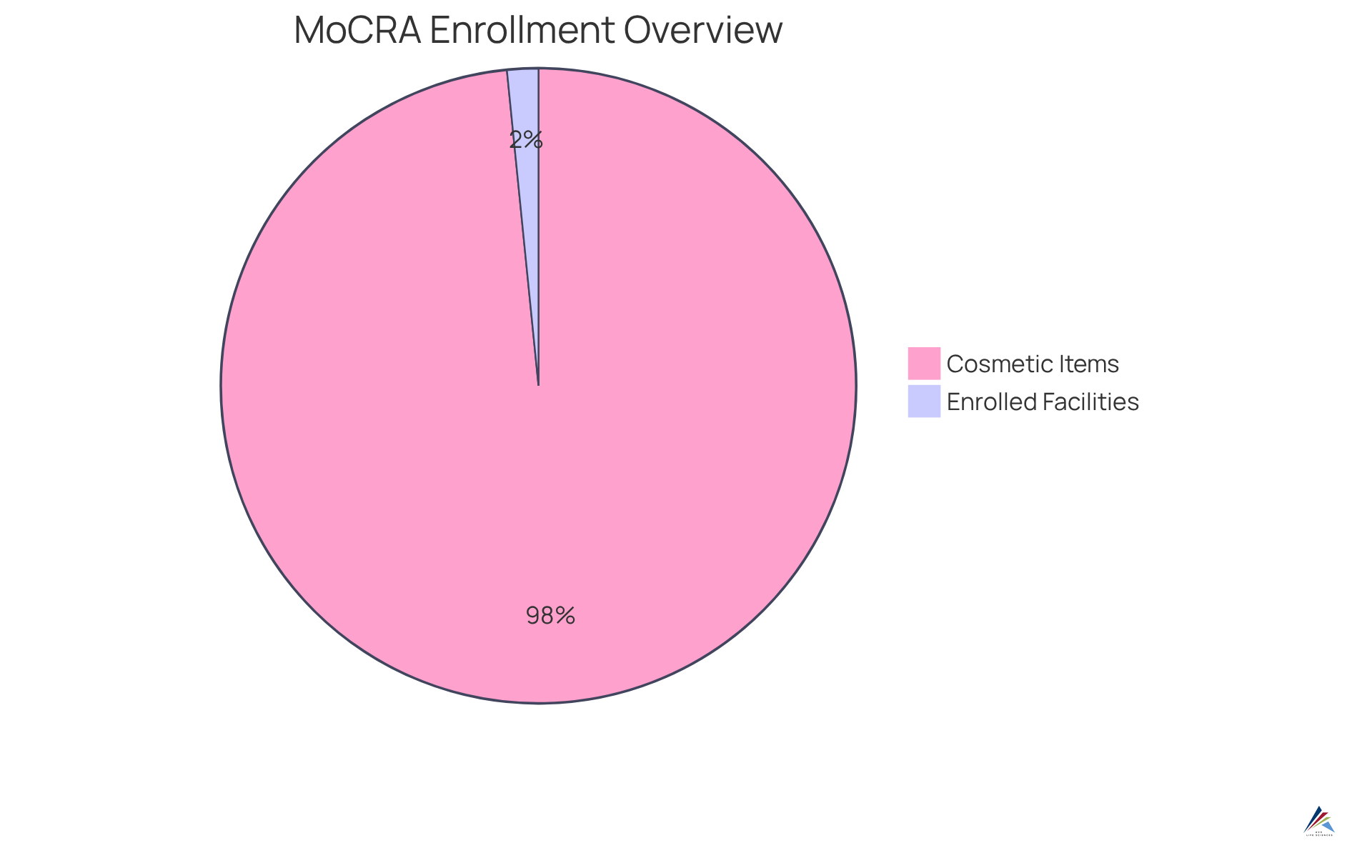
Key elements of MoCRA include the definitions of responsible individuals and the variety of items covered by this regulation. As of January 1, 2025, the FDA announced that 9,528 cosmetic manufacturing facilities have completed the enrollment process, and 589,762 cosmetic items are now included under the MoCRA framework. This significant rise in enrollments underscores the urgency for facilities to consult a guide to FDA facility registration under MoCRA and ensure compliance to avoid potential consequences.
In light of these developments, it is imperative for compliance officers to engage with the specifics of MoCRA and consider the implications for their operations. By prioritizing adherence to these regulations, facilities not only safeguard their certification but also enhance consumer trust in their products.
Complete Mandatory Facility Registration Requirements
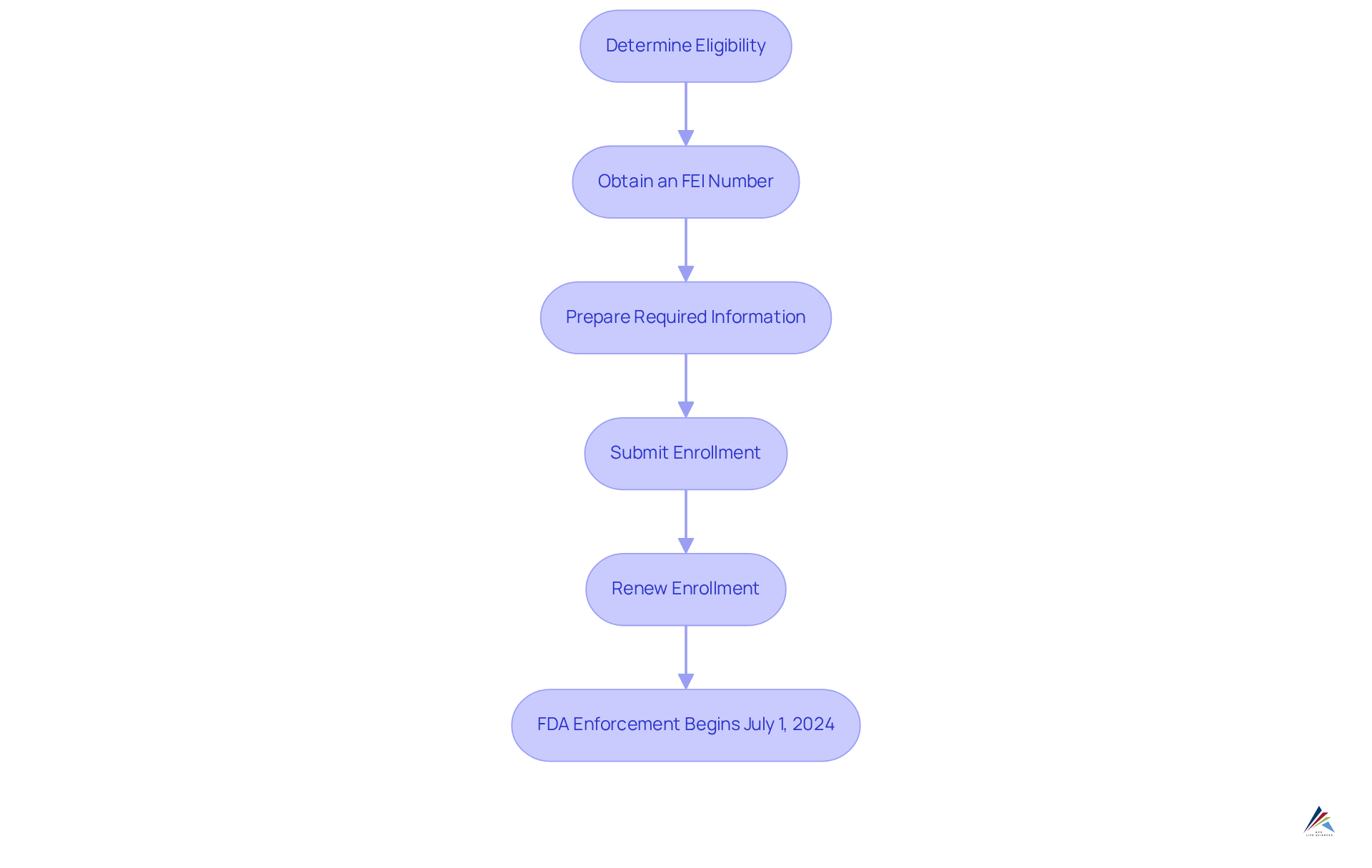
To register your facility with the FDA under the Modernization of Cosmetics Regulation Act (MoCRA), it is crucial to follow these essential steps:
- Determine Eligibility: Confirm that your facility qualifies as a cosmetic manufacturing or processing establishment, which encompasses any site that produces, repacks, or relabels cosmetic products.
- Obtain an FEI Number: Acquire a Facility Establishment Identifier (FEI) number, a unique identifier that is essential for the enrollment process.
- Prepare Required Information: Collect necessary details such as the facility's name, address, contact information, and the name of the owner or operator.
- Submit Enrollment: Utilize the FDA's Cosmetics Direct portal to electronically submit your enrollment. Ensure all information is accurate to prevent delays in processing.
- Renew Enrollment: Keep in mind that facility enrollment must be renewed every two years. Track your enrollment date to ensure continuous adherence.
Historically, prior to MoCRA, the Voluntary Cosmetic Registration Program (VCRP) recorded only 5,176 active establishment listings. This underscores the substantial increase in adherence under the new regulations. As of January 1, 2025, there are 9,528 active facility listings under MoCRA, reflecting a significant commitment to regulatory compliance within the cosmetic industry. ChemLinked recommends that all firms selling cosmetics in the U.S. utilize a guide to FDA facility registration under MoCRA to conduct a thorough regulatory review and meet current MoCRA requirements. Successful enrollments illustrate the effectiveness of these procedures, with numerous regulatory officers emphasizing the importance of careful preparation and adherence to guidelines to prevent potential setbacks. As noted by a compliance officer, "Thorough preparation is key to navigating the complexities of the application process effectively." Furthermore, it is essential to highlight that the FDA's enforcement for facility registration and item listing commences on July 1, 2024, underscoring the urgency of the registration process.
Execute Product Listing Procedures for Compliance
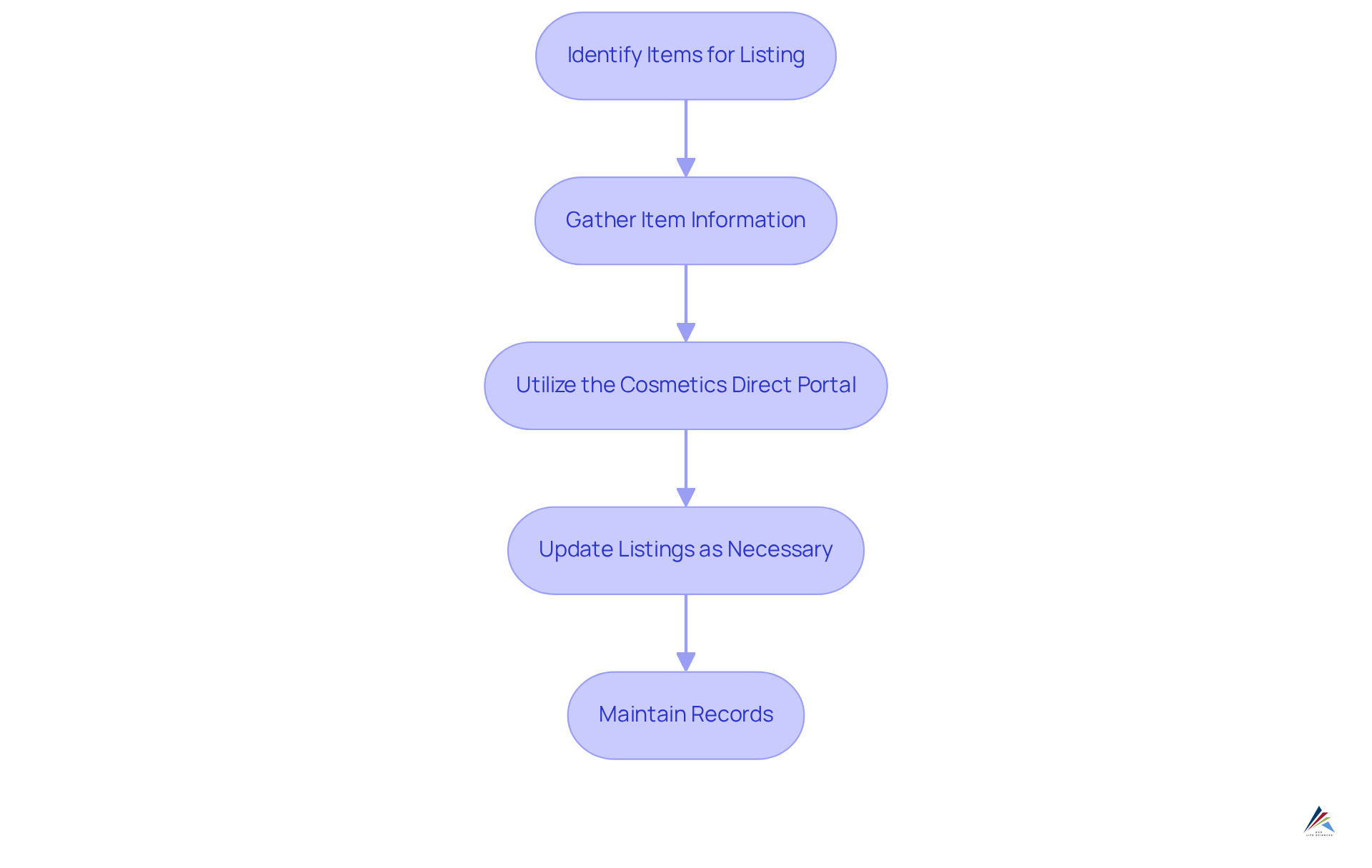
Once your facility is registered, the next step is to list your cosmetic items with the FDA. Follow these procedures to ensure compliance:
- Identify Items for Listing: Determine which cosmetic items you manufacture or process that require listing under MoCRA. As of July 2, 2025, there are more than 784,000 unique active item listings, emphasizing the significance of precise enrollment.
- Gather Item Information: Collect essential details for each item, including the name, ingredients, and the facility registration number (FEI). This information is crucial for maintaining compliance and facilitating audits.
- Utilize the : Access the FDA's Cosmetics Direct portal to submit your listing. Ensure that all information is complete and accurate, as the FDA does not issue certificates for these registrations, and incomplete submissions may lead to delays. The FDA encourages electronic submissions for efficiency and timeliness.
- Update Listings as Necessary: If there are alterations to your items, such as formulation or labeling, update your listings promptly to reflect these modifications. This proactive strategy aids in preventing regulatory problems and guarantees that your information stays up to date.
- Maintain Records: Keep thorough records of all item listings and any updates made, as these may be required for future audits or inspections. Creating a strong record-keeping system is crucial for showing adherence to MoCRA regulations.
Following these steps serves as a guide to FDA facility registration under MoCRA, helping you navigate the complexities of FDA product listings effectively, ensuring that your cosmetic products meet regulatory standards and contribute to consumer safety. Remember that the deadline for adherence was July 1, 2024, so prompt action is essential.
Implement Good Manufacturing Practices (GMP) for Compliance
Implementing Good Manufacturing Practices (GMP) in your facility is essential, as outlined in a guide to FDA facility registration under MoCRA. Here’s a structured approach:
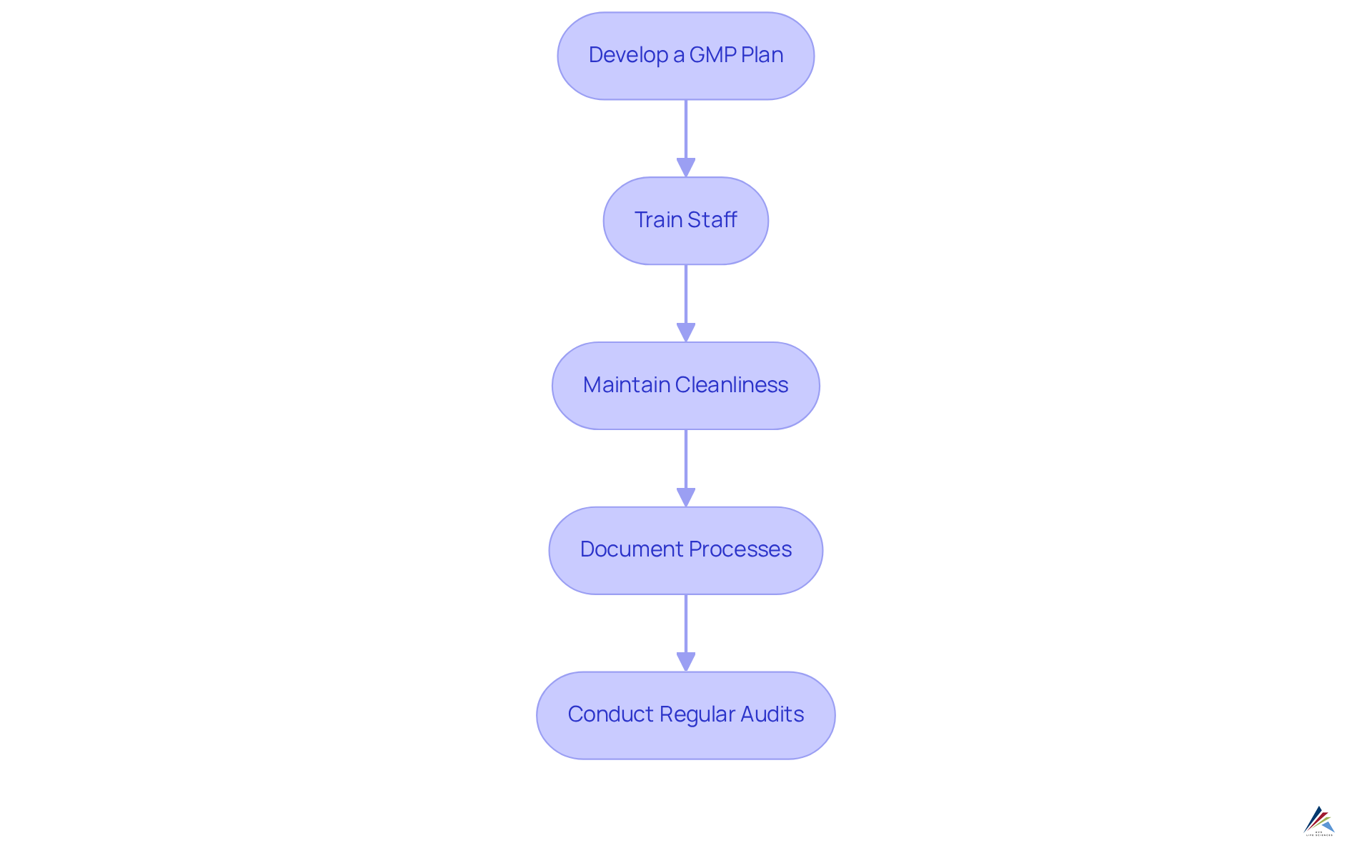
- Develop a GMP Plan: Formulate a comprehensive GMP plan that details procedures for every aspect of production, from raw material sourcing to processing and packaging. This plan should align with international standards, such as , to ensure consistent quality and adherence to FDA regulations.
- Train Staff: Provide thorough training for all employees on GMP principles, emphasizing their roles in upholding standards. Regular training sessions can enhance awareness and adherence to safety protocols, which is crucial for effective GMP implementation. AVS Life Sciences offers tailored consulting solutions to ensure your team is well-versed in these practices.
- Maintain Cleanliness: Implement stringent sanitation protocols to prevent contamination during production. Key GMP focus areas include cleanliness, equipment maintenance, ingredient quality, and staff training. Routine cleaning and sanitizing of equipment and production areas are essential to maintain safety and quality.
- Document Processes: Maintain meticulous records of all manufacturing processes, including batch records and quality control checks. This documentation is essential for demonstrating adherence during inspections and can significantly diminish the chance of recalls. Great documentation practices and adherence to Standard Operating Procedures (SOPs) are critical components of a successful GMP strategy and serve as a guide to FDA facility registration under MOCRA, particularly in addressing data integrity deviations.
- Conduct Regular Audits: Arrange internal assessments to evaluate adherence to GMP and pinpoint areas for enhancement. Addressing non-compliance issues promptly not only enhances operational efficiency but also builds consumer trust. As highlighted by industry specialists, regular audits and compliance with GMP regulations are crucial to prevent recalls. Engaging with AVS Life Sciences can provide you with expert solutions in GMP compliance, validation, and engineering, which include a guide to FDA facility registration under MOCRA tailored to your facility's needs.
By following these steps, cosmetic manufacturers can ensure safety and quality, ultimately fostering brand loyalty and consumer trust. For more information on how to get started with AVS Life Sciences, please refer to our user manuals.
Report Adverse Events Effectively
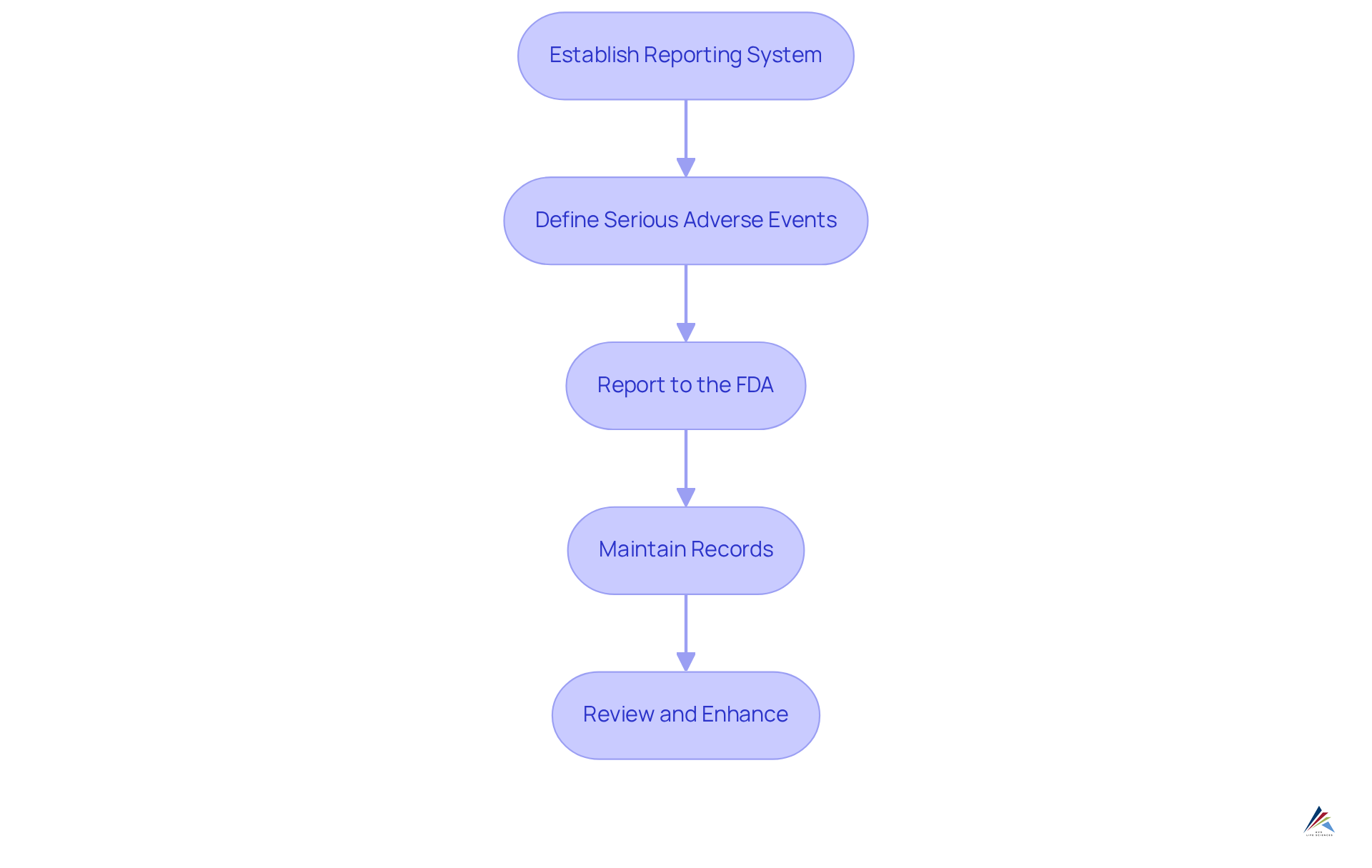
Under the Modernization of Cosmetics Regulation Act (MoCRA), implementing a comprehensive system for reporting serious adverse events linked to cosmetic products is essential. Adverse events can significantly impact consumer safety, as a study involving 400 respondents revealed a 33% prevalence of cosmetic-induced adverse events among users. To ensure compliance, consider the following steps:
- Establish a Reporting System: Create a structured process to receive and document reports of adverse events from consumers or healthcare professionals. This system should facilitate timely communication and data collection.
- Define Serious Adverse Events: Clearly understand what qualifies as a serious adverse event, including outcomes such as hospitalization, significant disability, or life-threatening experiences. This definition is crucial for precise reporting and adherence.
- Report to the FDA: Serious adverse events must be reported to the FDA within 15 business days of receipt. Utilize the FDA's MedWatch system for efficient submissions, ensuring that all necessary information is included. The FDA has revised the MedWatch Form 3500A to streamline the reporting procedure, which is vital for regulatory officers.
- Maintain Records: Keep meticulous records of all adverse event reports and your responses for a minimum of six years. These records are essential for audits and demonstrate compliance with regulatory requirements.
- Review and Enhance: Regularly assess adverse event reports to identify trends and areas for enhancement in safety and quality. This proactive approach can help mitigate risks and enhance consumer trust.
The FDA's recent updates serve as a guide to FDA facility registration under MoCRA, emphasizing the importance of these reporting requirements as the agency prepares to enforce its mandates starting December 29, 2023. By establishing a robust reporting system, companies can not only comply with regulations but also contribute to the overall safety and efficacy of cosmetic products in the market.
Conclusion
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a pivotal transformation in the regulatory framework governing cosmetic products in the U.S. By mandating that all cosmetic manufacturing facilities register with the FDA, this act not only bolsters consumer safety but also lays down a robust framework for the FDA to effectively monitor compliance. For any facility engaged in cosmetic production, understanding and adhering to MoCRA is imperative; non-compliance can result in severe penalties, including the suspension of certification.
Key steps outlined in this article underscore the critical nature of proper facility registration, product listing, and adherence to Good Manufacturing Practices (GMP). Facilities are required to:
- Ascertain their eligibility
- Obtain a Facility Establishment Identifier (FEI) number
- Submit precise enrollment information via the FDA's Cosmetics Direct portal
Moreover, maintaining comprehensive documentation and establishing a rigorous system for reporting adverse events are essential components of compliance. With deadlines looming, it is crucial for cosmetic manufacturers to act swiftly to avert regulatory setbacks.
Ultimately, the ramifications of MoCRA extend well beyond mere compliance; they cultivate consumer trust and safety in cosmetic products. By prioritizing adherence to these regulations, facilities not only safeguard their operations but also contribute to a more secure marketplace. Engaging with the guidelines and seeking expert solutions can significantly enhance compliance efforts, ensuring that manufacturers are adequately prepared for the evolving regulatory landscape.
Frequently Asked Questions
What is the Modernization of Cosmetics Regulation Act (MoCRA)?
MoCRA is a significant change in the regulatory framework for cosmetic products in the United States, aimed at enhancing consumer safety by requiring all cosmetic manufacturing facilities to register with the FDA.
What are the consequences of non-compliance with MoCRA?
Non-compliance with MoCRA can lead to substantial penalties, including the suspension of certification for cosmetic manufacturing facilities.
What key information must facilities know about MoCRA?
Facilities must understand the definitions of responsible individuals and the variety of items covered by MoCRA, as well as the importance of enrolling with the FDA to ensure compliance.
How many cosmetic manufacturing facilities have registered under MoCRA as of January 1, 2025?
As of January 1, 2025, 9,528 cosmetic manufacturing facilities have completed the enrollment process under MoCRA.
What steps are necessary for facility registration with the FDA under MoCRA?
The steps include determining eligibility, obtaining a Facility Establishment Identifier (FEI) number, preparing required information, submitting enrollment via the FDA's Cosmetics Direct portal, and renewing enrollment every two years.
What is an FEI number and why is it important?
An FEI number is a unique identifier required for the enrollment process of cosmetic facilities with the FDA, essential for tracking and compliance purposes.
How often must facility enrollment be renewed under MoCRA?
Facility enrollment must be renewed every two years to maintain compliance with MoCRA regulations.
When does the FDA's enforcement for facility registration and item listing begin?
The FDA's enforcement for facility registration and item listing under MoCRA begins on July 1, 2024.
How does the number of active facility listings under MoCRA compare to previous regulations?
Prior to MoCRA, the Voluntary Cosmetic Registration Program (VCRP) had only 5,176 active establishment listings, while under MoCRA, there are now 9,528 active facility listings, indicating a substantial increase in regulatory compliance.
What should compliance officers prioritize regarding MoCRA?
Compliance officers should prioritize understanding the specifics of MoCRA and ensuring adherence to its regulations to safeguard certification and enhance consumer trust in their products.
