9 Key Principles of ALCOA Data Integrity for Compliance Officers

Overview
This article delineates the essential principles of ALCOA data integrity, which are crucial for compliance officers operating within the life sciences sector. It underscores that adherence to the principles of:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
- Complete
- Enduring
- Available
is imperative for effective data management and regulatory compliance. By doing so, it significantly enhances the reliability and integrity of the information utilized in audits and decision-making processes. Compliance challenges in this sector are multifaceted, yet understanding and implementing these principles provides a robust framework for overcoming them. Engaging with these guidelines not only fosters compliance but also cultivates a culture of data integrity that is vital for organizational success. As such, it is essential for compliance officers to prioritize these principles, ensuring that the information they manage is not only compliant but also trustworthy and actionable.
Introduction
The integrity of data stands as a cornerstone in the life sciences sector, where compliance and regulatory standards dictate the success of operations. Understanding and implementing the nine key principles of ALCOA—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Enduring, and Available—can significantly enhance data management practices for compliance officers. Yet, the challenge lies in navigating the complexities of these principles amidst evolving regulations and the relentless pace of technological advancements.
How can organizations ensure that their data integrity practices not only meet current standards but also prepare them for future compliance demands? This inquiry is essential for fostering robust compliance solutions that adapt to the dynamic landscape of life sciences.
AVS Life Sciences: Comprehensive Solutions for ALCOA Data Integrity
AVS Life Sciences delivers a comprehensive suite of services vital for ensuring ALCOA data integrity across diverse sectors, including biopharmaceuticals, medical devices, and nutraceuticals. Their profound industry expertise, coupled with a strong focus on quality control and regulatory compliance, empowers organizations to effectively implement the principles of ALCOA data integrity within their information management frameworks. By leveraging their extensive knowledge in GxP regulatory services and validation solutions, compliance professionals are equipped to navigate the complexities of information integrity, ensuring that every aspect of information handling meets stringent regulatory standards. This alignment not only fosters trust but also drives operational excellence in a landscape where compliance is paramount.
Attributable: Ensuring Traceability in Data Management
Attributability is crucial in ensuring that every element of information can be traced back to its source, whether it be an individual or a system. Compliance officers must implement robust documentation practices that distinctly identify who generated, modified, or reviewed information. This traceability not only facilitates adherence to regulations but also enhances the reliability of the information during audits.
In the context of Computer System Validation (CSV), it is imperative to establish clear user requirement specifications (URS) and maintain comprehensive documentation throughout the validation stages, including:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
These practices ensure that all information generated during the validation process is attributable, thereby reinforcing the integrity and reliability of the systems in place, in line with ALCOA data integrity principles. By adhering to these thorough guidelines, regulatory personnel can ensure that their organizations uphold legal standards while sustaining high-quality data management practices.
Legible: Maintaining Clarity and Readability in Documentation
Legibility is paramount in the life sciences field, particularly for regulatory personnel, as it pertains to the clarity and readability of documentation. Ensuring that all records are articulated clearly and concisely, utilizing standard terminology and formats, not only facilitates understanding but also mitigates the risk of misinterpretation during audits or inspections. This is especially critical in the context of Computer System Validation (CSV), where documentation must accurately represent the multi-step process that encompasses:
- Planning
- Defining user requirements
- Design specifications
- Various qualification testing stages (IQ, OQ, PQ)
Each of these stages demands meticulous documentation to demonstrate compliance with FDA regulations and GXP standards. By upholding high legibility in documentation, compliance personnel can guarantee that validation results are readily interpretable, thereby bolstering quality assurance and regulatory compliance efforts. This practice is essential for effective communication during internal and external audits, ultimately enhancing the integrity and reliability of the systems in place.
Contemporaneous: Recording Data in Real-Time for Accuracy
Contemporaneous documentation is essential for capturing information as it is produced, a practice that holds critical importance in the life sciences sector, particularly during computer system validation (CSV) processes. Compliance officers must implement strategies that encourage real-time information entry, thereby minimizing the risk of errors or omissions. This principle becomes especially vital in environments where alcoa data integrity is crucial, such as clinical trials or manufacturing processes.
The CSV process encompasses several key stages:
- Planning
- Defining user requirement specifications (URS)
- Design specifications
- Building and configuring systems
- Various qualification testing phases, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)
Each stage underscores the necessity for accurate and timely documentation to ensure compliance with regulatory standards. By adhering to these practices, regulatory staff can uphold the integrity of information and ensure that alcoa data integrity is preserved, ultimately enhancing quality assurance within their organizations.
Original: Utilizing Original Data Sources for Authenticity
The principle of originality emphasizes the necessity of utilizing primary information sources rather than secondary or derived ones. Compliance officers must ensure that all information is sourced from original materials to maintain ALCOA data integrity, as this not only enhances the authenticity of the content but also supports adherence to regulatory requirements.
By collaborating with AVS Life Sciences, organizations can access customized consulting solutions that reinforce these principles. This collaboration ensures compliance with GXP and FDA regulations while promoting best practices in documentation and ALCOA data integrity. Engaging with AVS Life Sciences is a strategic step towards strengthening your compliance framework.
Accurate: Ensuring Data Reflects True and Precise Information
Precision in information management is paramount; it ensures that all documented details accurately reflect genuine values. Compliance officers must implement rigorous validation checks and conduct regular audits to guarantee that information remains accurate throughout its lifecycle. This principle is not merely a procedural necessity; it is essential for maintaining trust in the information utilized for regulatory submissions and informed internal decision-making. By prioritizing precision, organizations can enhance their credibility and foster a culture of compliance that resonates throughout their operations.
Complete: Capturing All Necessary Data for Compliance
Completeness is crucial in ensuring the gathering of all relevant information necessary for compliance. Compliance officers must establish robust protocols to guarantee that no essential information is overlooked during the data collection process. This principle holds particular weight in regulated environments, where the absence of critical information can lead to compliance failures. By prioritizing completeness, organizations can safeguard against potential risks and enhance their adherence to regulatory standards.
Enduring: Maintaining Data Integrity Over Time
Enduring alcoa data integrity signifies that records must be preserved in a manner that maintains their reliability over time. Compliance officers are tasked with implementing robust information retention policies and conducting regular reviews to ensure that records remain intact and accessible for audits and inspections. This principle is not merely a best practice; it is crucial for demonstrating compliance with regulatory requirements and maintaining alcoa data integrity.
Available: Ensuring Data Accessibility for Audits
Availability signifies the ease of access to information when required, particularly during audits. Compliance personnel must ensure that all pertinent data is organized in a manner that facilitates rapid retrieval. This principle is not merely a best practice; it is essential for demonstrating adherence to regulations and for enabling efficient audit processes.
Summary of ALCOA Principles: Essential for Compliance Officers
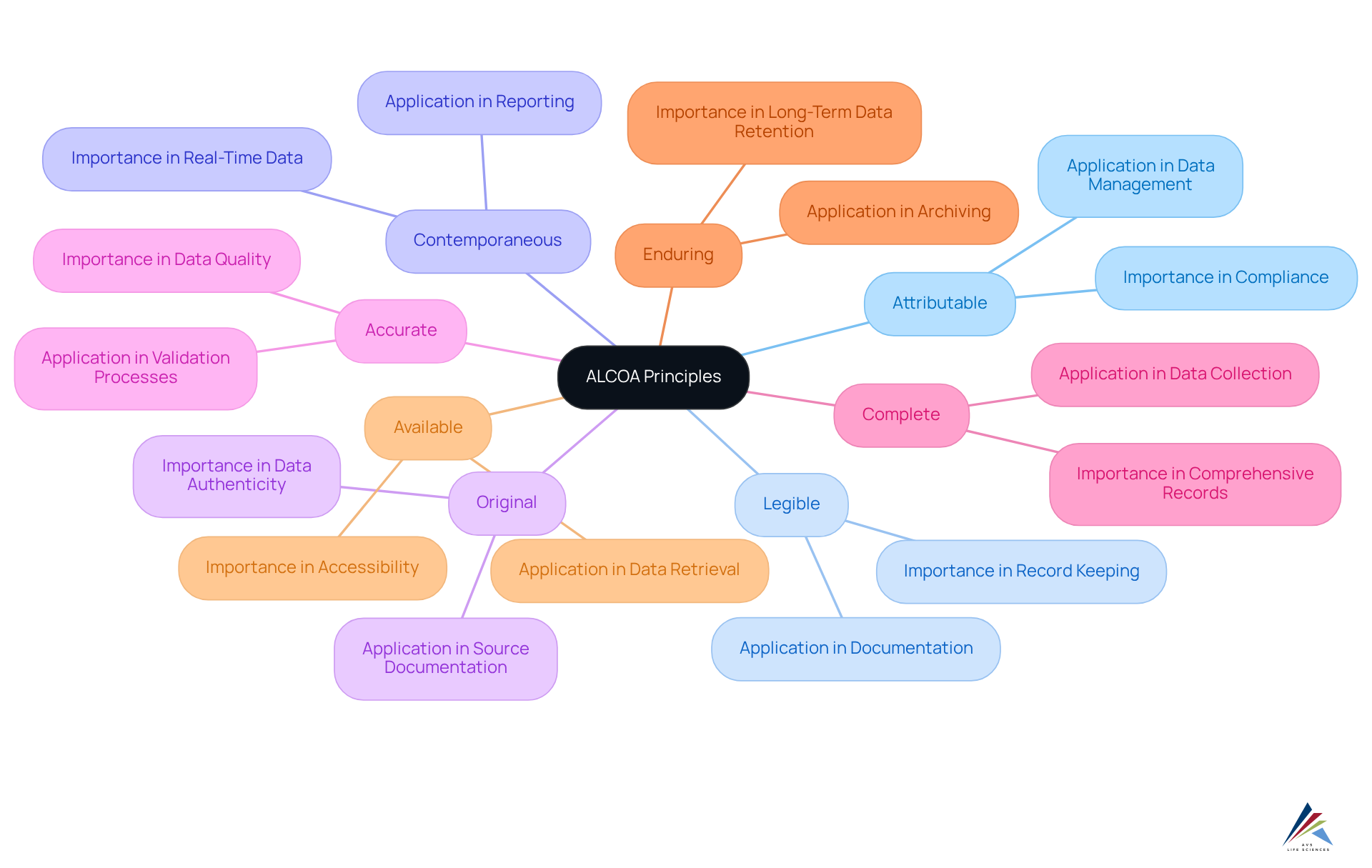
The principles of ALCOA data integrity—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Enduring, and Available—are essential for regulatory officers in the life sciences sector. Adhering to these principles enables organizations to uphold information integrity, particularly ALCOA data integrity, enhance compliance, and mitigate risks associated with oversight.
A compelling case study from AVS Life Sciences exemplifies this:
- The successful enhancement of a biotechnology GMP facility not only met compliance standards but also underscored the critical role of quality assurance in data management.
- This upgrade led to improved operational efficiency and a reduction in non-compliance incidents, thereby demonstrating AVS Life Sciences' unwavering commitment to assisting clients in navigating the complexities of regulatory environments.
Conclusion
The principles of ALCOA data integrity—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Enduring, and Available—serve as foundational pillars for compliance officers in the life sciences sector. Emphasizing these principles not only ensures adherence to stringent regulatory standards but also fosters a culture of quality and reliability within organizations. By effectively implementing these principles, compliance professionals can significantly enhance data integrity, thereby mitigating risks and improving overall operational efficiency.
Throughout the article, key insights have been shared regarding the importance of each ALCOA principle. From establishing traceability through robust documentation practices to ensuring real-time data recording for accuracy, compliance officers are equipped with essential strategies to uphold data integrity. The collaboration with AVS Life Sciences exemplifies how organizations can leverage expert solutions to reinforce their compliance frameworks, ultimately leading to improved outcomes in regulatory environments.
In conclusion, prioritizing ALCOA principles transcends mere compliance requirements; it cultivates trust and transparency in data management practices. As the landscape of regulatory oversight continues to evolve, embracing these principles empowers organizations to navigate complexities with confidence. A commitment to ALCOA data integrity is essential for achieving excellence and sustainability in the life sciences sector, urging compliance officers to take proactive steps in implementing these best practices.
Frequently Asked Questions
What services does AVS Life Sciences provide for ALCOA data integrity?
AVS Life Sciences offers a comprehensive suite of services aimed at ensuring ALCOA data integrity across sectors such as biopharmaceuticals, medical devices, and nutraceuticals, focusing on quality control and regulatory compliance.
Why is attributable data important in data management?
Attributable data is crucial because it ensures that every piece of information can be traced back to its source, enhancing reliability during audits and facilitating adherence to regulations.
What documentation practices should compliance officers implement for attributable data?
Compliance officers should implement robust documentation practices that clearly identify who generated, modified, or reviewed information, including maintaining comprehensive documentation throughout the Computer System Validation (CSV) stages such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
How does legibility impact documentation in the life sciences field?
Legibility is essential as it ensures clarity and readability of documentation, which helps mitigate misinterpretation during audits or inspections and supports compliance with regulatory standards.
What are the key stages that require meticulous documentation in Computer System Validation (CSV)?
The key stages that require thorough documentation in CSV include planning, defining user requirements, design specifications, and various qualification testing stages (IQ, OQ, PQ).
How does maintaining high legibility in documentation benefit compliance personnel?
Maintaining high legibility in documentation ensures that validation results are easily interpretable, bolstering quality assurance and regulatory compliance efforts, and enhancing communication during audits.
