9 Key Insights on PDUFA Meaning for Compliance Officers

Overview
The article underscores the critical importance of the Prescription Drug User Fee Act (PDUFA) for compliance officers within the pharmaceutical industry. It emphasizes the act's profound impact on regulatory processes and drug approval timelines. Understanding PDUFA is not merely advantageous; it is essential for compliance personnel to effectively navigate regulatory requirements, streamline submissions, and mitigate risks associated with drug approvals. This knowledge ultimately enhances operational efficiency and bolsters market competitiveness, positioning compliance officers as pivotal players in the industry.
Introduction
Understanding the intricacies of the Prescription Drug User Fee Act (PDUFA) is essential for compliance officers navigating the complex landscape of drug approval. This article provides key insights that illuminate the meaning of PDUFA, offering valuable strategies and best practices aimed at enhancing compliance and operational efficiency.
As regulatory requirements evolve, compliance professionals must adapt effectively to ensure their organizations meet these critical standards while minimizing the risk of costly delays.
How can they achieve this? By implementing proactive compliance solutions that align with PDUFA requirements, organizations can not only navigate challenges but also thrive in a dynamic regulatory environment.
AVS Life Sciences: Expert Guidance on PDUFA Compliance Strategies
excels in crafting tailored that help clients understand the , enabling them to skillfully navigate the complex drug approval landscape. By concentrating on biopharmaceuticals, , nutraceuticals, cosmetics, and food products, the team of seasoned experts provides critical insights into . This ensures that organizations align their submissions with , a necessity for regulatory officers focused on streamlining processes and minimizing the risk of .
Implementing best practices and successful compliance strategies empowers to enhance while maintaining a competitive edge in the market.
Engage with AVS Life Sciences today to transform your and secure your position in the industry.
Understanding PDUFA Dates: Key Milestones for Drug Approval
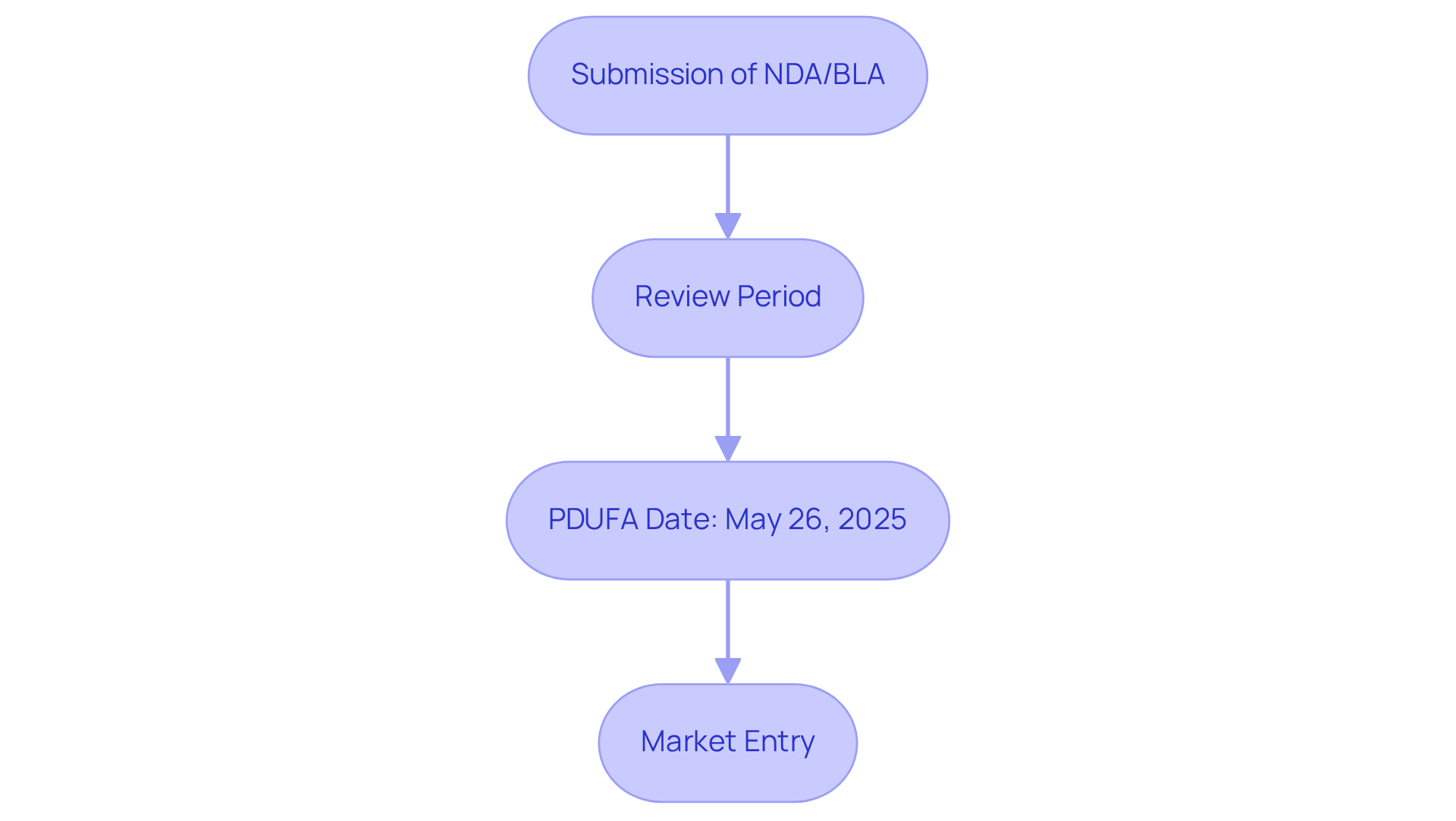
Key dates, which are essential for understanding , represent for the evaluation of (NDAs) and Biologics License Applications (BLAs). These dates function as pivotal milestones in the drug approval process, highlighting the PDUFA meaning and directly influencing the timeline for potential market entry.
For example, the PDUFA meaning regarding belzutifan, which is a treatment for advanced pheochromocytoma and paraganglioma, is that its date is set for May 26, 2025. If approved, belzutifan would become the first available therapy in the US for these rare neuroendocrine tumors, signifying a for both patients and stakeholders.
Compliance personnel play an essential role in , ensuring that all necessary documentation is submitted promptly. This proactive approach not only facilitates a smoother approval process but also aligns with the industry's best practices for .
AVS Life Sciences emphasizes , which can empower regulatory professionals to manage submission deadlines efficiently. To enhance their effectiveness, compliance professionals should consider leveraging tools that provide , ensuring they remain informed and prepared to navigate the complexities of drug development, thereby increasing their chances of achieving .
FDA's Role in PDUFA: Navigating Regulatory Requirements
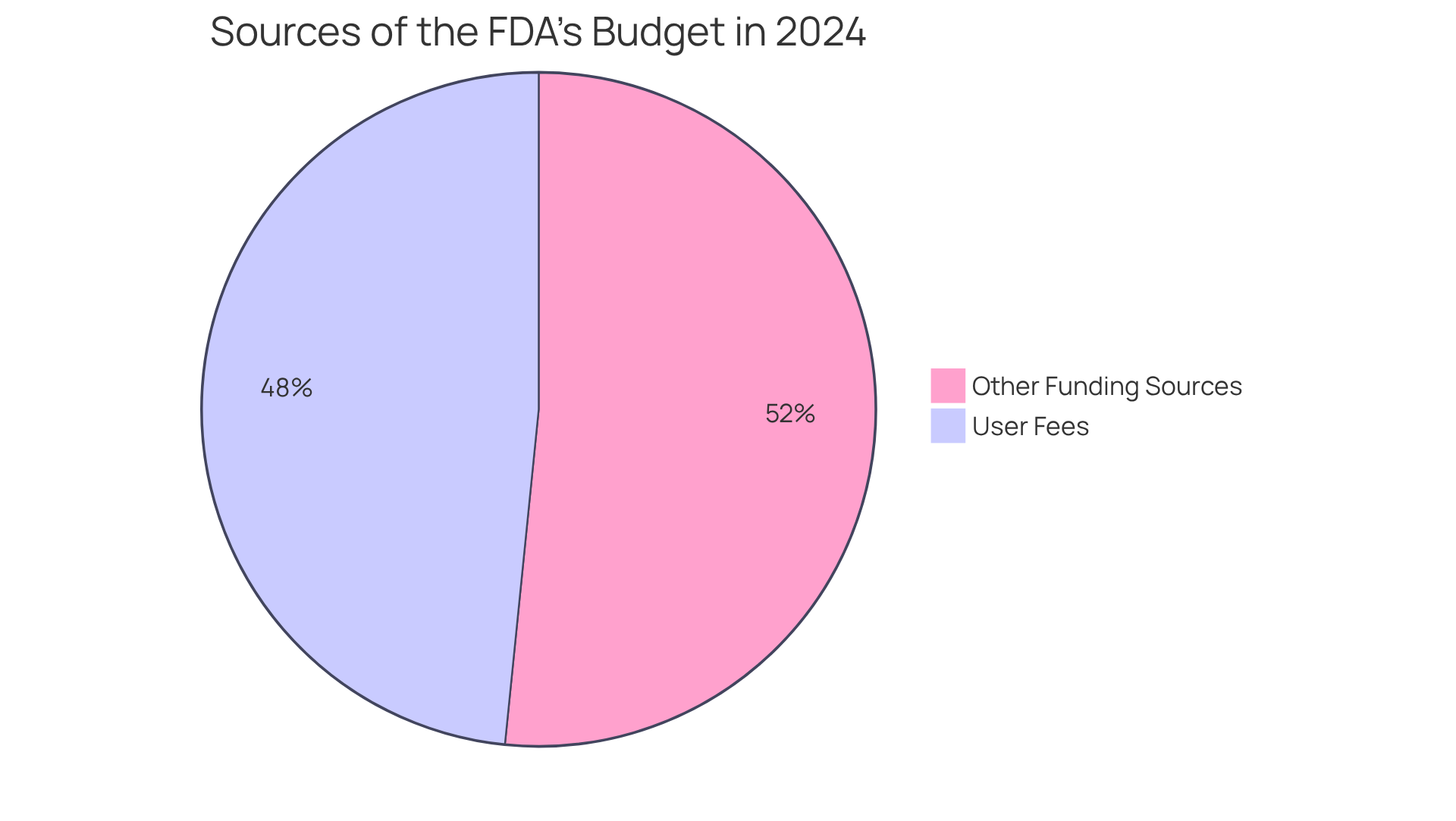
The FDA plays a pivotal role in the , collecting to strengthen the . In 2024, these user fees constituted nearly half of the FDA's total budget, amounting to approximately $3.328 billion. This funding significantly bolsters the agency's ability to recruit additional staff and . Notably, user fees account for two-thirds of the Human Drug Program budget, directly impacting the efficiency of drug evaluations.
Compliance officers must remain vigilant regarding the , as these changes can profoundly affect . For instance, the introduction of (REMS) under the fourth Prescription Drug User Fee Act illustrates the , as it has imposed additional to ensure adherence.
Grasping these dynamics is essential for upholding and facilitating timely product approvals.
Documentation Essentials: Ensuring Compliance in PDUFA Submissions
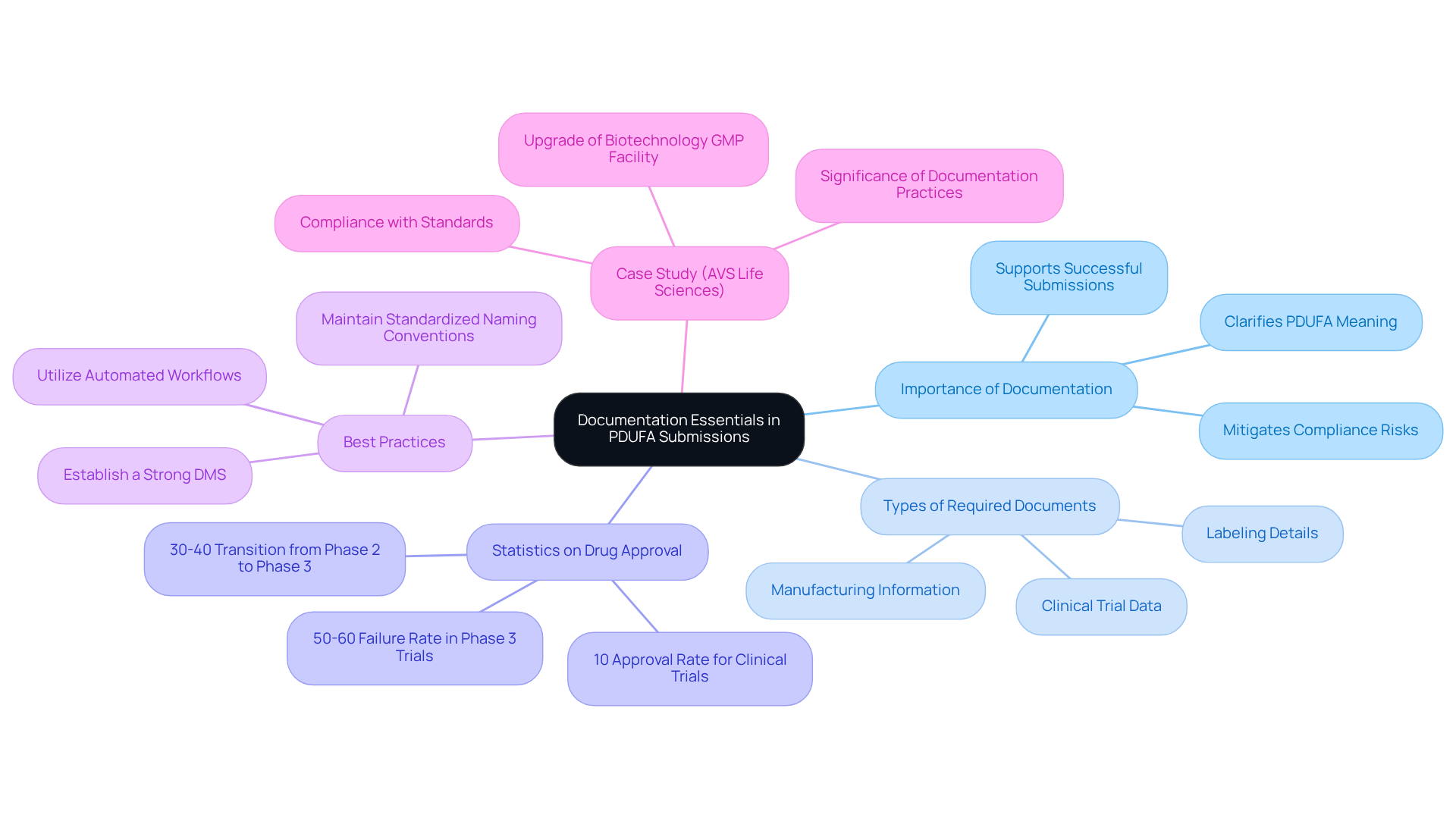
Effective documentation is fundamental to , as it helps clarify . Compliance officers must ensure that all required documents—ranging from to manufacturing information and labeling details—are complete, accurate, and submitted promptly. Statistics indicate that only about 10% of drugs entering clinical trials ultimately receive , highlighting the necessity of meticulous documentation to navigate this challenging landscape. Establishing a strong (DMS) can greatly improve , enabling smoother interactions with the FDA. Best practices include maintaining standardized naming conventions and comprehensive metadata to improve document searchability and retrieval. Successful organizations often utilize automated workflows to streamline the review and approval processes, ensuring that documentation is consistently up-to-date and readily accessible.
AVS Life Sciences exemplifies this approach through its successful upgrade of a biotechnology GMP facility, guiding a client from a Biosafety Level 1 to a Level 2 GMP standard. This transformation not only ensured compliance with standards but also highlighted the significance of throughout the process. Additionally, it is crucial to note that only 30%-40% of drugs transition from Phase 2 to Phase 3 trials, underscoring the critical nature of thorough documentation during this phase. By prioritizing these , regulatory agents can better position their organizations for successful submissions and ultimately enhance their chances of approval.
To enhance compliance efforts, officers should regularly review documentation processes, engage in , and within their teams to mitigate risks associated with poor documentation.
Impact of PDUFA on Drug Development Timelines: What Compliance Officers Need to Know
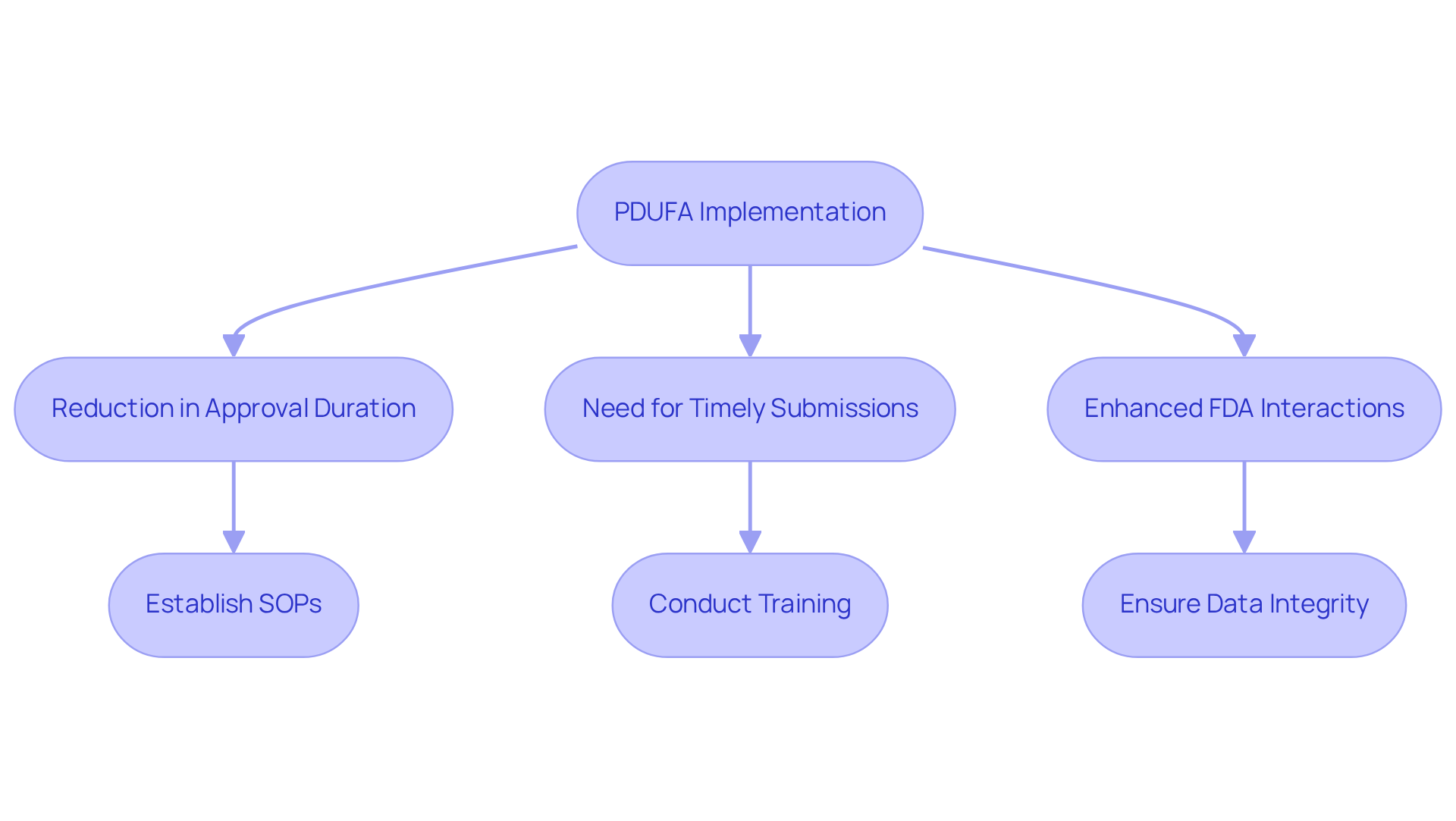
The , commonly known as PDUFA, holds significant as it has accelerated the , facilitating faster access to innovative therapies. Since its inception in 1992, this Act, often referred to as the PDUFA, has led to an average reduction in drug approval durations, with research indicating a decline from 24.2 months to 14.2 months between 1991 and 2002, primarily attributable to the PDUFA meaning and its implementation.
Compliance officers must understand how these timelines impact their projects, particularly the necessity for and the potential for expedited reviews. Notably, the third version of the Prescription Drug User Fee Act introduced initiatives that and , and the PDUFA meaning in the fourth version was to improve drug safety through increased user fees, ensuring that the FDA had the necessary resources for timely reviews.
By strategically aligning their development processes with , organizations can significantly enhance their chances of swiftly meeting market demands. This alignment is crucial, as achieving a regulatory date represents a , underscoring the commitment of development teams and the importance of meticulous project management in mitigating risks associated with approval timelines.
Furthermore, establishing robust , which include regular training sessions and , as well as ensuring data integrity, are vital components of maintaining compliance in this fast-paced environment.
Training Programs: Enhancing Understanding of PDUFA Regulations
focused on are essential for oversight personnel to remain informed about evolving requirements and best practices. AVS Life Sciences offers that encompass critical aspects of drug approval, including:
- Submission processes
- Documentation standards
- The latest
- A detailed explanation of
By prioritizing these training initiatives, organizations cultivate a robust culture of adherence while significantly enhancing operational efficiency. Recent statistics indicate that only a fraction of regulatory professionals possess thorough knowledge of , underscoring the urgent need for extensive educational materials.
Successful [training programs](https://lorman.com/blog/post/39-statistics-that-prove-the-value-of-employee-training?srsltid=AfmBOop8Hdyum_GxzN05DLbv2SjdG1SLBDVfOAMENk11aUVdG0dPaZAd), featuring hands-on workshops and practical case studies, have proven advantageous in bolstering the comprehension and implementation of . Insights from training specialists underscore the importance of continuous education and adaptability to legal changes, ensuring that professionals are well-equipped to navigate the complexities of the pharmaceutical landscape.
Challenges in PDUFA Compliance: Strategies for Risk Mitigation
Navigating adherence to the Prescription Drug User Charge Act presents considerable challenges, particularly due to the ever-evolving regulatory landscape and the intricate nature of required documentation. To effectively address these issues, regulatory officers must implement robust risk mitigation strategies. Regular evaluations are essential; they not only identify potential regulatory gaps but also reinforce compliance with the standards associated with PDUFA meaning. Maintaining open lines of communication with oversight organizations fosters transparency and allows for proactive resolution of issues before they escalate. Additionally, leveraging streamlines documentation processes and enhances tracking capabilities, ensuring that all legal obligations are met efficiently.
Statistics highlight the efficacy of these approaches:
- Organizations that conduct regular audits experience a .
- 65% of regulatory personnel affirm that .
As one regulatory officer noted, 'Being proactive in our approach has enabled us to manage approval requirements more smoothly, minimizing the risk of costly delays.' By prioritizing these , organizations can enhance their capacity to navigate regulatory challenges and maintain high adherence standards. To further elevate adherence initiatives, is recommended, as they specialize in GXP, FDA regulations, and the development of Standard Operating Procedures (SOPs). Their expertise in managing and executing Internal & External Auditing Techniques provides tailored solutions to ensure compliance with regulatory standards.
Collaboration with Regulatory Bodies: A Key to PDUFA Success
Cooperation with oversight organizations, particularly the FDA, is critical for achieving a . Compliance professionals must prioritize to clarify expectations and address potential concerns. Establishing facilitates smoother submissions and significantly enhances the likelihood of prompt approvals.
Insights from regulatory professionals reveal that —such as frequent updates and open dialogues about project timelines—can lead to improved submission outcomes. Regular and timely interactions with the FDA empower oversight personnel to navigate complex legal frameworks more adeptly, ensuring that all necessary documentation and data adhere to requirements. This strategic approach cultivates trust and collaboration, ultimately streamlining the approval process.
A compelling case study from AVS Life Sciences exemplifies this principle, highlighting their successful enhancement of a biotechnology GMP facility. By focusing on , AVS Life Sciences supported a leading biotechnology firm in elevating their manufacturing capabilities, resulting in a swift transition to a Level 2 GMP facility. This collaboration not only underscored the vital role of in achieving compliance success but also enabled the client to refine their production processes, ultimately .
Quality Management Systems: Supporting PDUFA Compliance
A robust is essential for ensuring compliance with the Prescription Drug User Fee Act (PDUFA), which is important for grasping the . Compliance officers must guarantee that their QMS not only meets but also integrates comprehensive processes for documentation, training, and continuous improvement. This includes critical elements such as:
- Data Integrity Deviations
- Investigations
- CAPA (Corrective and Preventive Actions)
Organizations that maintain a strong QMS can significantly enhance their ; indeed, 83% of organizations believe that a QMS solution aided their recovery from . This proactive strategy mitigates the risk of non-compliance during audits and cultivates a culture of quality across the organization.
For instance, companies that have adopted automated QMS solutions report enhanced data management and visibility, which are vital for fulfilling the obligations related to PDUFA meaning. Furthermore, empowered frontline workers play a pivotal role in maintaining high-quality standards and process efficiency, as they often serve as the first line of defense in identifying regulatory issues.
Experts emphasize the necessity of aligning QMS with compliance requirements. Notably, despite 94% of organizations having a dedicated quality function, product recalls continue to pose a significant challenge. This underscores the importance of ongoing evaluation and improvement of . By implementing structured methodologies such as CAPA and deviation management, organizations can effectively address the root causes of non-compliance and improve their overall adherence rates.
In summary, a well-aligned QMS not only facilitates compliance with industry standards but also empowers organizations to respond swiftly to regulatory changes while maintaining high-quality benchmarks. This ultimately safeguards their reputation and financial stability. To ensure your QMS not only meets but exceeds regulatory expectations, for customized consulting solutions.
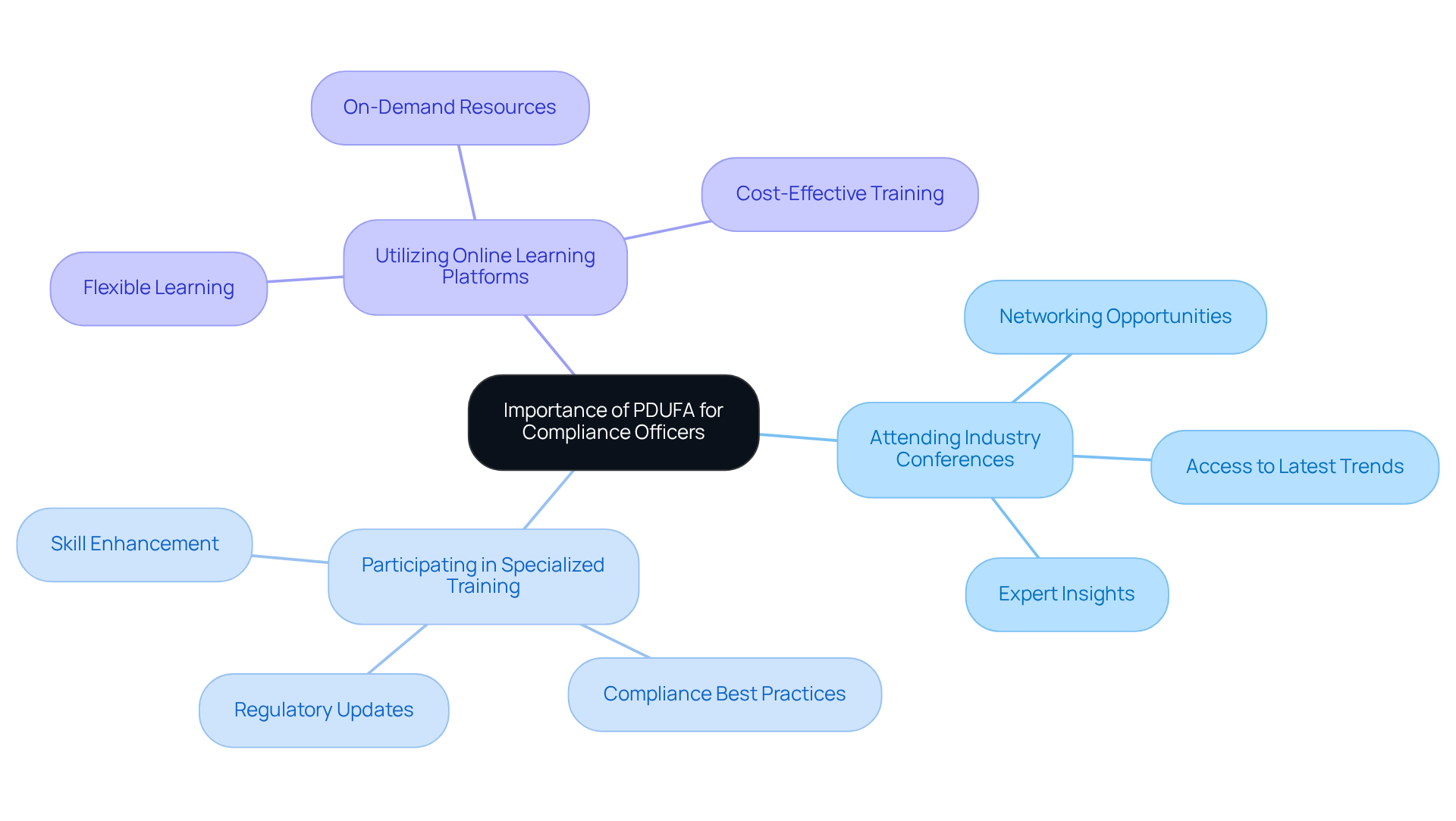
The Importance of PDUFA for Compliance Officers: Continuous Learning and Adaptation
For , mastering the PDUFA process involves grasping the , which represents an ongoing journey that demands continuous learning and adaptability. As regulations evolve, it is imperative for these professionals to stay informed about changes and best practices. Engaging in opportunities—such as:
- Attending
- Participating in
- Utilizing
can significantly enhance their expertise. This proactive approach not only equips regulatory agents with the essential skills to navigate the requirements but also fosters a within their organizations. Industry specialists emphasize that is critical; it empowers regulatory professionals to adeptly manage the complexities of changing oversight environments, including the data management challenges outlined in the FDA's guidance, and to implement best practices that ensure organizational adherence to the PDUFA meaning standards. By prioritizing professional development, compliance officers can elevate their effectiveness, ultimately contributing to their organizations' success in maintaining compliance and ensuring .
Conclusion
Understanding the intricacies of the Prescription Drug User Fee Act (PDUFA) is essential for compliance officers aiming to enhance their organizational effectiveness in the drug approval process. This act plays a critical role in expediting drug approvals, making it imperative for regulatory professionals to grasp its significance to navigate the complexities of compliance successfully.
Compliance officers must prioritize:
- Timely submissions
- Maintaining thorough documentation
- Engaging in continuous training to stay aligned with evolving FDA regulations
Collaboration with regulatory bodies, particularly the FDA, is vital for facilitating smoother interactions and improving approval outcomes. Furthermore, the implementation of robust Quality Management Systems (QMS) and proactive risk mitigation strategies are essential components for achieving compliance and ensuring quality throughout the drug development lifecycle.
Mastering PDUFA compliance demands a commitment to ongoing education and adaptability. By investing in training programs, leveraging expert guidance, and fostering a culture of compliance within their organizations, regulatory professionals can significantly enhance their chances of meeting regulatory expectations and facilitating timely market access for innovative therapies. Embracing these strategies not only boosts operational efficiency but also positions organizations to thrive in a competitive landscape, ultimately benefiting patients and stakeholders alike.
Frequently Asked Questions
What is PDUFA and why is it important in drug approval?
PDUFA stands for the Prescription Drug User Fee Act, which establishes critical deadlines for the evaluation of New Drug Applications (NDAs) and Biologics License Applications (BLAs). Understanding PDUFA is essential for navigating the drug approval process and influencing the timeline for potential market entry.
How does AVS Life Sciences assist with PDUFA compliance strategies?
AVS Life Sciences provides tailored regulatory strategies that help clients understand PDUFA, enabling them to navigate the drug approval landscape effectively. Their experts focus on aligning submissions with FDA expectations to minimize delays in drug approvals.
What are the key roles of compliance personnel in relation to PDUFA dates?
Compliance personnel oversee critical deadlines related to PDUFA, ensuring that all necessary documentation is submitted promptly. This proactive approach facilitates a smoother approval process and aligns with industry best practices for regulatory adherence.
What is the significance of the FDA's user fees in the drug evaluation process?
The FDA collects user fees from pharmaceutical firms, which constitute a significant part of its budget. In 2024, these fees accounted for nearly half of the FDA's total budget, enhancing the agency's ability to recruit staff and improve review capabilities, thus impacting the efficiency of drug evaluations.
What are Risk Evaluation and Mitigation Strategies (REMS) and how do they relate to PDUFA?
REMS are additional compliance obligations introduced under the fourth Prescription Drug User Fee Act. They illustrate the evolving requirements of the FDA, which compliance officers must navigate to ensure adherence and facilitate timely product approvals.
How can compliance professionals enhance their effectiveness in managing submission deadlines?
Compliance professionals can enhance their effectiveness by leveraging tools that provide real-time updates on regulatory dates, allowing them to stay informed and prepared to navigate the complexities of drug development and improve their chances of achieving timely market access.
