9 Key Elements of a Clinical Evaluation Report for Compliance

Overview
The key elements of a Clinical Evaluation Report (CER) for compliance are critical in addressing the challenges faced in the medical device industry. These elements include:
- Formulation of a Clinical Evaluation Plan
- Thorough literature reviews
- Comprehensive clinical data documentation
- Adherence to regulatory standards
- Effective risk management strategies
- Ongoing post-market surveillance
Each of these components plays a vital role in ensuring the report's credibility and compliance with regulations. Ultimately, this not only enhances patient safety but also facilitates market access for medical devices. Engaging with these components is essential for compliance solutions that foster trust and reliability in the healthcare sector.
Introduction
The landscape of medical device regulation presents an increasingly complex challenge, where compliance is paramount for success in the industry. For manufacturers striving to navigate this intricate terrain effectively, a thorough understanding of the key elements of a Clinical Evaluation Report (CER) is essential. This article explores the nine fundamental components that not only ensure adherence to regulatory standards but also bolster the credibility and market readiness of medical devices. As we delve deeper, we will consider the potential challenges that may arise in creating a compliant CER and how stakeholders can leverage these insights to enhance their evaluation processes.
AVS Life Sciences: Comprehensive Guidance for Clinical Evaluation Reports
AVS Life Sciences provides comprehensive support for the creation of clinical evaluation reports (CERs), ensuring that clients adhere to regulatory standards across various sectors, including biopharmaceuticals, medical products, and nutraceuticals. Given that a significant portion of medical equipment requires a clinical evaluation report for market access, AVS's expertise in providing this report becomes indispensable in this field. Their team develops tailored strategies that align with Good Manufacturing Practices (GMP) and ISO standards, facilitating a more efficient assessment process for medical devices and pharmaceuticals.
A critical initial step in the clinical evaluation report (CER) process is defining the scope and formulating a Clinical Evaluation Plan (CEP), which AVS assists clients in crafting. By focusing on quality management and compliance with standards, AVS Life Sciences empowers clients to navigate the complexities of clinical evaluations confidently. The company underscores the importance of systematic literature searches and the engagement of skilled CER authors and evaluators, guaranteeing that the documentation is both compliant and robust.
This commitment to excellence and fosters smoother compliance approvals under frameworks such as the Medical Device Regulation (MDR), ultimately advancing patient safety. For further insights on regulatory compliance, consider subscribing to our newsletter!
Clinical Evaluation Plan (CEP): Foundation for Effective Reporting
A Clinical Assessment Plan (CAP) serves as the essential document delineating the objectives, methodology, and scope of the clinical assessment process. It meticulously outlines the data collection methods, appraisal strategies, and analysis techniques that will be utilized to evaluate the safety and effectiveness of the medical device. A robust CAP not only ensures compliance with legal standards but also enhances the credibility of the clinical evaluation report by providing a structured framework for the evaluation process.
To further bolster your endeavors in creating an effective CAP, consider for customized consulting solutions in quality management and compliance. Our team of industry-leading professionals stands ready to assist you in navigating the complexities of regulatory landscapes, ensuring a successful market entry for your innovations.
Literature Review: Critical Component for Evidence-Based Evaluation
A thorough literature review is essential for gathering existing clinical data and evidence relevant to the medical device being assessed. This process not only identifies pertinent studies and publications but also critically evaluates their quality and relevance to the current assessment. By combining this information, the literature review supports the assertions made in the Clinical Assessment Report, ensuring that the assessment is firmly grounded in strong evidence and meets compliance expectations. The standard of the literature review directly influences the results of the clinical evaluation report, as it establishes the trustworthiness of the information supporting the product's safety and effectiveness.
Consequently, performing a comprehensive literature review is not merely a compliance necessity; it is a fundamental phase in the evidence-based assessment process that can greatly influence the success of the medical product in the market. AVS Life Sciences exemplifies the importance of quality in literature reviews, as demonstrated by their successful upgrade of a biotechnology GMP facility. Throughout this project, they encountered difficulties such as ensuring adherence to legal standards and maintaining complete traceability. By addressing these challenges, AVS Life Sciences not only completed the project on schedule and within budget but also captured important lessons learned that can inform best practices in conducting literature reviews.
Their impressive and extensive training programs further support effective methodologies for conducting literature reviews, reinforcing their commitment to excellence, as evidenced by achieving zero findings during audits.
Clinical Data Documentation: Essential for Validating Claims
Clinical data documentation is essential for the clinical evaluation report (CER), as it serves as the foundation for substantiating safety and effectiveness claims of medical devices. This documentation must be comprehensive, precise, and compliant with standards, ensuring that all pertinent data is accurately captured and clearly presented. Key components of a CER include:
- Device Description
- State of the Art
- Clinical Background
- Clinical Data Analysis
Efficient documentation not only simplifies the assessment process but also significantly influences the clinical evaluation report submissions, thereby reducing the risks associated with adherence failures. Frequent updates to the clinical evaluation report throughout the product lifecycle are crucial to maintain compliance and integrate any new clinical data or modifications in the apparatus. Best practices involve:
- Conducting thorough literature reviews
- Engaging qualified evaluators
- Aligning with the Clinical Evaluation Plan (CEP)
By prioritizing meticulous documentation, manufacturers can enhance the credibility of their clinical evaluation reports, ultimately supporting successful market access and compliance with the EU Medical Device Regulation (MDR).
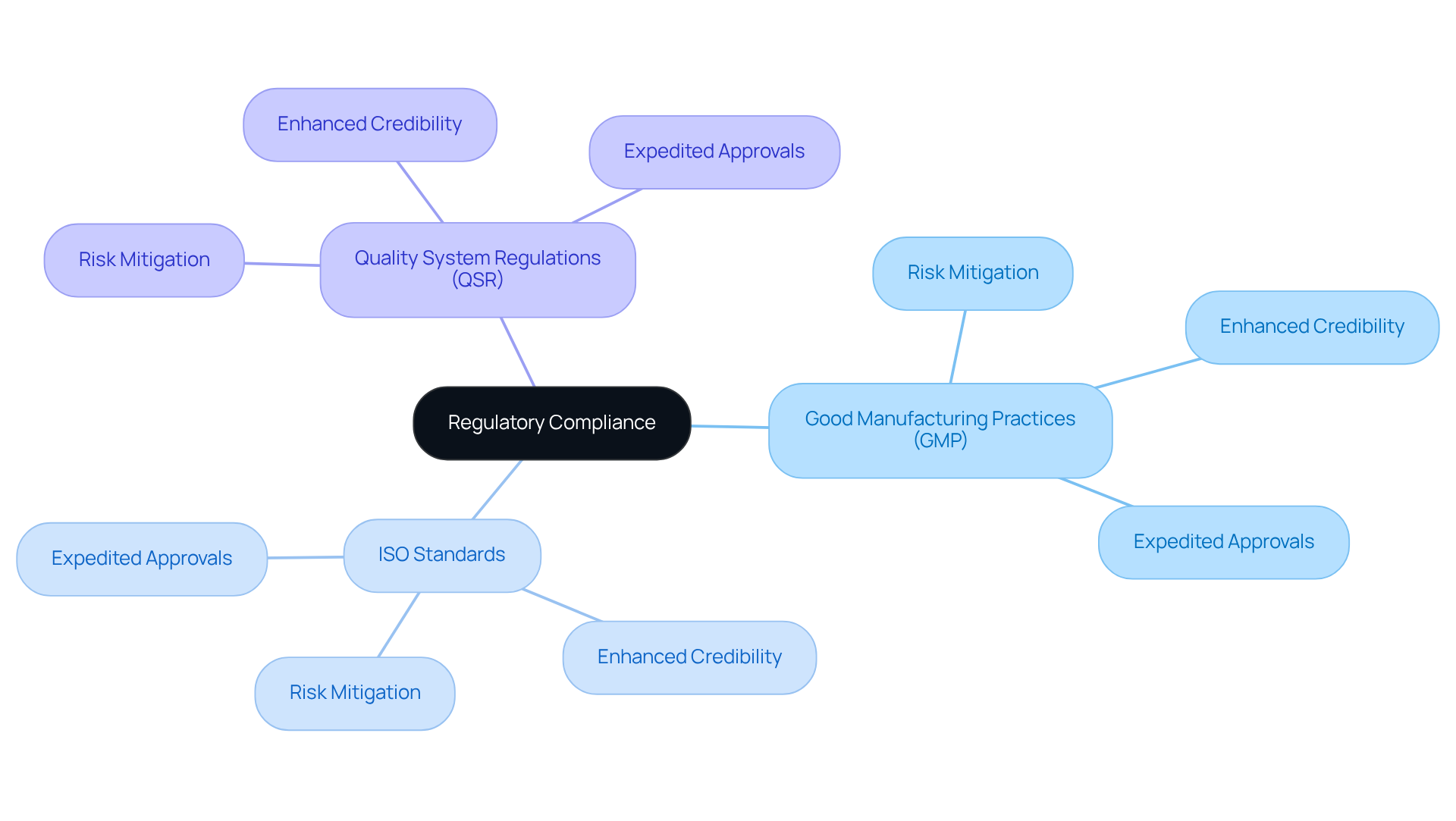
Regulatory Compliance: Ensuring Adherence to Standards
Adherence to regulations is paramount in the preparation of the clinical evaluation report, ensuring that assessments align with the laws, guidelines, and standards established by governing bodies. For manufacturers, following Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR) is essential. By prioritizing these compliance measures, organizations can significantly mitigate the risk of costly delays and penalties, facilitating a more efficient pathway to market for their medical devices.
Viewing compliance not merely as an expense but as a can yield substantial long-term benefits. Successful adherence to GMP and ISO standards not only enhances the credibility of clinical evaluation reports but also fosters trust among stakeholders, ultimately contributing to a more robust oversight framework in the life sciences sector. This compliance can lead to expedited approvals, ensuring that products meet the required standards of effectiveness and reliability, which is critical for timely market entry.
To explore how AVS Life Sciences can aid you in navigating regulatory compliance and quality management, consider engaging with our consulting services.
Risk Management: Identifying and Mitigating Safety Concerns
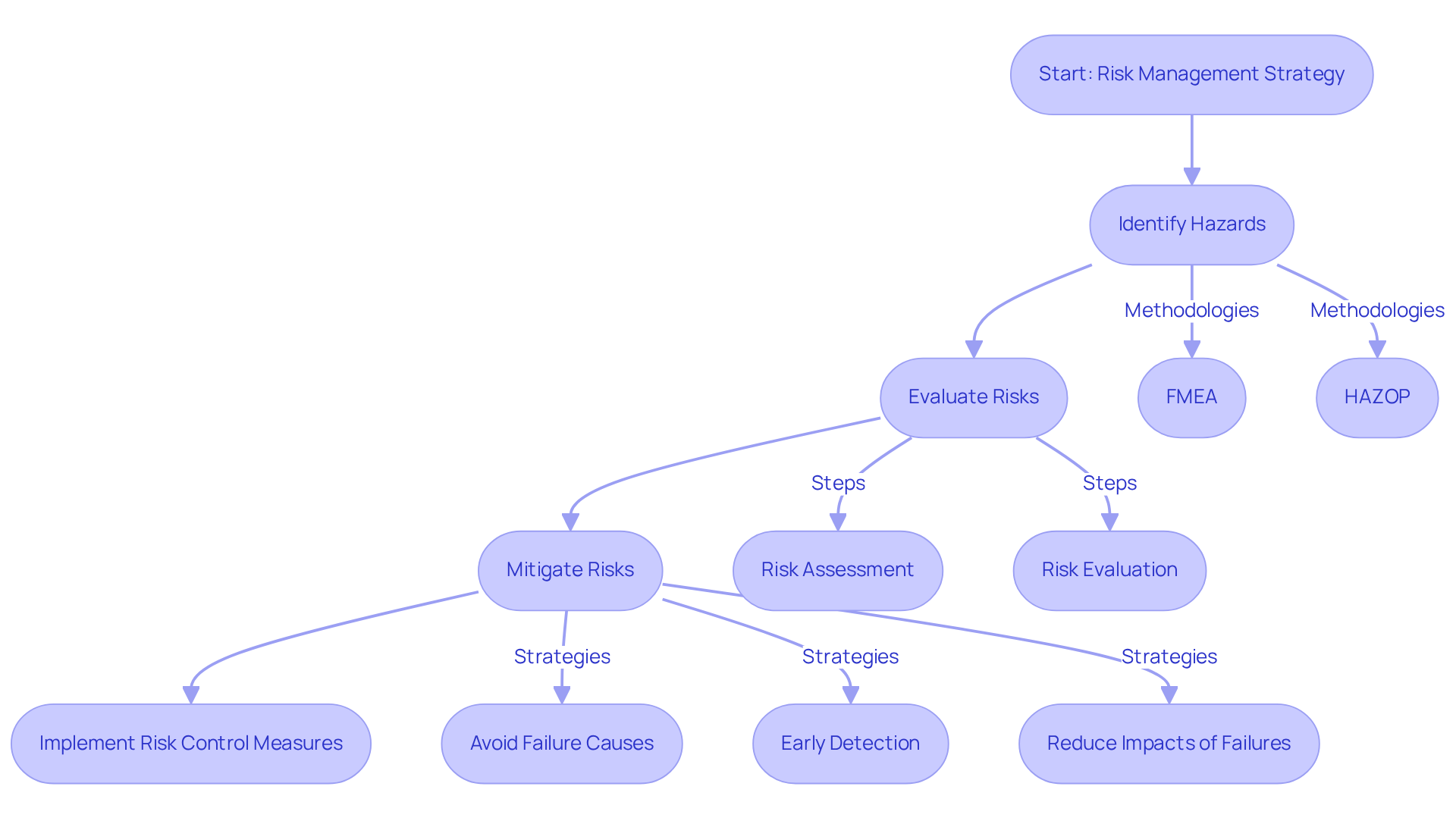
Risk management represents a systematic strategy that encompasses the identification, evaluation, and mitigation of potential hazards associated with medical instruments. This process is crucial to the clinical evaluation report, ensuring that risks are thoroughly addressed throughout the evaluation lifecycle. By implementing robust risk management strategies, including methodologies such as Failure Mode and Effects Analysis (FMEA) and Hazard and Operability Study (HAZOP), manufacturers can significantly bolster the safety profile of their devices. This enhancement not only increases the likelihood of regulatory approval but also promotes market success.
AVS Life Sciences underscores the necessity of a comprehensive Risk Management Plan, detailing how risk control measures will be executed and monitored. This plan should include:
- Meticulous documentation of risk analysis, assessment, and controls
- Considerations for post-production risks
- Definition of the scope, intended use, roles, risk acceptability criteria, and verification methods for risk control measures, in alignment with ISO 14971:2007 standards
As we approach 2025, the significance of risk management in safeguarding medical equipment cannot be overstated. With an estimated 98,000 annual deaths linked to , the urgency for effective risk management practices is more critical than ever. Integrating risk management into the clinical evaluation report not only protects patients but also ensures adherence to regulatory requirements, thereby guaranteeing that manufacturers meet essential protection standards.
Industry specialists emphasize that the identification of risk issues must commence at the trial's inception, reinforcing the notion that proactive risk management is vital for maintaining high standards of patient protection and equipment efficacy. Furthermore, fostering a culture of security and transparency within organizations is imperative for effective risk management strategies, enabling better navigation through the complexities of medical equipment assessments and ultimately enhancing patient outcomes.
Conclusion and Recommendations: Guiding Future Actions
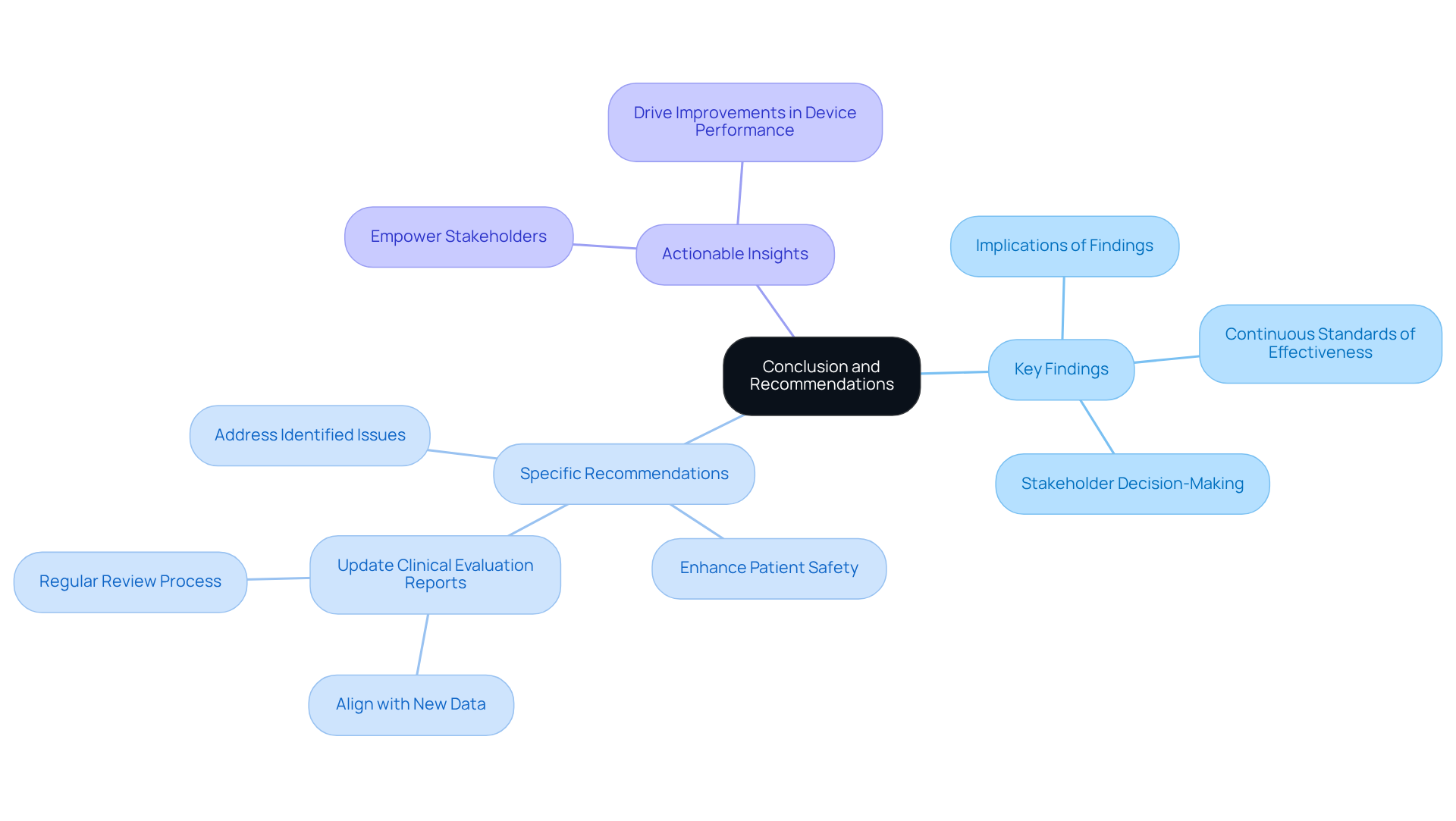
The conclusion and recommendations section of the clinical evaluation report is essential for synthesizing the evaluation's key findings and delivering actionable insights for stakeholders. This section must articulate the implications of the findings clearly, outlining to address any identified issues or concerns.
By providing clearly articulated suggestions, the clinical evaluation report directs future actions and reinforces the medical equipment's commitment to continuous standards of effectiveness and reliability. Effective conclusions drawn from the clinical evaluation report significantly influence stakeholder decision-making, ensuring alignment on necessary actions to maintain compliance and enhance patient safety.
In 2025, the emphasis on actionable insights in clinical assessments is more crucial than ever, as they empower stakeholders to make informed choices that drive ongoing improvements in medical device performance.
Summary of Clinical Evaluation Process: Providing Context
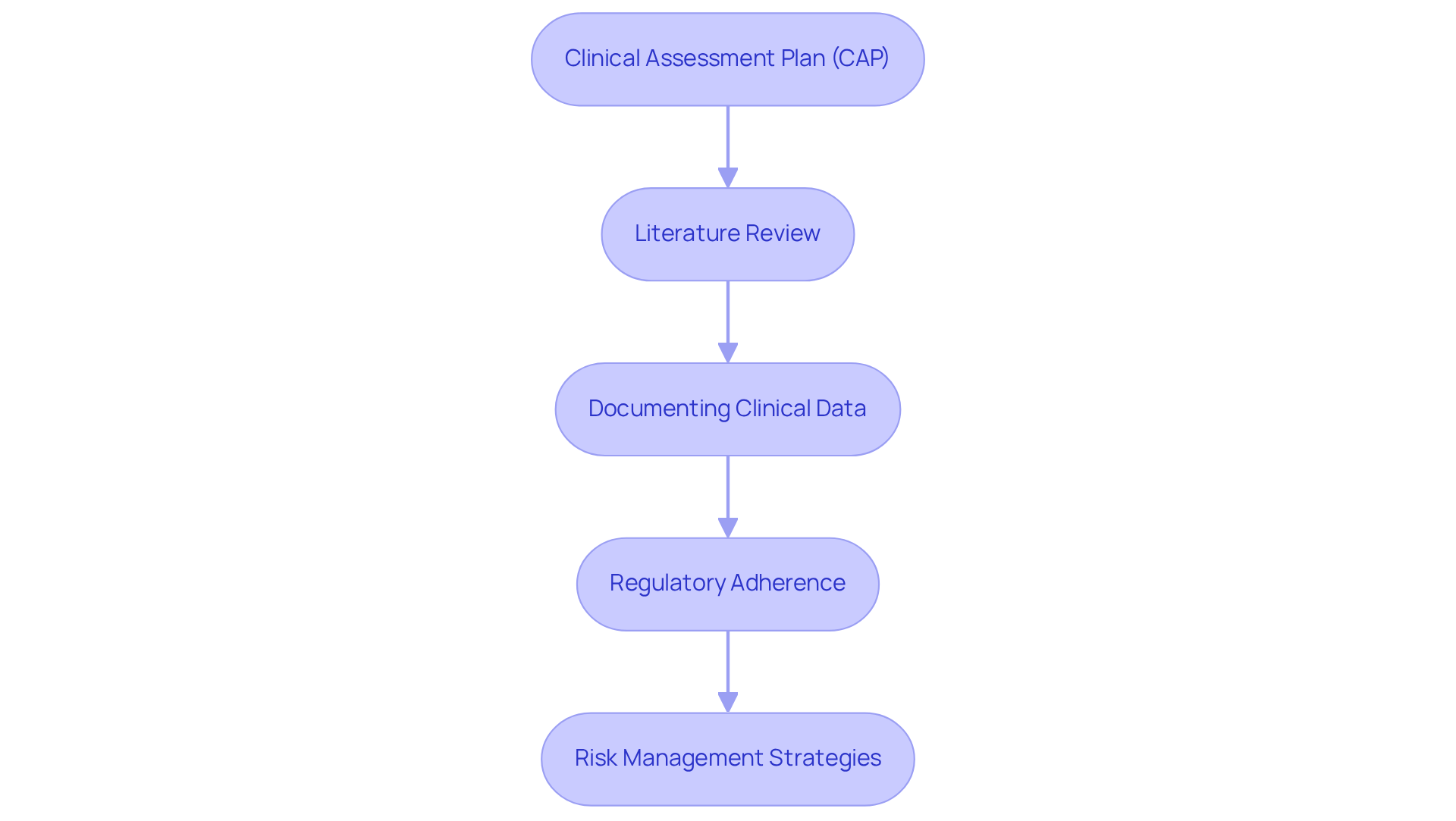
The clinical evaluation report encompasses multiple critical stages, including:
- The formulation of the Clinical Assessment Plan (CAP)
- Conducting a thorough literature review
- Documenting clinical data
- Assessing regulatory adherence
- Implementing effective risk management strategies
Each stage is vital for ensuring that the clinical evaluation report is both comprehensive and compliant with regulatory standards. By grasping the complexities of this process, stakeholders can appreciate the importance of each step outlined in the clinical evaluation report to achieve successful outcomes. As Richard P. Feynman astutely noted, intellectual integrity in research is paramount, underscoring the necessity for meticulousness at every stage of the clinical evaluation report.
Prioritizing these stages enables pharmaceutical companies to bolster their compliance efforts, which is essential for the clinical evaluation report, and ultimately enhance patient well-being and product efficacy. For , connect with AVS Life Sciences to initiate the enhancement of your clinical assessment process.
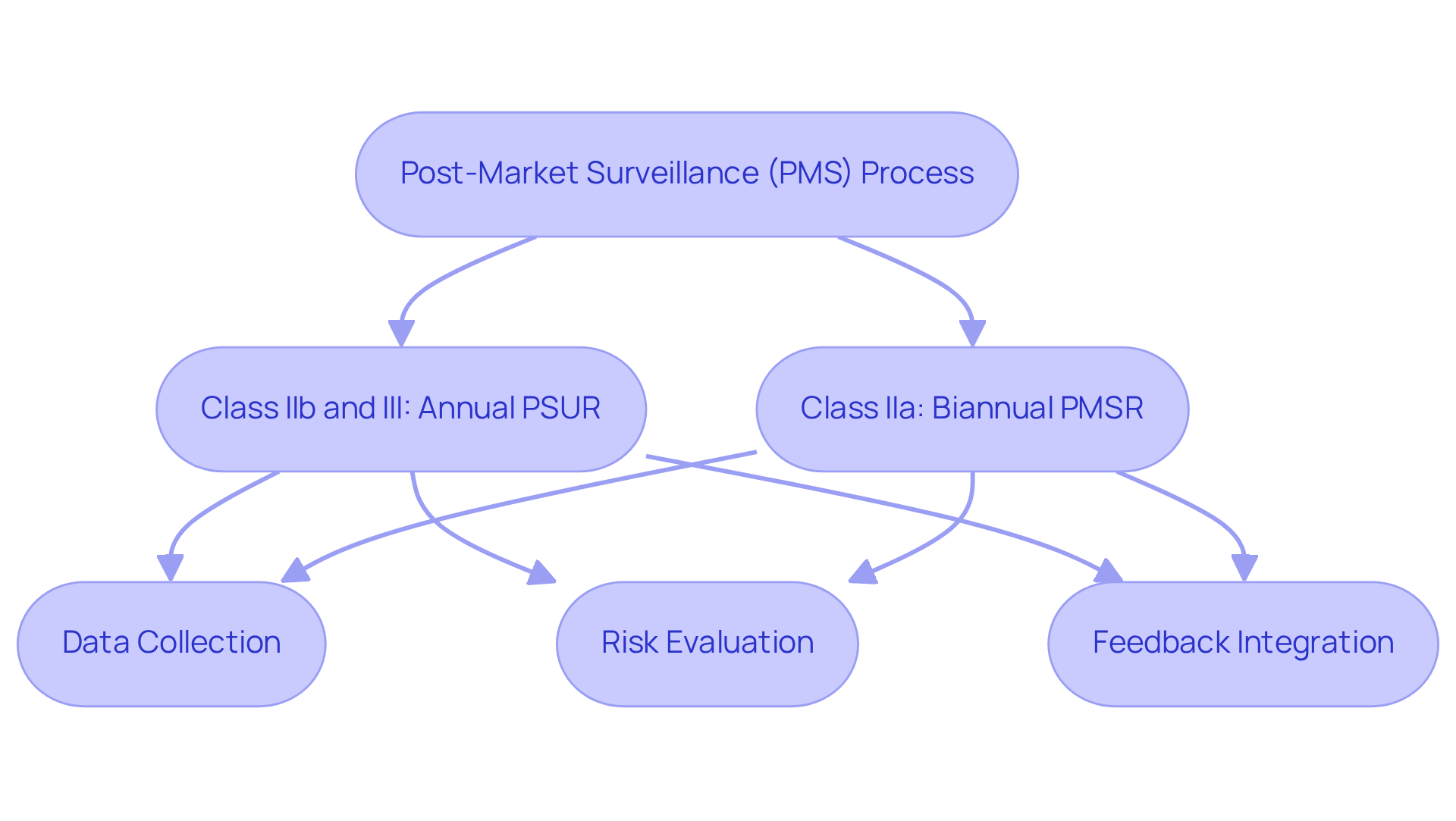
Post-Market Surveillance: Ensuring Ongoing Safety Monitoring
Post-market surveillance (PMS) is a critical element of the clinical evaluation report process, integrating the ongoing assessment of the security and efficacy of medical instruments in real-world settings. This continuous observation is vital for , enabling manufacturers to implement corrective actions promptly. By developing robust PMS strategies, manufacturers can ensure their products consistently meet safety and efficacy standards, thereby safeguarding patient health and ensuring compliance with regulatory requirements.
Effective PMS not only boosts regulatory compliance rates but also cultivates trust among stakeholders. For instance, manufacturers of Class IIb and III devices are required to submit Periodic Safety Update Reports (PSURs) annually, while Class IIa devices necessitate a Post-Market Surveillance Report (PMSR) at least every two years. These mandates underscore the importance of a systematic PMS strategy that includes regular risk evaluations and trend analysis to detect emerging issues.
Strategies for continuous monitoring should encompass the establishment of dedicated teams responsible for data collection and analysis, ensuring that feedback from healthcare professionals and users is systematically integrated into the PMS process. This proactive engagement is essential for ensuring compliance and enhancing device security.
Incorporating insights from ongoing PMS activities into the clinical evaluation report is imperative. As noted by industry specialists, a well-executed PMS can lead to fewer defects and improved user protection, ultimately diminishing litigation risks. Ramya Sriram emphasizes that "medical equipment startups and manufacturers should adopt the PMS process as a comprehensive strategy to optimize the benefits including fewer medical product flaws, enhanced user safety, and, in some instances, reduced litigation risks." Therefore, producers should view PMS as a holistic process that not only fulfills compliance obligations but also contributes to the overall success of their medical devices. Furthermore, AVS Life Sciences offers comprehensive quality management and compliance solutions that can assist manufacturers in developing effective PMS strategies tailored to their specific needs.
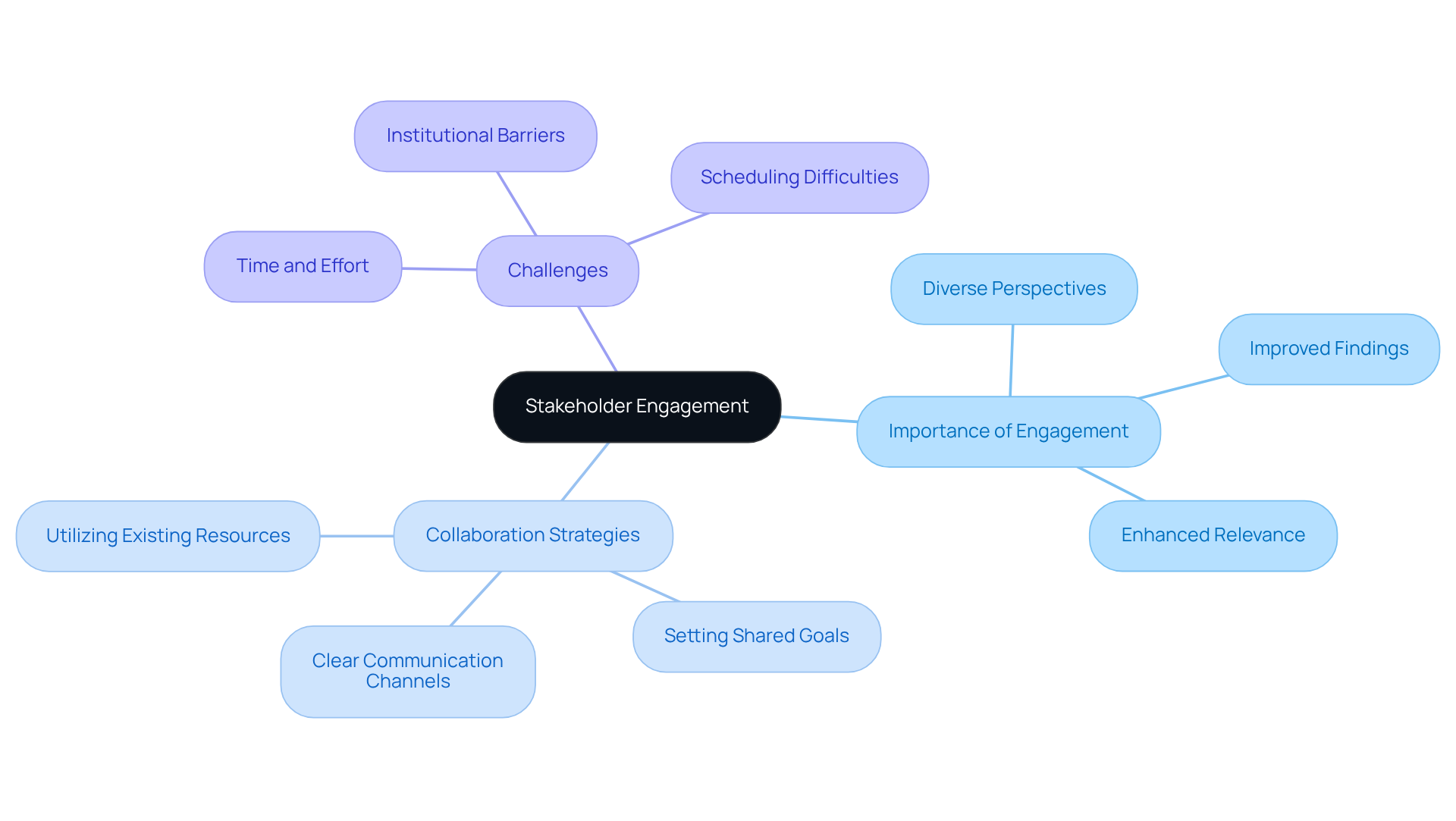
Stakeholder Engagement: Involving Relevant Parties in Evaluation
Stakeholder involvement is essential in the clinical evaluation report process, as it ensures that healthcare professionals, patients, and regulatory bodies provide their valuable insights. This inclusive approach not only enriches the assessment by incorporating diverse perspectives but also enhances its relevance to real-world applications, reflecting AVS Life Sciences' in life sciences consulting.
Research demonstrates that engaging healthcare professionals early in the assessment process can significantly improve the quality of findings, as their expertise helps frame pertinent questions and tackle potential challenges. A systematic review revealed that 80% of articles reported engagement with patients and the public, highlighting the critical nature of stakeholder involvement.
Moreover, effective collaboration strategies, such as establishing clear communication channels and setting shared goals, can alleviate logistical barriers and enhance stakeholder satisfaction. Specific facilitators for patient engagement include:
- Utilizing existing resources
- Setting clear objectives
- Treating patients with respect
As the landscape of clinical evaluation reports for medical devices evolves in 2025, the significance of stakeholder involvement remains paramount, driving the development of more effective and user-centered solutions. However, challenges such as additional time and effort, institutional barriers, and difficulties in scheduling meetings must be addressed to ensure meaningful engagement, reinforcing AVS Life Sciences' role as a leading provider of quality management and regulatory compliance solutions.
Conclusion
The significance of a Clinical Evaluation Report (CER) in ensuring compliance and enhancing patient safety is paramount. This essential document serves as a comprehensive framework for assessing the safety and effectiveness of medical devices, guiding manufacturers through the complexities of regulatory requirements. By grasping the critical elements involved in creating a CER, stakeholders can adeptly navigate the path to market access while adhering to established guidelines and standards.
Key points discussed in the article underscore the necessity of a well-structured Clinical Evaluation Plan, thorough literature reviews, meticulous clinical data documentation, and robust risk management strategies. Each of these components is pivotal in developing a credible CER that not only meets regulatory expectations but also fosters trust among stakeholders. Collaborating with experts like AVS Life Sciences can further streamline this process, equipping manufacturers with the essential tools and knowledge to succeed.
As the landscape of medical device evaluation evolves, the emphasis on compliance and quality management remains critical. Manufacturers are urged to adopt best practices in clinical evaluations, actively engage stakeholders, and implement effective post-market surveillance strategies. By doing so, they not only enhance the safety and efficacy of their products but also contribute to a more reliable healthcare environment. Embracing these principles will ultimately lead to improved patient outcomes and a stronger foundation for the future of medical innovation.
Frequently Asked Questions
What services does AVS Life Sciences provide regarding clinical evaluation reports (CERs)?
AVS Life Sciences offers comprehensive support for creating clinical evaluation reports (CERs) to help clients comply with regulatory standards in biopharmaceuticals, medical products, and nutraceuticals.
Why are clinical evaluation reports important for medical equipment?
A significant portion of medical equipment requires a clinical evaluation report for market access, making the expertise of AVS Life Sciences in providing these reports indispensable.
What is the role of a Clinical Evaluation Plan (CEP) in the CER process?
The Clinical Evaluation Plan (CEP) is a critical initial step that defines the scope and outlines the objectives and methodology of the clinical evaluation process, which AVS assists clients in crafting.
How does AVS Life Sciences ensure compliance in clinical evaluations?
AVS Life Sciences focuses on quality management, compliance with standards, systematic literature searches, and the engagement of skilled CER authors and evaluators to ensure robust and compliant documentation.
What is the significance of a literature review in the clinical evaluation process?
A thorough literature review is essential for gathering existing clinical data and evidence related to the medical device, ensuring that the assessment is evidence-based and compliant with expectations.
How does the quality of the literature review impact the clinical evaluation report?
The standard of the literature review directly influences the results of the clinical evaluation report, as it establishes the trustworthiness of the information supporting the product's safety and effectiveness.
What challenges did AVS Life Sciences face during their project involving a biotechnology GMP facility?
AVS Life Sciences faced challenges related to ensuring adherence to legal standards and maintaining complete traceability during the upgrade of the biotechnology GMP facility.
What is AVS Life Sciences' track record in terms of client satisfaction and compliance?
AVS Life Sciences has an impressive 80% repeat business rate and has achieved zero findings during audits, demonstrating their commitment to excellence and effective methodologies.
