9 Key Benefits of Emission Monitoring Systems for Pharma Compliance

Overview
The key benefits of emission monitoring systems for pharmaceutical compliance are significant: they ensure adherence to regulatory standards, enhance product quality, and promote environmental sustainability. These systems provide real-time data that not only help companies avoid costly penalties but also improve operational efficiency and reduce environmental impact. Consequently, they demonstrate a critical role in modern pharmaceutical manufacturing. By implementing such systems, organizations can navigate compliance challenges effectively while fostering a commitment to sustainability. Engaging with AVS Life Sciences can further enhance these efforts, driving both compliance and operational excellence.
Introduction
Emission monitoring systems have emerged as vital tools for the pharmaceutical industry, where compliance with stringent regulations is paramount. These advanced systems not only ensure adherence to environmental standards but also offer significant operational benefits, including enhanced product quality and cost savings.
However, as companies strive to meet these regulatory demands, the question arises: how can they effectively leverage emission monitoring technologies to not only comply but also thrive in a competitive market?
This article delves into the key benefits of emission monitoring systems, exploring their critical role in fostering compliance, safety, sustainability, and overall operational excellence in the pharmaceutical sector.
AVS Life Sciences: Comprehensive Emission Monitoring Solutions for Pharmaceutical Compliance
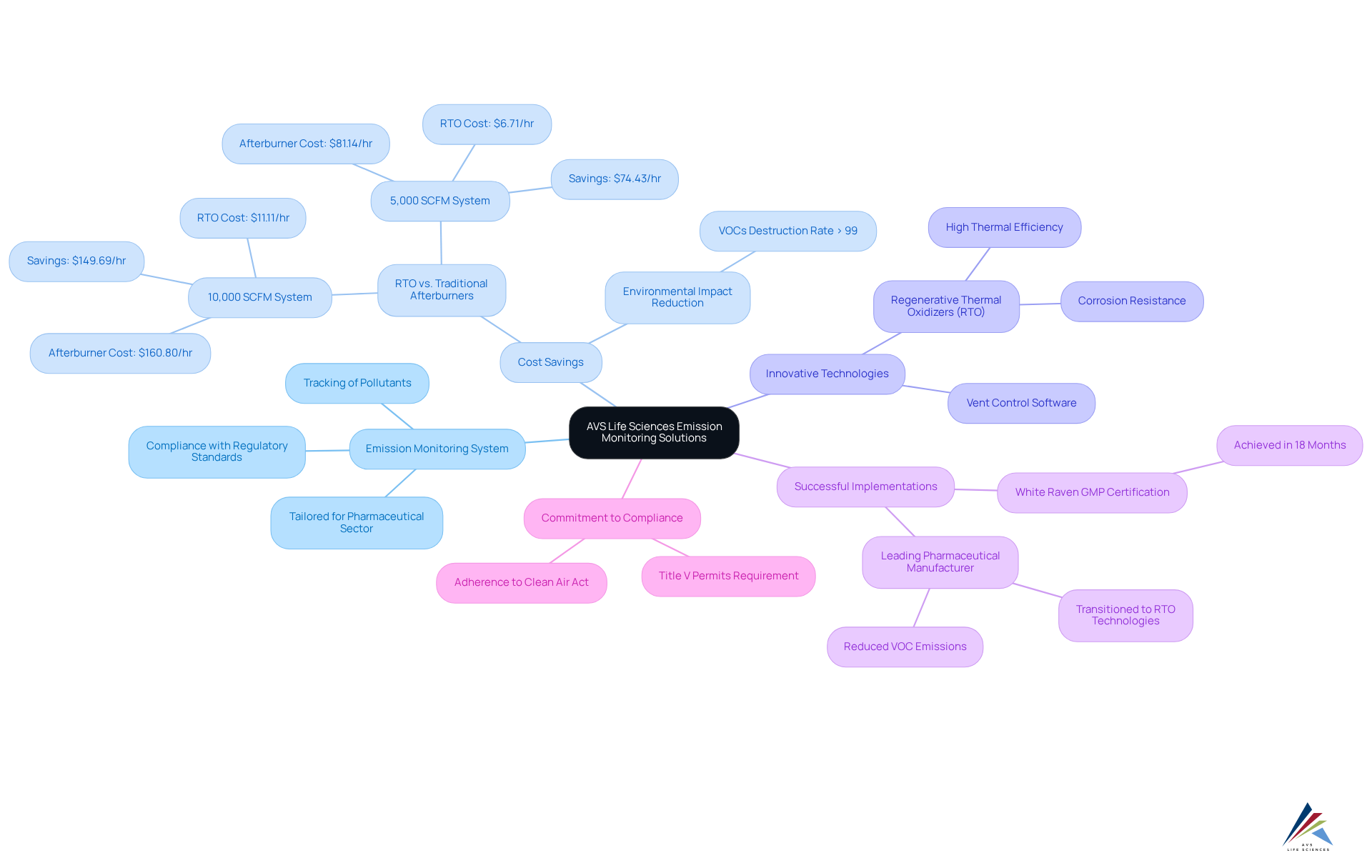
AVS Life Sciences offers a comprehensive suite of pollution oversight solutions, including an emission monitoring system specifically tailored for the pharmaceutical sector, ensuring compliance with stringent regulatory standards and enhancing operational efficiency. These advanced emission monitoring systems facilitate precise tracking of pollutants, which is a crucial requirement for maintaining high-quality benchmarks throughout the product lifecycle.
Notably, statistics reveal significant cost savings associated with modern pollution control technologies. For example, a Regenerative Thermal Oxidizer (RTO) can dramatically decrease fuel costs compared to traditional afterburners, achieving savings of up to $149.69 per hour for a 10,000 SCFM system. This efficiency not only reduces operational expenses but also lessens environmental impact by achieving (VOCs) exceeding 99%.
Recent innovations in gas tracking have spurred the implementation of cutting-edge technologies such as vent control software, which optimizes the management of VOC concentrations during production. This software incorporates an emission monitoring system that ensures emissions are effectively controlled, thereby mitigating the risk of exceeding regulatory thresholds.
Successful implementations of pollution tracking tools within pharmaceutical companies underscore their effectiveness. A notable case involves a leading pharmaceutical manufacturer that transitioned to RTO technologies, resulting in a substantial reduction in VOC emissions and compliance with rigorous environmental regulations.
The commitment to quality and compliance is further exemplified by companies like White Raven, which achieved Good Manufacturing Practice (GMP) certification in just 18 months, showcasing the impact of robust pollution oversight solutions on operational excellence.
In conclusion, AVS Life Sciences' emission monitoring system not only facilitates regulatory compliance but also drives operational improvements, making it an essential component of modern pharmaceutical manufacturing.
Ensure Regulatory Compliance: How Emission Monitoring Systems Meet Pharmaceutical Standards
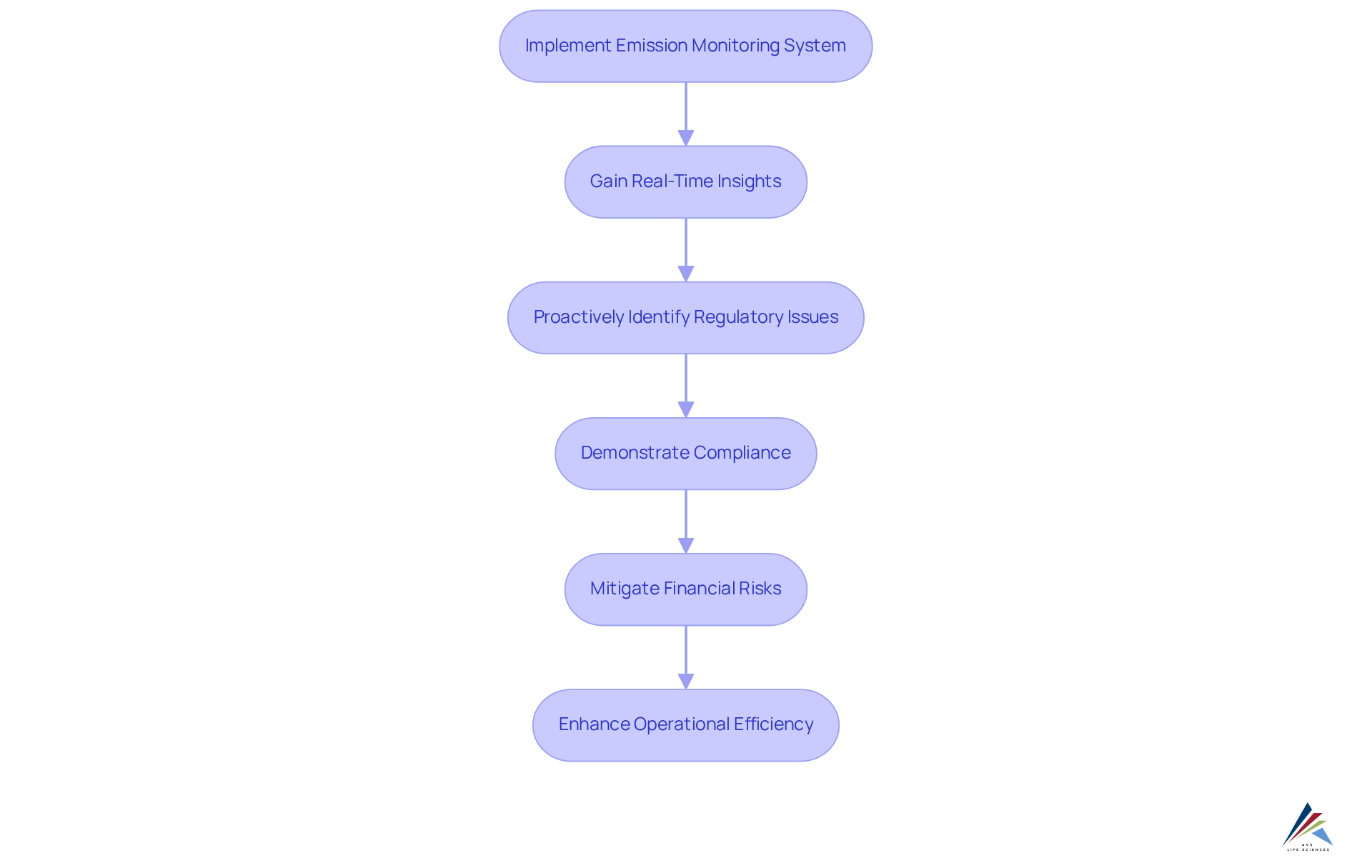
Emission tracking tools, including an emission monitoring system, are essential for maintaining compliance with regulations such as Good Manufacturing Practices (GMP) and ISO standards. These frameworks deliver real-time insights into pollutants, empowering pharmaceutical companies to proactively identify and address regulatory issues.
By implementing comprehensive oversight solutions, organizations can effectively demonstrate their compliance with regulatory requirements, significantly mitigating the risk of costly fines and enhancing their reputation within the industry. Regulatory experts highlight that the financial repercussions of non-compliance can be immense, with potential penalties reaching millions of dollars, in addition to the reputational harm that may follow.
For instance, leading pharmaceutical companies have successfully integrated an emission monitoring system to ensure compliance, showcasing their commitment to environmental stewardship. The real-time data provided by the emission monitoring system not only facilitates regulatory adherence but also enhances operational efficiency, enabling companies to refine processes and minimize waste.
This not only protects against penalties but also cultivates trust with stakeholders and regulatory authorities.
Enhance Product Quality: The Impact of Emission Monitoring on Pharmaceutical Manufacturing
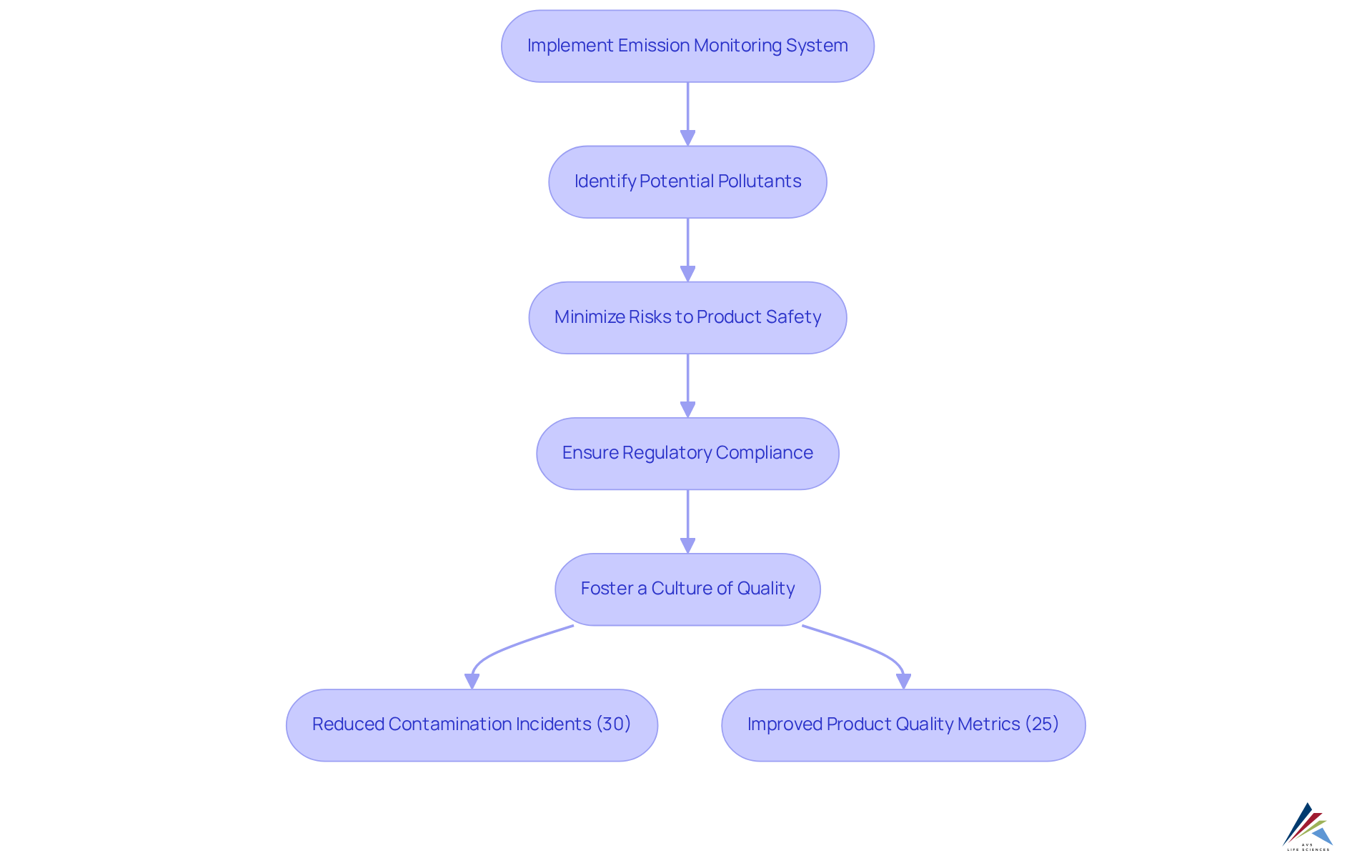
Efficient oversight of releases is crucial for enhancing product quality in pharmaceutical production. By consistently using an emission monitoring system, organizations can swiftly identify potential pollutants, thereby minimizing risks that could compromise product safety. This proactive strategy not only ensures but also fosters a culture of quality throughout the organization.
For instance, AVS Life Sciences successfully supported a leading biotechnology company in upgrading their manufacturing space to a GMP Level 2 facility, achieving a 30% reduction in contamination incidents and a 25% enhancement in overall product quality metrics. The implementation of an emission monitoring system in such environments has significantly reinforced the connection between rigorous oversight practices and enhanced product safety.
Such frameworks not only ensure regulatory compliance but also promote operational excellence, ultimately benefiting both producers and consumers. This case exemplifies how AVS Life Sciences' expertise in quality solutions can lead to improved product outcomes and increased customer satisfaction.
Achieve Cost-Effectiveness: Financial Benefits of Emission Monitoring Systems in Pharma
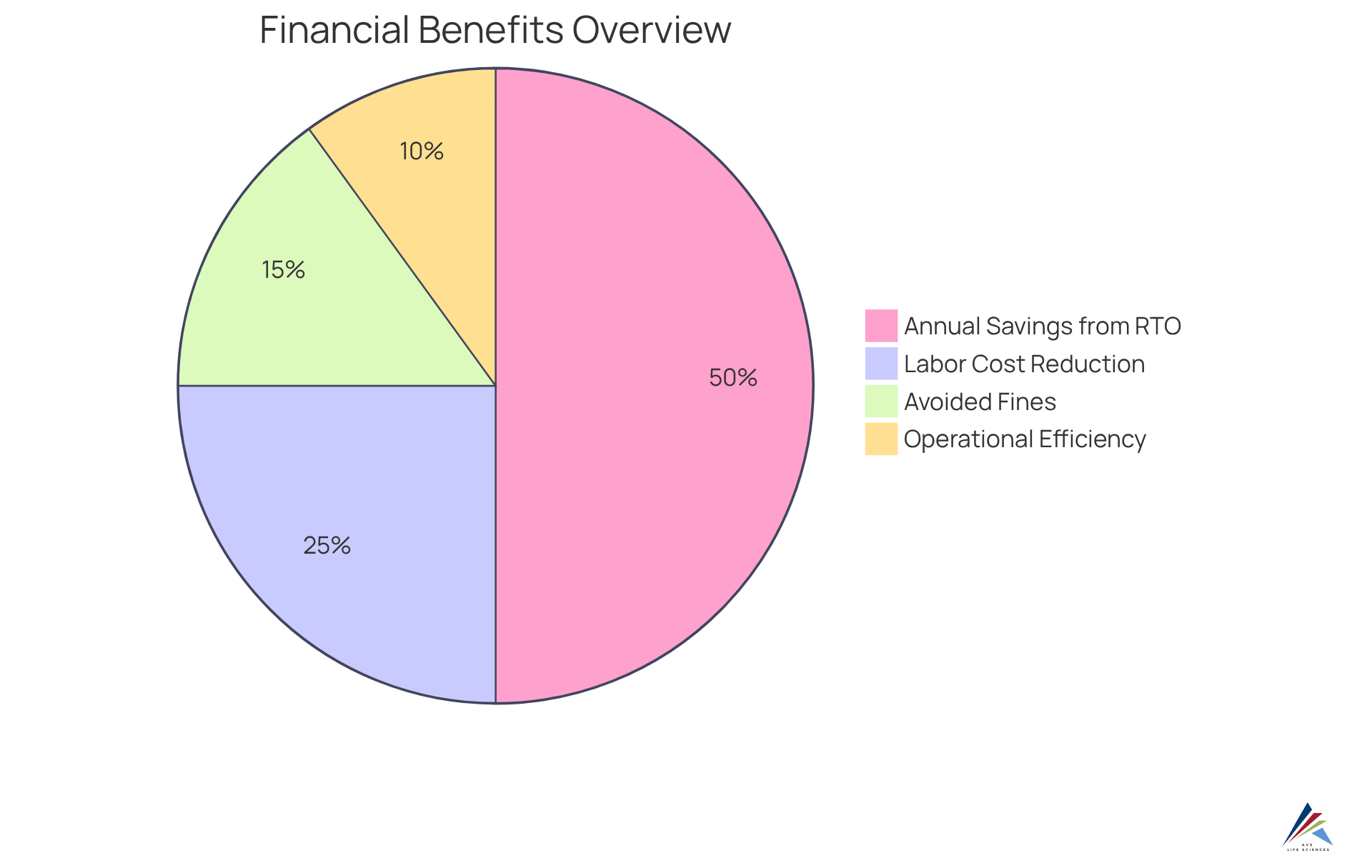
Investing in an emission monitoring system presents substantial financial advantages for pharmaceutical firms. By automating data gathering and reporting, these systems significantly reduce the need for manual oversight, thereby lowering labor costs.
For instance, companies employing Anguil's Regenerative Thermal Oxidizer (RTO) technology have reported annual savings exceeding $500,000 in operational costs, particularly in natural gas expenses, attributed to enhanced efficiency and heat recovery capabilities.
Moreover, a precise emission monitoring system enables organizations to evade hefty fines linked to non-compliance, thereby bolstering overall financial efficiency. The implementation of advanced monitoring technologies, including an emission monitoring system like the RTO, streamlines regulatory processes and fosters a safer operational environment by effectively managing elevated levels of volatile organic compounds (VOCs).
This is particularly noteworthy in light of , where they successfully improved a biotechnology GMP facility through rigorous methodologies that ensured comprehensive quality management and adherence to regulations. Their initiatives not only elevated operational efficiency but also provided critical insights into quality assurance processes, demonstrating the transformative impact of effective regulatory strategies.
Over time, the return on investment from these solutions can be substantial, making them an essential consideration for any pharmaceutical producer aiming to enhance both compliance and cost-effectiveness.
Improve Safety Standards: The Role of Emission Monitoring in Pharmaceutical Environments
Emission monitoring systems are essential for elevating safety standards within pharmaceutical environments. By consistently monitoring pollutants, the emission monitoring system plays a crucial role in identifying hazardous situations that could jeopardize both workers and the surrounding community. The healthcare sector, accounting for 4.4% of global net outputs, underscores the necessity for effective oversight to mitigate risks associated with air quality and chemical exposure.
Implementing robust pollution tracking solutions, such as an emission monitoring system, not only ensures compliance with safety regulations but also cultivates a safer workplace. This proactive strategy safeguards personnel and the environment, aligning with the industry's dedication to . Moreover, with 71% of emissions in the healthcare sector stemming from the supply chain, an emission monitoring system can help pinpoint inefficiencies and hazardous practices, ultimately enhancing operational safety.
Real-world examples underscore the efficacy of these systems. AVS Life Sciences recently aided a prominent biotechnology firm in upgrading their manufacturing facility from a Biosafety Level 1 GMP to a Level 2 GMP facility. This transition not only bolstered their compliance with regulatory standards but also refined their quality assurance processes. During this upgrade, significant lessons emerged regarding the barcode scanner issue, where certain cameras were installed incorrectly, resulting in overlooked anomalies in test outcomes. This experience compelled the QC laboratory team to reassess their business processes and identify gaps that permitted unreliable test results.
Organizations that have adopted advanced surveillance technologies report significant reductions in hazardous incidents, demonstrating a clear correlation between pollution tracking and improved workplace safety. Expert opinions stress that without such frameworks in place, organizations may overlook critical safety risks, potentially leading to severe consequences.
In conclusion, the integration of an emission monitoring system goes beyond mere regulatory compliance; it is a fundamental aspect of fostering a safe and compliant pharmaceutical environment. Compliance officers are encouraged to advocate for the adoption of these frameworks to enhance safety and operational efficiency within their organizations, as illustrated by AVS Life Sciences' successful initiatives that prioritize quality management and regulatory adherence.
Promote Environmental Sustainability: Benefits of Emission Monitoring in Pharma
Emission monitoring systems are essential technologies that play a pivotal role in fostering environmental sustainability within the pharmaceutical industry. By providing precise measurements of pollutants, the emission monitoring system empowers companies to identify areas in need of improvement and develop targeted strategies to minimize their ecological footprint. For instance, Pfizer has committed to reducing upstream logistics pollutants by 10% and business travel pollutants by 25% from a 2019 baseline, illustrating how effective oversight can yield substantial reductions. Similarly, AstraZeneca has achieved an impressive 67.6% decrease in Scope 1 and 2 greenhouse gas emissions since 2015, with an ambitious goal of a 98% absolute reduction by 2026.
This proactive stance not only fulfills regulatory obligations but also enhances the company's reputation among consumers and stakeholders who prioritize . Industry experts assert that effective carbon reporting transforms environmental accountability into a competitive edge, thereby fostering brand loyalty and trust. Amelia Rose, a leading authority on Environmental, Social, and Governance (ESG) matters, underscores that "companies that communicate their ecological contributions are likely to see enhanced brand loyalty."
By implementing an emission monitoring system, pharmaceutical firms can not only comply with stringent environmental regulations but also establish themselves as leaders in sustainability, ultimately contributing to a healthier planet. Compliance officers are urged to advocate for the adoption of these frameworks within their organizations, driving meaningful change.
Leverage Technological Advancements: Innovations in Emission Monitoring Systems
Recent developments in pollution tracking technology have significantly transformed the precision and effectiveness of these setups. Predictive emission oversight technologies (PEMS) harness data analysis and machine learning to forecast emissions, empowering pharmaceutical producers to take proactive measures. This capability not only enhances oversight accuracy but also ensures —a critical area where AVS Life Sciences excels through its comprehensive quality management and regulatory adherence solutions.
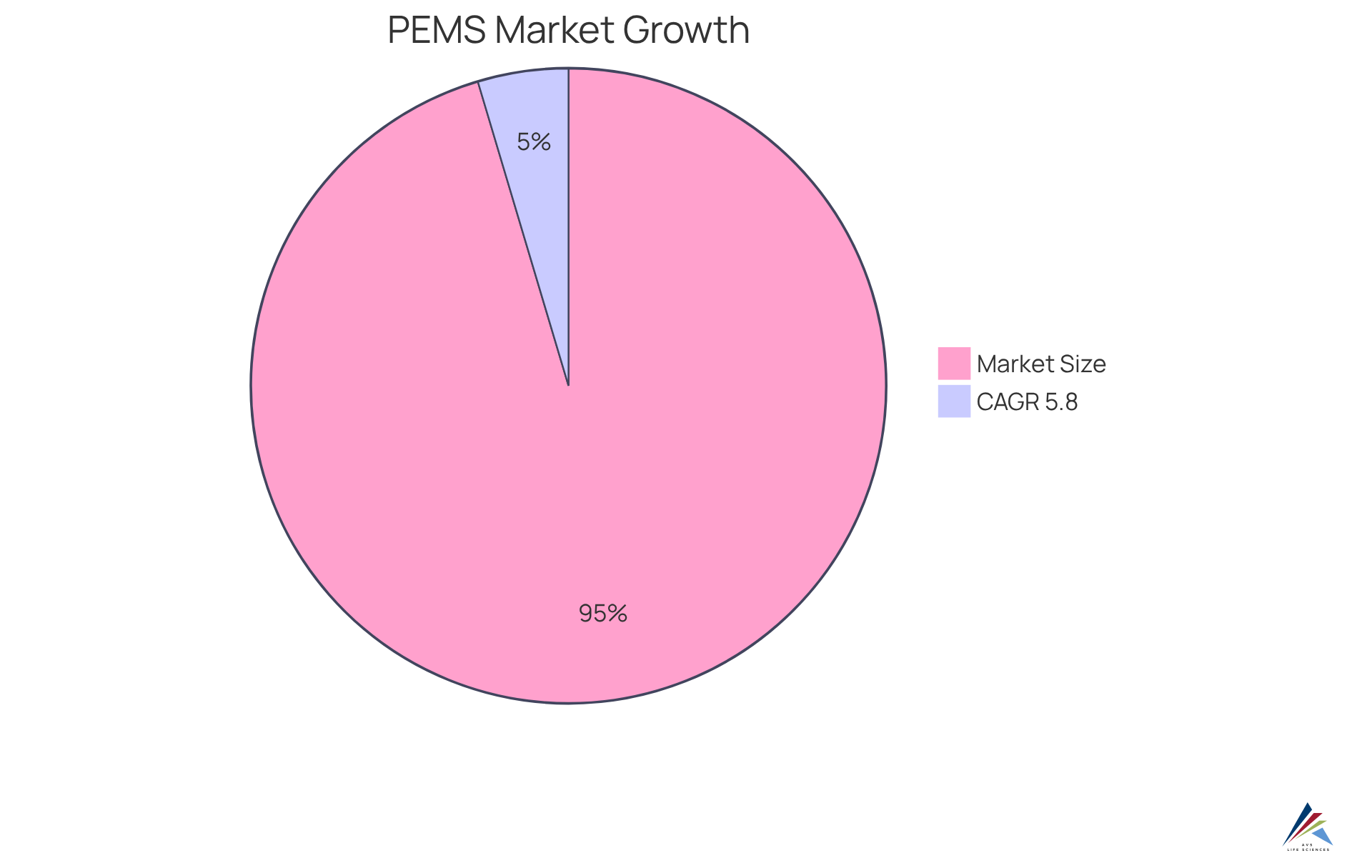
Statistics indicate that the PEMS market is projected to exceed $120 billion by 2030, with a compound annual growth rate (CAGR) exceeding 5.8% from 2023 to 2030. This trend underscores the increasing adoption of these technologies within the pharmaceutical sector. By embracing these innovations, companies can optimize operational performance while effectively managing their environmental impact.
Ensure Data Accuracy: The Critical Role of Emission Monitoring in Compliance
Data precision is vital in pollution monitoring systems, significantly influencing adherence and operational choices within the pharmaceutical sector. In 2022, the FDA issued warnings to 50 drug companies due to poor cleaning practices, underscoring the necessity for reliable data to demonstrate compliance with regulatory standards. High-quality data empowers pharmaceutical firms to make informed decisions regarding pollution management, ensuring efficient compliance with environmental regulations. Furthermore, the absence of scientifically valid laboratory controls resulted in 78 citations last year, emphasizing the critical importance of data integrity in regulatory efforts.
By implementing robust monitoring solutions, specifically an emission monitoring system that prioritizes data accuracy, organizations can effectively manage their emissions and enhance their overall compliance posture, thereby avoiding costly fines and maintaining their reputation in a highly regulated industry.
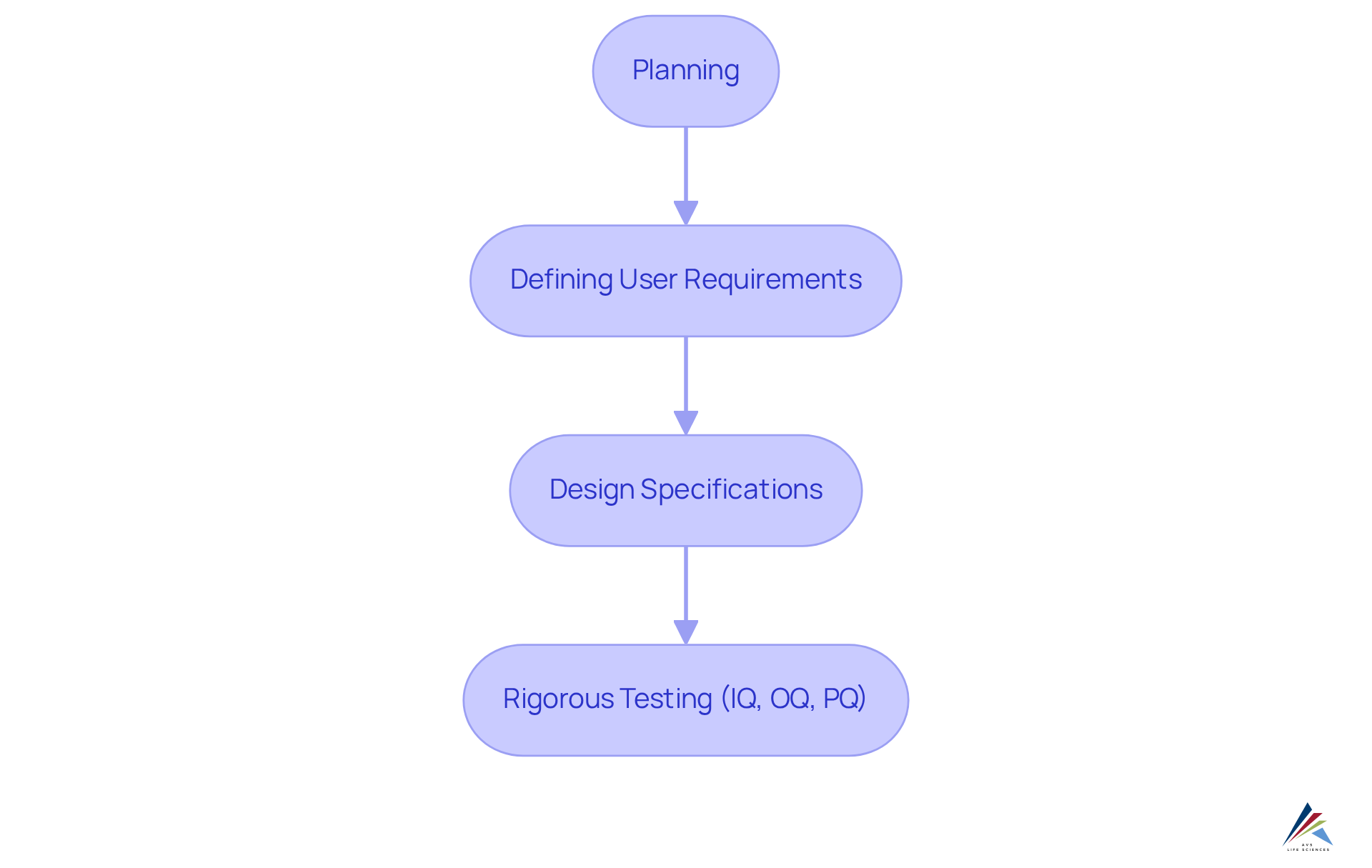
To achieve this, organizations should adopt a structured approach akin to the computer application validation (CSV) process, which encompasses stages such as:
- Planning
- Defining user requirements
- Design specifications
- Rigorous testing (IQ, OQ, PQ)
By adhering to these best practices, organizations can ensure that their operations function as intended and that they are well-prepared to meet regulatory demands.
Provide Training and Support: Essential for Effective Emission Monitoring Implementation
Thorough training and assistance are vital for the effective execution of the emission monitoring system in the pharmaceutical industry. Workers must acquire the to manage these systems efficiently, analyze data accurately, and respond swiftly to regulatory matters. Investing in robust training programs significantly enhances oversight capabilities, ensuring that staff are well-prepared to tackle regulatory challenges.
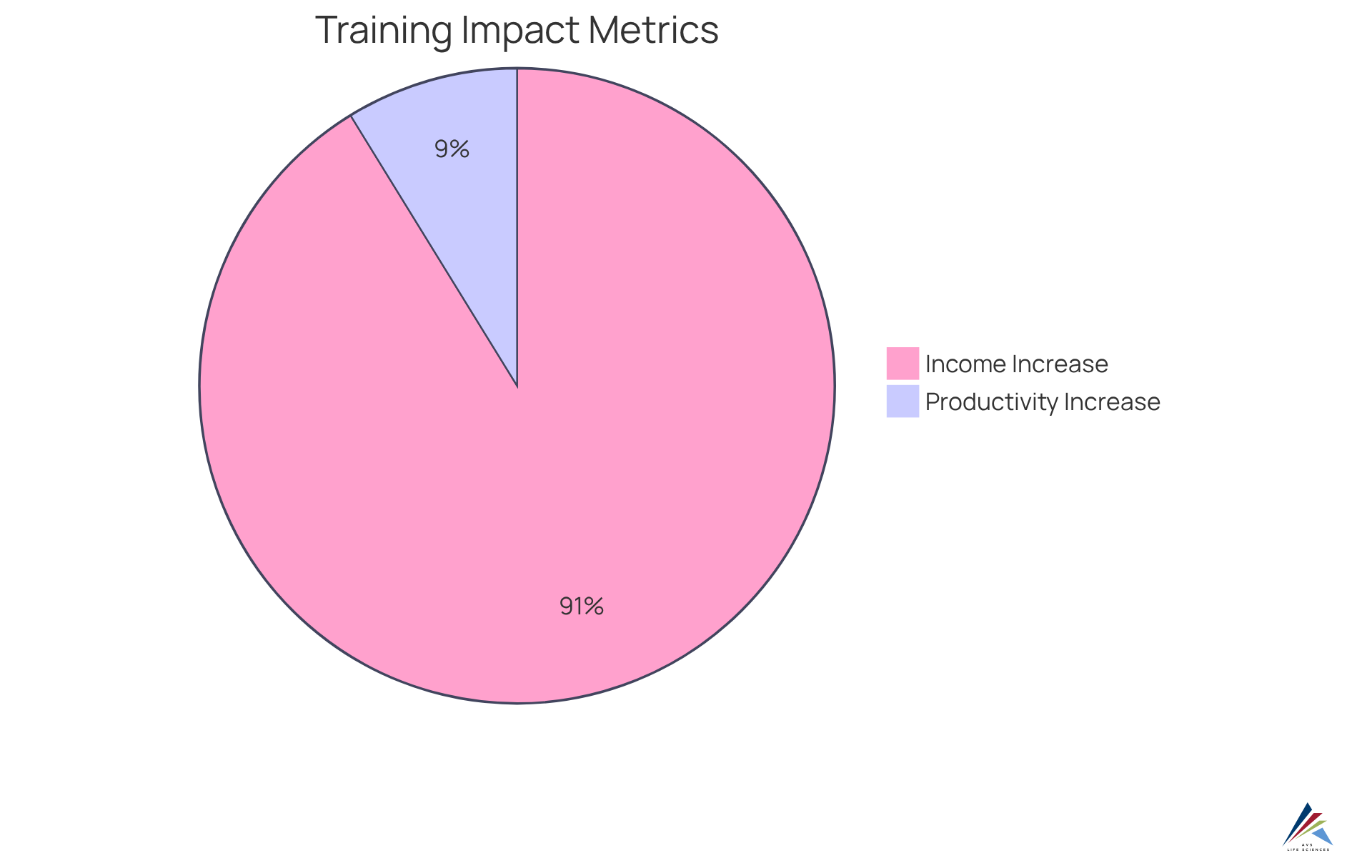
Organizations that prioritize employee education within their emission monitoring system have reported improved adherence rates and fewer instances of non-conformity. Notably, statistics reveal that companies with effective training programs experience a 21% increase in productivity and a remarkable 218% higher income per employee compared to those lacking structured training.
Furthermore, ongoing education fosters a culture of compliance, empowering employees to take responsibility for their roles in upholding regulatory standards. By equipping their workforce with the appropriate tools and knowledge, pharmaceutical companies can not only enhance their oversight but also contribute to a more sustainable and responsible industry.
Reinforce Compliance: The Comprehensive Benefits of Emission Monitoring Systems
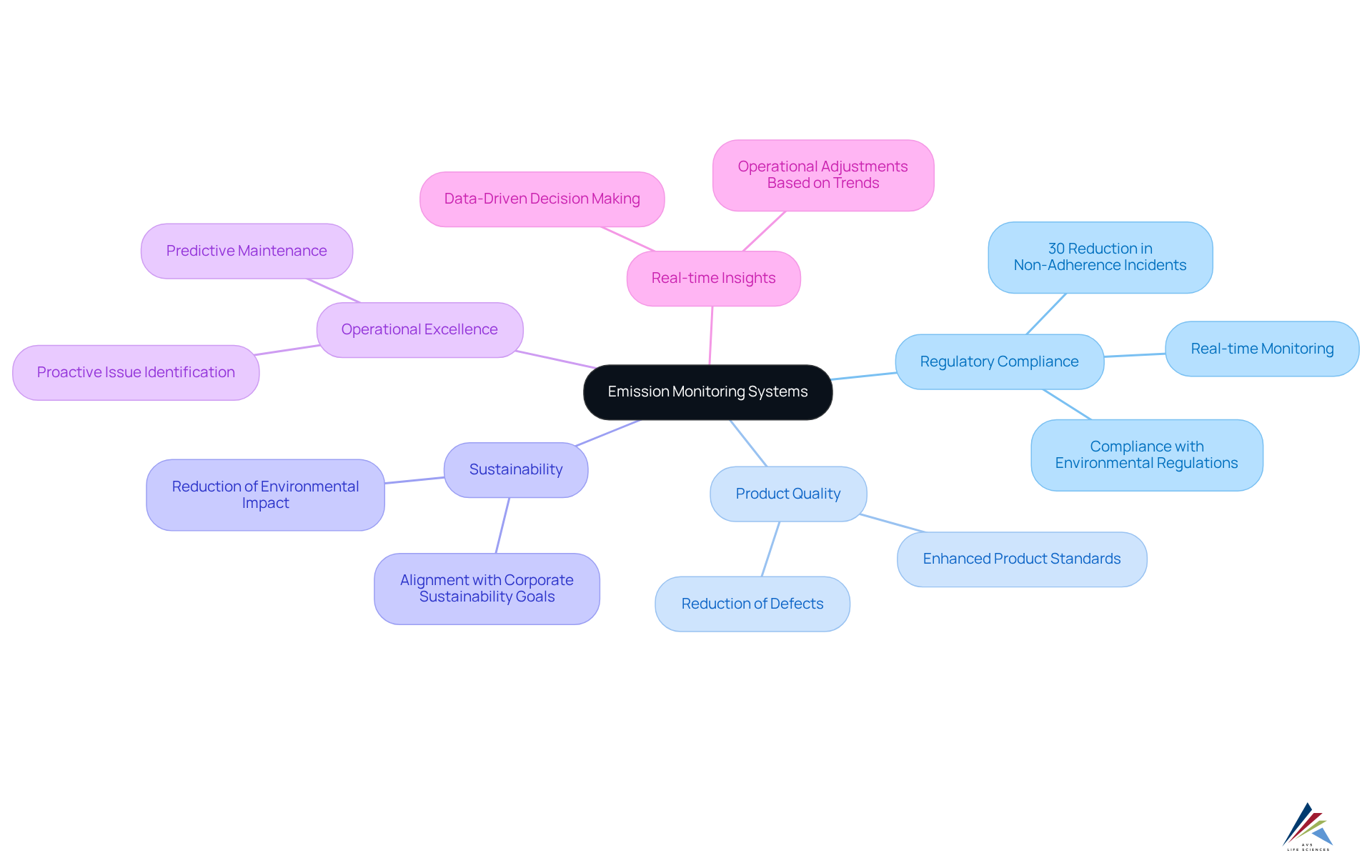
The pharmaceutical sector benefits greatly from emission monitoring systems, which are essential for enhancing adherence and provide numerous advantages that extend beyond mere regulatory compliance. The emission monitoring system not only ensures adherence to stringent environmental regulations but also elevates product quality and fosters sustainability.
Companies employing advanced Continuous Emissions Tracking Systems (CETS) have reported notable improvements in adherence rates; studies indicate that organizations implementing robust tracking programs can reduce non-adherence incidents by as much as 30%. Moreover, the emission monitoring system contributes to operational excellence by providing real-time insights that inform decision-making and operational adjustments. This proactive approach enables pharmaceutical manufacturers to identify potential issues before they escalate, thereby mitigating risks and ensuring .
As noted by industry experts, "Effective environmental tracking necessitates comprehensive preparation and planning," which highlights the importance of integrating the emission monitoring system into operational frameworks. Navigating compliance complexities is further supported by insights gained from pollution assessments. By understanding emission trends, companies can make informed adjustments to their processes, aligning with both regulatory mandates and corporate sustainability goals.
The integration of cloud-based data management platforms enhances this capability, enabling advanced trend analysis and predictive maintenance of monitoring equipment. In conclusion, the strategic deployment of an emission monitoring system not only bolsters compliance but also drives operational excellence, positioning pharmaceutical companies for sustained success in an ever-evolving regulatory landscape.
Conclusion
The integration of emission monitoring systems within the pharmaceutical industry transcends mere regulatory compliance; it signifies a substantial leap forward in operational efficiency, product quality, and environmental stewardship. By harnessing these systems, companies can not only meet stringent industry standards but also elevate their overall performance and sustainability initiatives.
This article has underscored the pivotal advantages of emission monitoring systems, including their crucial role in:
- Ensuring regulatory compliance
- Enhancing product quality
- Achieving cost-effectiveness
- Upholding safety standards
Furthermore, the embrace of innovative technologies alongside comprehensive training programs highlights the necessity of precise data and effective implementation in attaining compliance and operational excellence.
Given these insights, it is essential for pharmaceutical organizations to prioritize the integration of emission monitoring systems. By doing so, they not only mitigate the risk of regulatory penalties but also establish themselves as frontrunners in sustainability and quality management. The adoption of these systems will ultimately foster a healthier environment and a more accountable industry, reinforcing the indispensable link between compliance and corporate success.
Frequently Asked Questions
What solutions does AVS Life Sciences offer for emission monitoring in the pharmaceutical sector?
AVS Life Sciences provides a comprehensive suite of pollution oversight solutions, including an emission monitoring system specifically tailored for the pharmaceutical sector, ensuring compliance with regulatory standards and enhancing operational efficiency.
How do modern pollution control technologies impact operational costs?
Modern pollution control technologies, such as Regenerative Thermal Oxidizers (RTO), can significantly decrease fuel costs compared to traditional afterburners, achieving savings of up to $149.69 per hour for a 10,000 SCFM system, while also reducing environmental impact by achieving destruction rates of volatile organic compounds (VOCs) exceeding 99%.
What innovations have been made in gas tracking for emissions management?
Recent innovations include the implementation of vent control software that optimizes the management of VOC concentrations during production, incorporating an emission monitoring system to ensure effective emissions control and mitigate the risk of exceeding regulatory thresholds.
Can you provide an example of successful implementation of pollution tracking tools in the pharmaceutical industry?
A leading pharmaceutical manufacturer transitioned to RTO technologies, resulting in a substantial reduction in VOC emissions and compliance with rigorous environmental regulations, demonstrating the effectiveness of pollution tracking tools.
How do emission monitoring systems help with regulatory compliance in pharmaceuticals?
Emission monitoring systems are essential for maintaining compliance with regulations such as Good Manufacturing Practices (GMP) and ISO standards, providing real-time insights into pollutants and enabling proactive identification and resolution of regulatory issues.
What are the financial implications of non-compliance with emission regulations?
The financial repercussions of non-compliance can be immense, with potential penalties reaching millions of dollars, in addition to significant reputational harm.
How does emission monitoring contribute to product quality in pharmaceutical manufacturing?
Consistent use of an emission monitoring system allows organizations to swiftly identify potential pollutants, minimizing risks that could compromise product safety, thereby enhancing overall product quality metrics and fostering a culture of quality.
What impact did AVS Life Sciences have on a biotechnology company’s manufacturing facility?
AVS Life Sciences supported a biotechnology company in upgrading their manufacturing space to a GMP Level 2 facility, resulting in a 30% reduction in contamination incidents and a 25% enhancement in overall product quality metrics.
