9 Essential Strategies for Effective Deviation Management

Overview
The primary objective of this article is to delineate critical strategies for effective deviation management within organizations, with a specific focus on the life sciences sector. It underscores the necessity of establishing explicit policies, defining roles and responsibilities, implementing comprehensive training programs, maintaining meticulous documentation, and leveraging technology to bolster compliance and operational efficiency. These measures collectively contribute to cultivating a culture of quality and continuous improvement, essential for navigating the complexities of the industry.
Introduction
Effective deviation management stands as a cornerstone for organizations aiming to uphold compliance and ensure product integrity within the highly regulated life sciences sector. By implementing strategic practices, companies can adeptly navigate the complexities of discrepancies, thereby enhancing operational efficiency and cultivating a culture of continuous improvement. Yet, as challenges emerge, the pressing question arises: how can organizations effectively balance adherence to regulatory standards with the imperative for innovation and flexibility in their processes? This article delves into nine essential strategies that empower organizations to master deviation management, ensuring they remain resilient and compliant in an ever-evolving landscape.
AVS Life Sciences: Comprehensive Solutions for Deviation Management
AVS Life Sciences offers a robust framework for addressing compliance challenges that integrates quality oversight, regulatory adherence, and engineering solutions. By leveraging our global consulting expertise in GxP, ISO, and QSR regulations, we empower organizations to navigate the complexities of discrepancies through effective deviation management, ensuring alignment with industry standards.
Our consultants have consistently proven their capability to meet project deadlines while maintaining operational efficiency, as demonstrated by successful project outcomes, including validation support that achieved zero findings during audits.
Through AVS's comprehensive , clients can significantly enhance their operational efficiency and ensure effective deviation management throughout the product lifecycle. This is crucial for mitigating risks and cultivating a culture of continuous improvement, prompting organizations to engage with our services for optimal results.
Establish Clear Policies and Procedures for Deviation Management
To effectively address compliance challenges, organizations must establish and implement clear policies and procedures. These guidelines should delineate the processes for recognizing, recording, and managing discrepancies, emphasizing the importance of deviation management, ensuring that all personnel understand their roles and responsibilities. Regular assessments and revisions of these policies are crucial; research indicates that approximately 40% of companies refresh their management procedures annually. This practice not only aligns with but also bolsters compliance within the pharmaceutical sector. Clear procedures are essential for maintaining quality standards and ensuring that deviation management is conducted consistently and effectively, ultimately safeguarding product integrity and patient safety.
AVS Life Sciences offers comprehensive GXP regulatory services, including Quality Event Management and Compliance Audits, to assist organizations in developing and executing these critical policies. By leveraging technology-driven solutions, entities can streamline policy oversight and remain compliant in a rapidly changing regulatory landscape.
Consider the impact of effective compliance strategies: organizations that prioritize these practices not only enhance their operational efficiency but also build trust with stakeholders. Engage with AVS Life Sciences to fortify your compliance framework and ensure your organization thrives in today’s complex regulatory environment.
Define Roles and Responsibilities in Deviation Management
In the realm of deviation management, it is imperative to delineate specific roles and responsibilities for all team members involved in the computer system validation process. This includes assigning accountability for variance reporting, investigation, and resolution—critical components of the validation phases outlined in the V-Model from the GAMP 5 Guide. By clarifying these roles, organizations can ensure that discrepancies are addressed promptly and effectively, thereby mitigating the risk of non-compliance.
Each stage of the validation process—planning, defining user requirement specifications (URS), design specifications, building and configuring the system, and conducting installation (IQ), operational (OQ), and performance qualification (PQ) testing—demands clear accountability to guarantee that all actions are documented and adhere to regulatory standards.
This systematic approach not only facilitates but also supports deviation management, fostering a comprehensive understanding of the validation process and ensuring that all team members are synchronized in their responsibilities.

Implement Training and Competency Programs for Staff
Organizations must implement comprehensive training and competency programs focused on deviation management, highlighting the critical importance of GXP and FDA regulations. These programs should encompass the necessary policies, procedures, and tools that empower staff to effectively recognize and manage discrepancies through deviation management, including adherence to (SOPs) and data integrity practices.
Regular training sessions are vital; they reinforce compliance and keep staff informed about regulatory changes, ensuring that they remain well-versed in the latest documentation practices and internal auditing techniques.
By investing in these training initiatives, organizations not only enhance their compliance posture but also foster a culture of accountability and excellence.
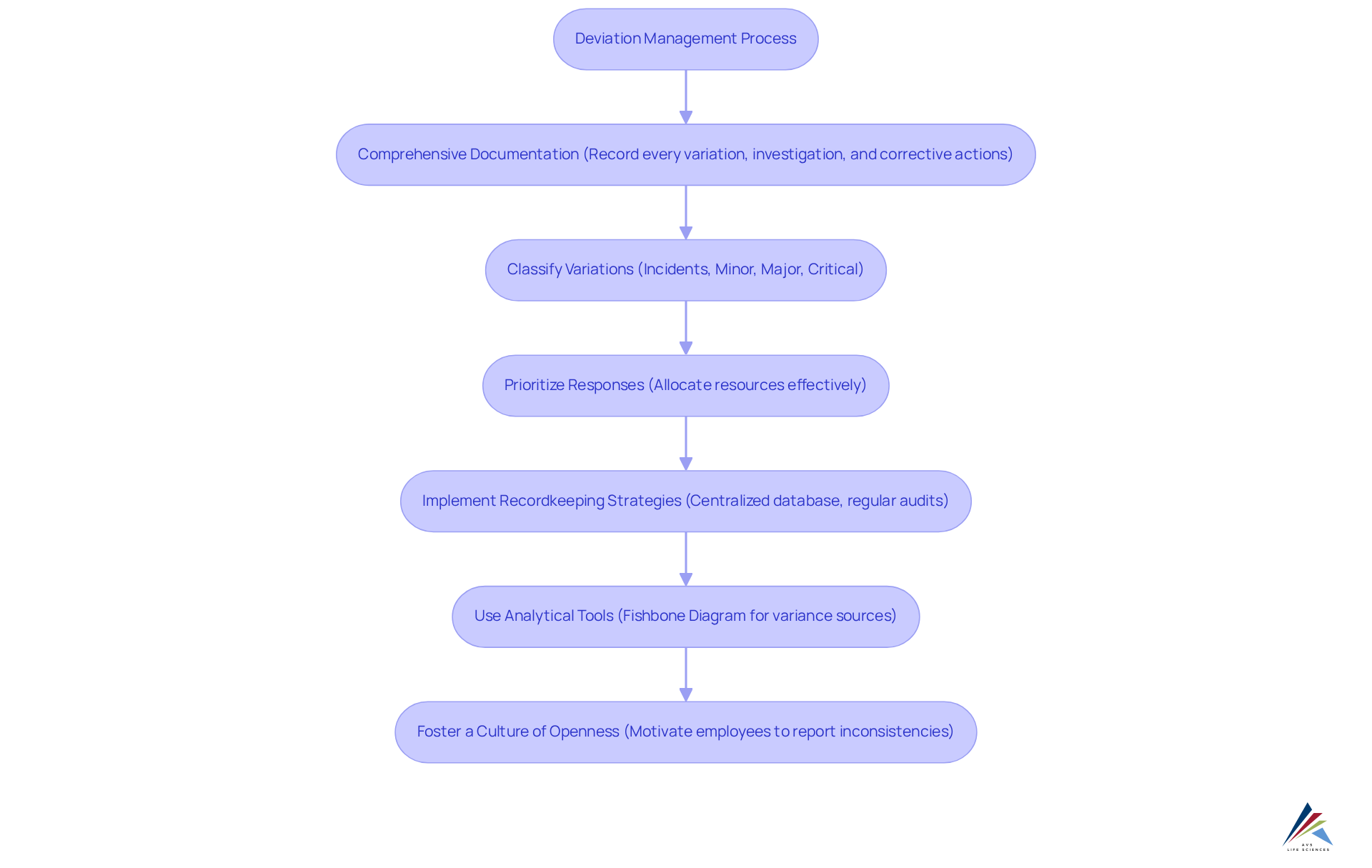
Maintain Comprehensive Documentation and Recordkeeping
Thorough documentation and careful recordkeeping are essential components of efficient deviation management in the pharmaceutical industry. Organizations must meticulously record every variation as part of their deviation management, detailing its nature, the investigation process, and the corrective actions implemented. This comprehensive documentation not only ensures compliance with regulatory standards but also serves as a valuable resource for deviation management while identifying trends and driving future improvements.
By utilizing deviation management to classify variations into incidents, minor, major, and critical categories, companies can prioritize their responses and allocate resources effectively. In our collaboration with a leading biotechnology company, we implemented successful recordkeeping strategies, including a centralized database and regular audits, which significantly enhanced the accuracy and accessibility of records.
Furthermore, we employed analytical tools such as the Fishbone Diagram to examine the sources of variances, determining whether they originated from personnel, processes, or equipment. This strategic approach allowed us to capture important lessons learned, prompting the QC laboratory team to evaluate their business processes and identify gaps that contributed to unreliable test results.
Moreover, fostering a culture of openness and responsibility motivates employees to participate in deviation management by reporting inconsistencies swiftly, which is crucial for upholding high-quality standards. Ultimately, not only safeguard compliance but also contribute to the overall efficiency and reliability of pharmaceutical operations.
Conduct CAPA and Root Cause Analysis for Continuous Improvement
Organizations face significant compliance challenges that necessitate thorough CAPA and root cause analysis, alongside effective deviation management for every irregularity. By leveraging AVS Life Sciences' expertise in deviation management, entities can effectively identify the underlying causes of discrepancies. This process not only involves recognizing these root causes but also focuses on deviation management by implementing to prevent recurrence. Concentrating on ongoing enhancement and applying effective methods from AVS Life Sciences enables organizations to improve their quality assurance systems. As a result, they can significantly lessen the chance of future discrepancies, fostering a culture of compliance and excellence.
Engage Third-Party Consultants for Expert Deviation Management Support
Engaging third-party consultants can significantly improve a company's deviation management efforts. These experts bring specialized knowledge and extensive experience, enabling organizations to identify gaps in their processes and implement effectively. Their objective viewpoint aids in conducting more comprehensive audits and regulatory evaluations.
For instance, a global pharmaceutical firm that collaborated with specialist advisors successfully eliminated substantial discrepancies and CAPA backlogs, demonstrating the efficacy of external assistance in maintaining regulatory compliance and operational efficiency.
Furthermore, consultants can provide customized training programs that address organizational policies, regulatory requirements, and best practices, ensuring that staff are well-prepared for deviation management. It is crucial that variances are reported promptly after they occur, ideally within 2 to 4 hours, to uphold regulations and ensure product quality.
By leveraging the knowledge and techniques of experienced experts, companies can cultivate a culture of continuous improvement, enhance communication and teamwork among staff, and utilize automated tools for real-time monitoring of inconsistencies, ultimately strengthening regulatory adherence.
Conduct Regular Audits and Reviews of Deviation Management Processes
Frequent evaluations and assessments of deviation management procedures are essential for ensuring adherence and operational efficiency in the life sciences field. These evaluations must rigorously assess compliance with established policies and procedures, identify areas needing improvement, and confirm that corrective actions are effectively implemented. By conducting these evaluations, organizations can proactively address discrepancies through deviation management, fostering a culture of continuous improvement that aligns with industry standards.
To enhance the effectiveness of these audits, AVS Life Sciences recommends employing specific methodologies, such as:
- Risk-based assessments
- Root cause analysis
Furthermore, organizations should consider best practices, including:
- Engaging cross-functional teams in the audit process
- Routinely refreshing training for staff on protocol handling
This proactive approach not only enhances compliance but also fortifies overall operational integrity, ultimately leading to improved outcomes in product quality and safety.
Utilize Technology Solutions for Streamlined Deviation Management
Implementing advanced technology solutions is essential for optimizing variance oversight processes, significantly enhancing both efficiency and accuracy. Organizations can adopt software tools designed for real-time monitoring of discrepancies, which not only automate documentation but also improve communication among team members. Research indicates that incorporating personal data in training datasets can yield a 10% improvement in accuracy, underscoring the effectiveness of customized software solutions in managing discrepancies. This technology integration enables faster response times to deviations, thereby improving deviation management and ensuring that regulatory standards are met more effectively.
As Shaibal Barua notes, understanding the factors contributing to compliance challenges is crucial for effective management. Moreover, utilizing these tools can enhance deviation management, leading to a more streamlined workflow, reducing the risk of human error and facilitating better oversight of compliance-related activities. To maximize benefits, entities should consider implementing specific software solutions, such as [insert specific software tools], which are designed to . Consequently, organizations can uphold high-quality standards while adeptly managing the complexities of regulatory requirements.
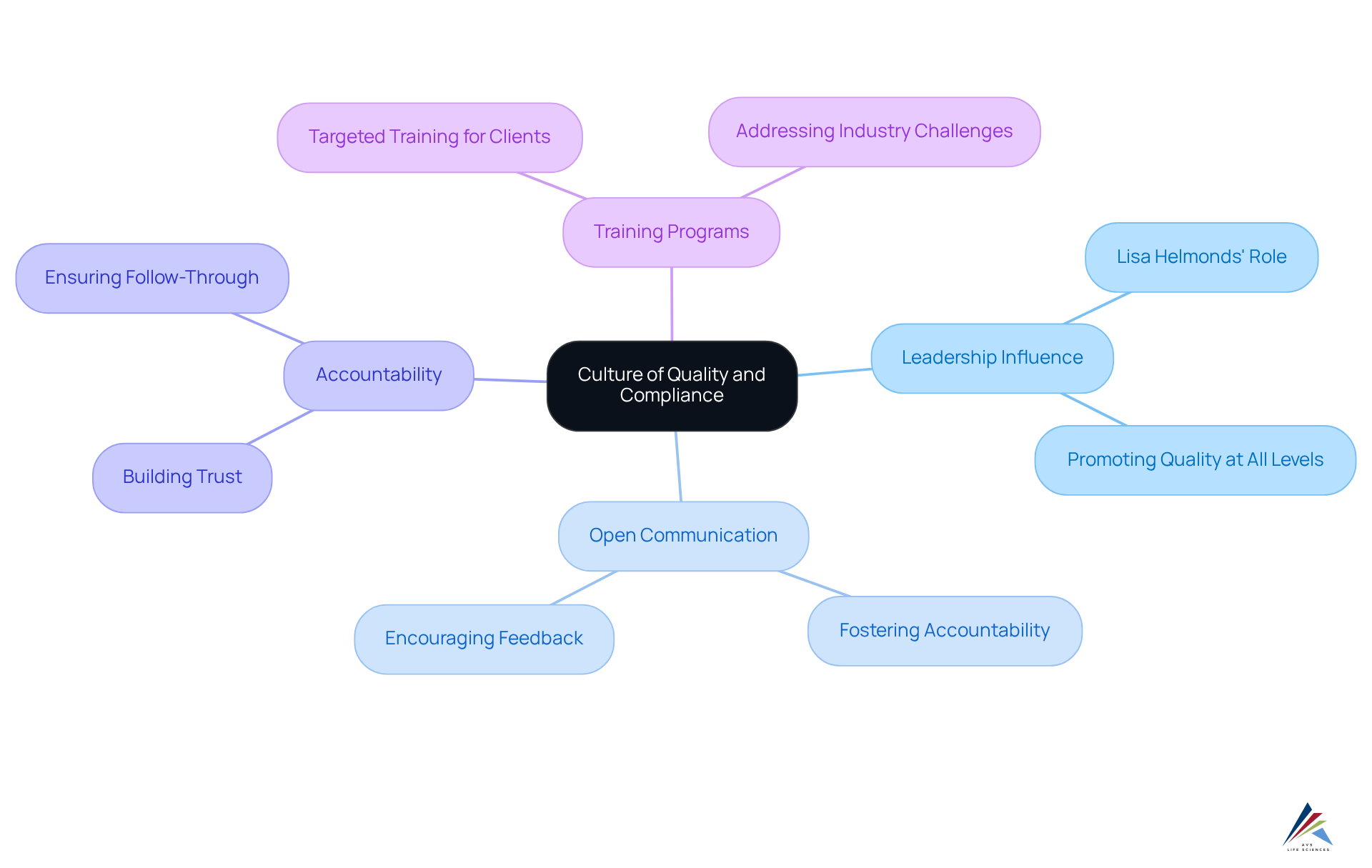
Foster a Culture of Quality and Compliance in Deviation Management
Nurturing a culture of excellence and adherence within the entity is essential for effective variation oversight. Leadership plays a pivotal role in this endeavor, as exemplified by professionals like Lisa Helmonds, VP of Quality & Operations at AVS Life Sciences. It is imperative that leaders promote the importance of compliance and quality at all levels, fostering an environment of open communication and accountability.
With over 30 years of experience in Manufacturing and Quality Assurance, Lisa focuses on developing relationships with consulting clients and implementing . She possesses a profound understanding of the challenges facing the pharmaceutical and medical device industries.
By instilling a culture that prioritizes quality, organizations can significantly enhance their deviation management, thereby ensuring long-term success.
Conclusion
Effective deviation management is crucial for organizations striving to uphold compliance and maintain high-quality standards in the pharmaceutical industry. By adopting a comprehensive framework that emphasizes clear policies, defined roles, and a culture of quality, businesses can adeptly navigate the complexities of regulatory challenges while ensuring patient safety and operational excellence.
This article outlines essential strategies for successful deviation management, including:
- The establishment of robust policies
- Implementation of training programs
- The significance of thorough documentation
Engaging third-party consultants and conducting regular audits significantly enhance an organization’s ability to identify discrepancies and implement corrective actions. Moreover, leveraging technology solutions streamlines processes, facilitating real-time monitoring and more efficient management of deviations.
Ultimately, fostering a culture of quality and compliance is paramount. Organizations that prioritize these strategies not only improve their deviation management but also build trust with stakeholders and enhance their overall operational integrity. By committing to these best practices, businesses can ensure long-term success and resilience in an ever-evolving regulatory landscape.
Frequently Asked Questions
What solutions does AVS Life Sciences offer for deviation management?
AVS Life Sciences provides a robust framework that integrates quality oversight, regulatory adherence, and engineering solutions to effectively manage compliance challenges and discrepancies throughout the product lifecycle.
How does AVS Life Sciences ensure project efficiency and compliance?
AVS Life Sciences employs global consulting expertise in GxP, ISO, and QSR regulations, consistently meeting project deadlines while achieving operational efficiency, as evidenced by successful project outcomes with zero findings during audits.
Why is establishing clear policies and procedures important for deviation management?
Clear policies and procedures are essential for recognizing, recording, and managing discrepancies, ensuring that all personnel understand their roles and responsibilities, and maintaining compliance with evolving regulatory requirements.
How often should organizations reassess their deviation management policies?
Research indicates that approximately 40% of companies refresh their management procedures annually to align with evolving regulatory requirements and bolster compliance.
What services does AVS Life Sciences provide to assist with regulatory compliance?
AVS Life Sciences offers comprehensive GxP regulatory services, including Quality Event Management and Compliance Audits, to help organizations develop and execute critical policies for effective deviation management.
What is the significance of defining roles and responsibilities in deviation management?
Clearly delineating roles and responsibilities ensures accountability for variance reporting, investigation, and resolution, which is crucial for addressing discrepancies promptly and effectively, thus mitigating the risk of non-compliance.
What stages of the validation process require clear accountability?
The validation process includes planning, defining user requirement specifications (URS), design specifications, building and configuring the system, and conducting installation (IQ), operational (OQ), and performance qualification (PQ) testing.
How does AVS Life Sciences support organizations in maintaining compliance?
By leveraging technology-driven solutions and providing guidance on establishing policies, AVS Life Sciences helps organizations streamline oversight and ensure compliance in a rapidly changing regulatory landscape.
