8 Key Quality by Design Strategies for Effective Validation

Overview
The article examines eight essential strategies for implementing Quality by Design (QbD) in the validation processes within the pharmaceutical sector. By addressing compliance challenges, these strategies—such as:
- Establishing a robust design space
- Conducting comprehensive knowledge reviews
- Integrating QbD into quality management systems
collectively enhance product quality and compliance. Furthermore, they significantly reduce batch failures, thus tackling major obstacles in the validation landscape. Ultimately, these insights provide a pathway for industry professionals to engage effectively with compliance solutions, ensuring a more robust validation framework.
Introduction
In an industry where precision and compliance are paramount, the integration of Quality by Design (QbD) strategies has emerged as a transformative approach for validation in the pharmaceutical and biotechnology sectors. By adopting these innovative practices, organizations not only enhance product quality but also streamline their compliance processes, leading to significant reductions in batch failures.
However, the journey to effective validation is fraught with challenges, particularly in fragmented operations where technology integration and knowledge transfer can hinder progress.
How can organizations overcome these obstacles to fully realize the benefits of QbD in their validation efforts? This question serves as a critical starting point for understanding the necessity of robust compliance solutions in today’s complex regulatory landscape.
AVS Life Sciences: Comprehensive QbD Solutions for Validation
AVS Life Sciences offers a comprehensive suite of solutions for quality by design for validation (qbd) tailored for efficient verification in the pharmaceutical and biotechnology sectors. This innovative approach integrates management principles with regulatory compliance and emphasizes quality by design for validation (qbd), empowering clients to navigate the complexities of validation processes effectively.
Organizations that adopt quality by design for validation (qbd) strategies experience a [significant reduction in batch failures and deviations](https://pharmalesson.com/pharmaceutical-development-quality-by-design-summary), thereby enhancing product quality and compliance. By leveraging extensive industry knowledge, AVS Life Sciences facilitates the implementation of quality by design for validation (qbd) strategies that enhance quality and streamline compliance throughout the lifecycle of their products.
Emphasizing quality by design for validation (qbd) cultivates a proactive quality culture, ensuring that products satisfy established quality attributes and regulatory requirements from the very beginning. This dedication positions AVS Life Sciences as a frontrunner in executing quality by design for validation (qbd) strategies within the pharmaceutical evaluation landscape.
Yet, challenges persist in the broader adoption of quality by design for validation (qbd), particularly in fragmented operations where and knowledge transfer can pose difficulties. To successfully implement quality by design for validation (qbd) strategies, organizations must develop a robust Quality Management System (QMS) that enables real-time adjustments and continuous improvement, along with services such as:
- Compliance Audits & Gap Assessments
- Quality Event Management

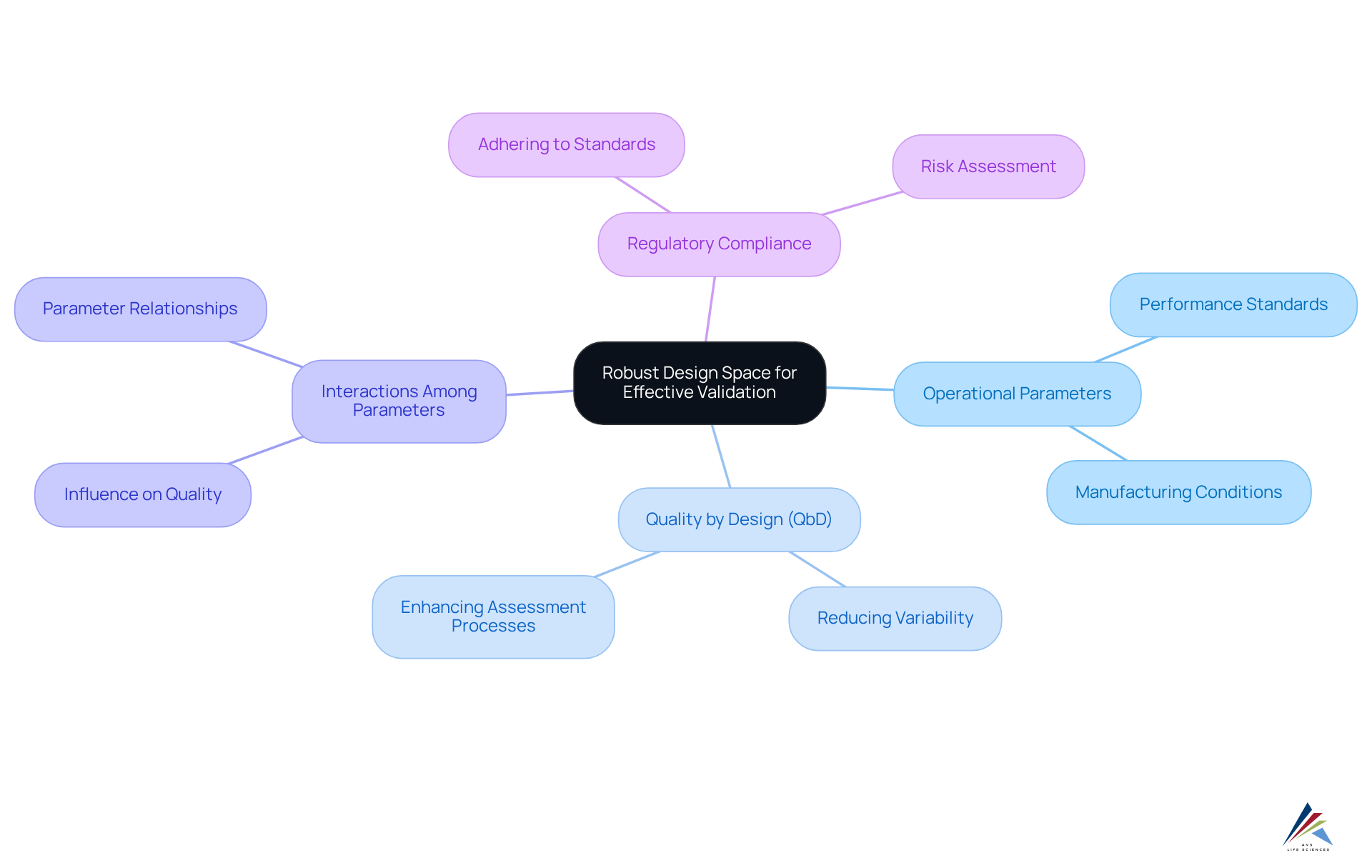
Establish a Robust Design Space for Effective Validation
Establishing a robust design space is essential for effective validation in pharmaceutical manufacturing, especially when applying quality by design for validation (qbd). This process involves recognizing and outlining the operational parameters under which an item can be manufactured, all while adhering to stringent performance standards.
Understanding the interactions among different process parameters is crucial, as these connections significantly influence the quality by design for validation (qbd) of the outcome. By clearly defining a design area, organizations can enhance their assessment processes by implementing quality by design for validation (qbd), reduce variability, and ensure that items consistently meet regulatory requirements.
This tactical approach not only improves operations but also contributes to higher success rates, ultimately leading to enhanced standards and reliability. As Robert L. Peters aptly states, 'Design creates culture. Culture shapes values. Values determine the future,' emphasizing the profound impact that a can have on the overall quality and efficacy of pharmaceutical products.
Conduct Thorough Existing Knowledge Reviews for Validation Success
Conducting thorough reviews of current knowledge is essential for success in verification. This involves methodically collecting and examining historical data, prior assessment outcomes, and relevant scientific literature. By doing so, organizations can identify best practices, recognize potential pitfalls, and pinpoint areas for improvement.
For instance, AVS Life Sciences recently assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade enabled the client to manufacture medication with lentivirus vector material. Notably, this project adhered to strict timelines and budgets, while emphasizing the importance of thorough documentation and traceability, as acknowledged by the client’s quality assurance team.
Incorporating these insights into current verification strategies enhances decision-making processes and significantly increases the likelihood of successful outcomes. Statistics indicate that organizations utilizing historical data in their verification efforts experience higher success rates, underscoring the essential role of informed knowledge assessments. Insights from industry leaders further highlight that a strong understanding of previous assessment efforts can lead to more effective strategies, ultimately fostering a culture of continuous improvement and innovation in assessment practices.
To maximize validation success, organizations should regularly review and update their , ensuring that lessons learned are effectively integrated into future projects.

Implement Design Deconstruction to Meet Specifications
Implementing design deconstruction demands a meticulous analysis of item specifications, breaking them down into individual components or processes. This method not only allows teams to assess the impact of each component on overall item standards and adherence but also addresses compliance challenges head-on. By verifying each element individually, organizations can systematically ensure that the final outcome meets all regulatory requirements and standards. This approach significantly enhances adherence and profoundly influences the overall quality of the item, as each verified component plays a critical role in fulfilling the stringent requirements of the pharmaceutical sector with a focus on quality by design for validation (qbd). Ultimately, this structured analysis not only but also fortifies the integrity of the entire process, establishing a robust framework for success.

Utilize NIRS for Effective Sample Interface Design
Integrating Near-Infrared Spectroscopy (NIRS) into sample interface design empowers organizations to monitor and analyze both the physical and chemical properties of samples in real-time. This advanced method provides essential insights into item excellence and consistency, which supports the principles of quality by design for validation (QbD) and enables teams to make knowledgeable choices during the validation process.
By utilizing NIRS, companies can greatly improve their adherence to compliance standards, including GXP and FDA guidelines, while also enhancing overall item excellence. The ability of NIRS to provide rapid results with minimal sample preparation makes it an invaluable tool in the pharmaceutical landscape.
Research has demonstrated that NIRS can effectively track essential characteristics, ensuring that items meet strict standards. Moreover, NIRS is acknowledged by oversight organizations like the FDA, which declares that "NIRS is a highly relevant tool for achieving control when built-in standards are favored over testing standards," emphasizing its function in attaining built-in standards over testing standards.
This dedication to maintaining standards not only simplifies verification processes but also encourages increased trust in product integrity. Additionally, validated NIRS methods provide efficient monitoring with minimal sample destruction, enhancing cost-effectiveness in pharmaceutical operations.
The verification process for NIRS devices, which includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), ensures adherence to industry standards and aligns with AVS Life Sciences' comprehensive quality management and compliance solutions by implementing quality by design for validation (QbD).
To maximize the benefits of NIRS, organizations should also implement and standard operating procedures (SOPs) to support their assessment strategies.

Conduct Feasibility Studies to Assess Validation Strategies
Carrying out feasibility studies is essential for evaluating the practicality of proposed verification strategies, particularly regarding resource allocation, timelines, and regulatory compliance. By systematically identifying potential challenges and limitations, organizations can refine their assessment approaches, ensuring they are well-prepared for the complexities of the evaluation process. This proactive strategy not only mitigates risks but also significantly enhances the likelihood of . As the pharmaceutical landscape evolves in 2025, the ability to assess the practicality of verification strategies will be vital for maintaining compliance and achieving operational efficiency.

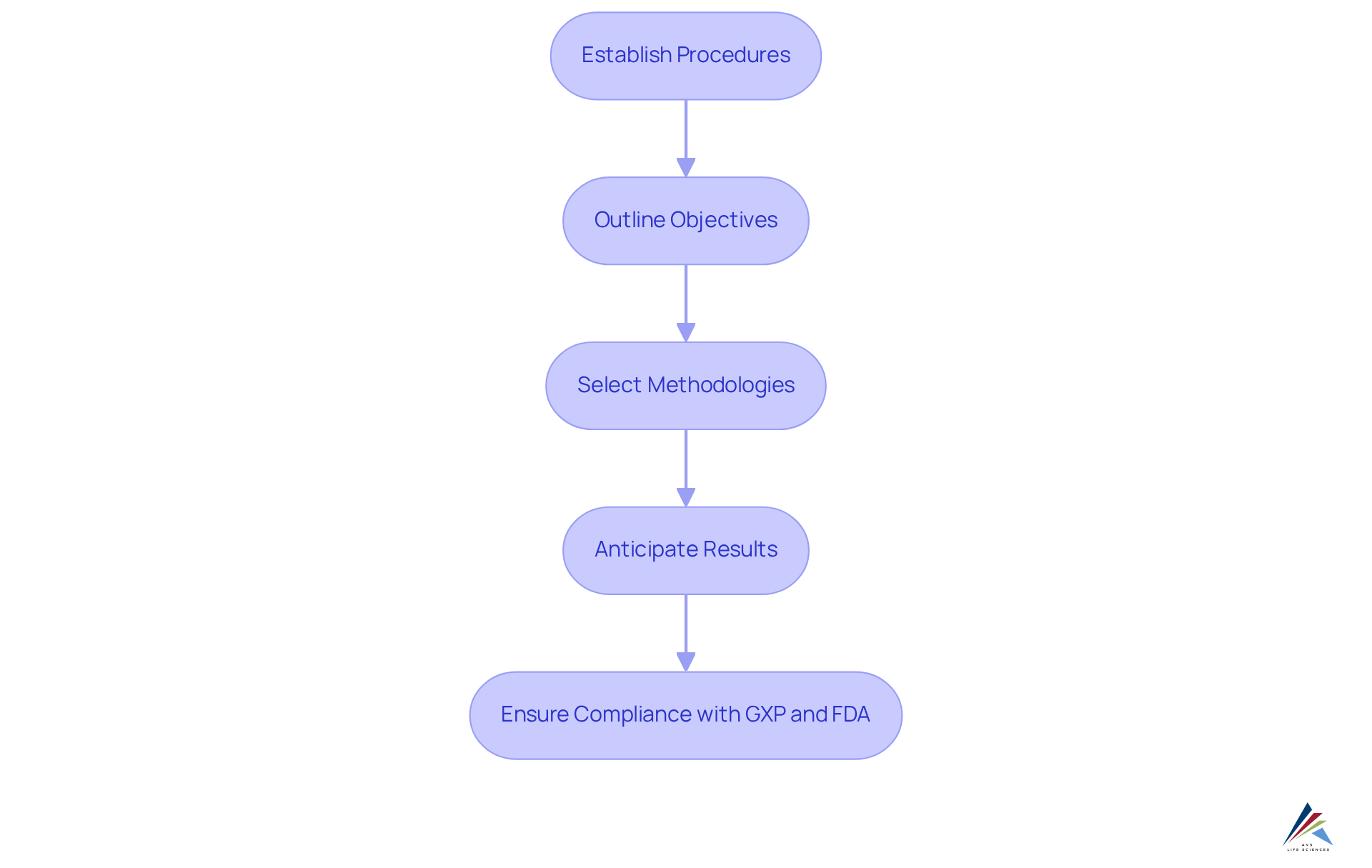
Develop and Design Experiments for Robust Validation
Establishing organized procedures for developing and designing experiments is essential for achieving robust verification in the pharmaceutical sector. These protocols must clearly outline objectives, methodologies, and anticipated results, ensuring that experiments generate .
Research indicates that well-structured verification experiments significantly boost success rates, with systematic approaches yielding more consistent and credible outcomes. By adhering to established scientific principles and compliance standards, including GXP and FDA regulations, organizations can enhance the reliability of their verification results.
Moreover, effective experimental design not only minimizes variability but also aligns closely with regulatory expectations, thereby reinforcing the integrity of the verification process. The systematic nature of organized protocols is vital for ensuring that verification efforts are both effective and compliant with necessary standards.
Ensure Validation and Implementation of QbD Principles
Incorporating Quality by Design (QbD) principles into every phase of the development process is essential for addressing compliance challenges effectively. This approach requires:
- A clear outline of attribute characteristics
- The creation of design areas
- The execution of comprehensive verification tasks
By integrating quality by design for validation (qbd) principles into their validation strategies, organizations significantly enhance their quality and compliance outcomes. This proactive methodology not only improves the likelihood of successful compliance submissions but also aligns with the FDA's emphasis on patient-centered drug development, which incorporates patient feedback into the Target Product Quality Profile (TPQP).
Moreover, the implementation of QbD has been shown to decrease batch failures by 40%, underscoring its critical role in ensuring item consistency and compliance success rates. Ultimately, a commitment to embedding quality attributes throughout product development cultivates a culture of , leading to superior outcomes in the highly regulated life sciences industry. Engaging with AVS Life Sciences can further empower organizations to navigate these complexities and achieve excellence in compliance.

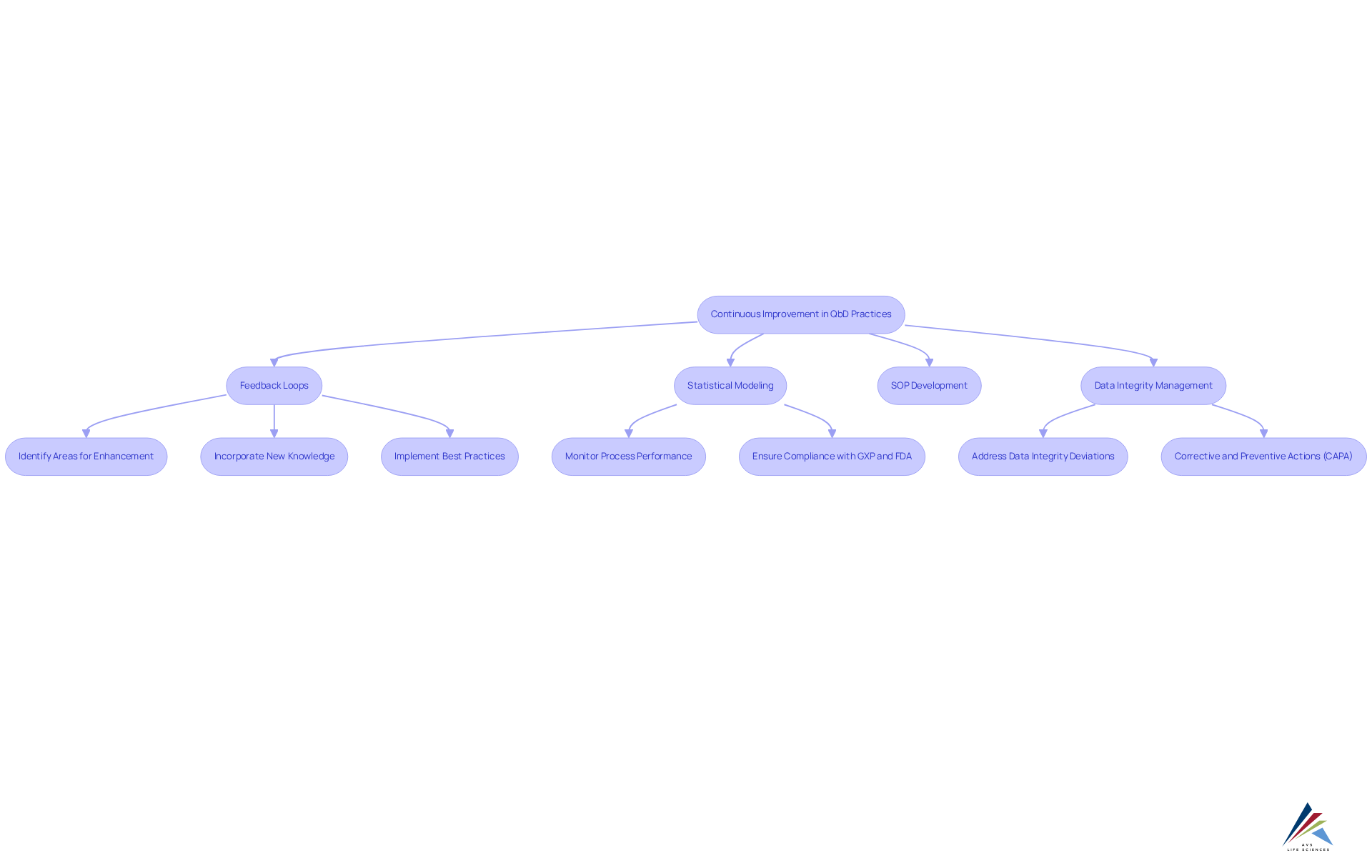
Prioritize Continuous Improvement in QbD Practices
Focusing on ongoing enhancement in quality by design for validation (qbd) practices is essential for organizations navigating the complexities of compliance. This entails frequently assessing and revising assurance strategies to incorporate new knowledge, technologies, and compliance obligations, particularly those specified by GXP and FDA guidelines. To effectively address compliance challenges, organizations should establish feedback loops that facilitate the identification of areas for enhancement and the implementation of . This includes the development of robust Standard Operating Procedures (SOPs). Furthermore, it is crucial to address Data Integrity Deviations and Corrective and Preventive Actions (CAPA) as integral components of these practices.
Incorporating statistical modeling, such as Control Charts and Capability Indices, plays a vital role in monitoring process performance and ensuring adherence to regulatory standards. This dedication to ongoing enhancement guarantees that verification processes remain efficient and aligned with industry standards, particularly in relation to quality by design for validation (qbd) and Good Manufacturing Practices (GMP). As noted by Mark Alasandro, "A statistical modeling tool is presented that enables real-time viewing of how changes in method, process, and stability variability/bias impact product acceptance rate."
AVS Life Sciences is committed to offering expert solutions in GMP adherence and confirmation, ensuring that organizations can effectively manage the intricacies of compliance. By leveraging these strategies, organizations not only enhance their operational efficiency but also solidify their standing in a competitive landscape.
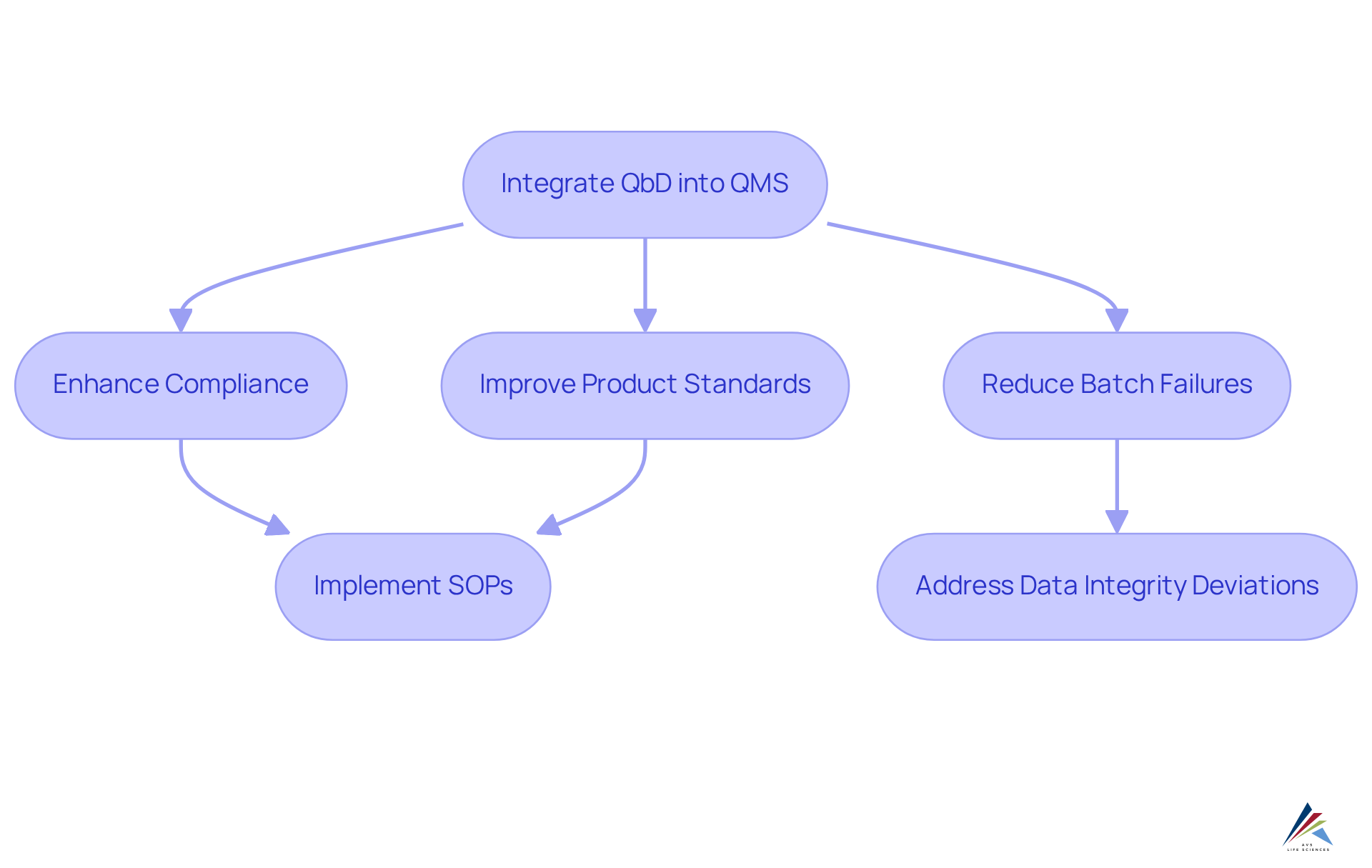
Integrate QbD into Quality Management Systems for Compliance
Incorporating quality by design for validation (QbD) into management systems is essential for aligning assessment processes with established standards and regulatory requirements. This strategic integration ensures that considerations of quality by design for validation (QbD) permeate every aspect of development and validation. By embracing a comprehensive management approach, organizations can significantly bolster their compliance efforts, resulting in enhanced product standards and a reduction in batch failures.
Notably, QbD has demonstrated the capability to decrease batch failures by up to 40%, optimizing processes through real-time monitoring and adaptive control with advanced Process Analytical Technology (PAT) tools. This proactive methodology not only meets compliance standards but also fosters a culture of continuous improvement, ultimately yielding superior outcomes in the life sciences sector.
To further , organizations must implement robust Standard Operating Procedures (SOPs) and effectively address Data Integrity Deviations. AVS Life Sciences emerges as a committed partner in this endeavor, providing expert consulting in validation, quality compliance, and regulatory strategies across biopharmaceuticals, medical devices, and nutraceuticals. This partnership ensures that organizations uphold compliance with FDA regulations and GXP standards throughout the drug development lifecycle.
Conclusion
Adopting Quality by Design (QbD) strategies for validation is essential for organizations seeking to elevate product quality and compliance within the pharmaceutical and biotechnology sectors. By embedding QbD principles into their workflows, companies can adeptly navigate the intricacies of validation, minimize batch failures, and cultivate a proactive quality culture that guarantees products adhere to regulatory requirements from the outset.
This article delineates several pivotal strategies for effective QbD implementation, such as:
- Establishing a robust design space
- Performing comprehensive knowledge reviews
- Leveraging innovative techniques like Near-Infrared Spectroscopy (NIRS)
Each of these strategies streamlines the validation process, empowering organizations to make informed decisions that enhance compliance and operational efficiency. Furthermore, the significance of continuous improvement in QbD practices is paramount, as it enables organizations to adapt to the shifting landscape of regulations and industry standards.
Ultimately, integrating QbD into quality management systems lays the groundwork for achieving exceptional outcomes in the life sciences industry. By prioritizing these strategies, organizations can not only refine their validation processes but also fortify their competitive advantage in an ever-evolving environment. Collaborating with expert partners such as AVS Life Sciences can further bolster these initiatives, ensuring that companies are well-prepared to tackle the challenges of compliance and quality in their operations.
Frequently Asked Questions
What is the focus of AVS Life Sciences in the pharmaceutical and biotechnology sectors?
AVS Life Sciences focuses on providing comprehensive quality by design for validation (QbD) solutions tailored for efficient verification, integrating management principles with regulatory compliance to enhance product quality and streamline compliance.
What are the benefits of adopting quality by design for validation (QbD) strategies?
Organizations that adopt QbD strategies experience a significant reduction in batch failures and deviations, leading to enhanced product quality and compliance throughout the lifecycle of their products.
How does AVS Life Sciences help organizations implement QbD strategies?
AVS Life Sciences leverages extensive industry knowledge to facilitate the implementation of QbD strategies, fostering a proactive quality culture that ensures products meet established quality attributes and regulatory requirements from the outset.
What challenges exist in the broader adoption of QbD?
Challenges include fragmented operations where technology integration and knowledge transfer can be difficult, hindering the successful implementation of QbD strategies.
What is essential for effective validation in pharmaceutical manufacturing?
Establishing a robust design space is essential, which involves recognizing and outlining operational parameters while adhering to stringent performance standards to enhance assessment processes and reduce variability.
Why is understanding process parameters important in QbD?
Understanding the interactions among different process parameters is crucial as these connections significantly influence the quality of the outcome in QbD, ensuring that items consistently meet regulatory requirements.
What role do thorough existing knowledge reviews play in validation success?
Conducting thorough reviews of current knowledge helps organizations identify best practices, recognize potential pitfalls, and pinpoint areas for improvement, ultimately enhancing decision-making processes and increasing the likelihood of successful outcomes.
Can you provide an example of AVS Life Sciences' impact on a client's project?
AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, allowing them to manufacture medication with lentivirus vector material while adhering to strict timelines and budgets.
How can organizations maximize validation success?
Organizations should regularly review and update their knowledge management practices, ensuring that lessons learned from past projects are effectively integrated into future verification efforts.
