8 Key Insights on the Cosmetic Ingredient Disclosure Bill

Overview
The Cosmetic Ingredient Disclosure Bill is a pivotal legislation aimed at enhancing consumer safety by mandating full transparency of cosmetic ingredients on product packaging and online platforms. This article underscores the bill's critical requirements, detailing the consequences of non-compliance and emphasizing the vital role of consumer advocacy in promoting safer products.
Ingredient disclosure is not merely a regulatory obligation; it is essential for maintaining consumer trust and ensuring industry accountability. By fostering transparency, the bill empowers consumers to make informed choices, thereby driving demand for safer cosmetic products.
As compliance officers, it is imperative to recognize the significance of this legislation and to implement robust strategies that align with its requirements, ultimately contributing to a safer marketplace.
Introduction
The introduction of the Cosmetic Ingredient Disclosure Bill signifies a pivotal shift in the cosmetics industry, demanding heightened transparency and accountability from manufacturers. This legislation aims not only to safeguard consumer health through comprehensive ingredient disclosure but also presents a unique opportunity for brands to cultivate trust and loyalty among their clientele.
As companies prepare to navigate the complexities of compliance, they face a pressing question: how will they adapt to these new regulations while maintaining their competitive edge in an increasingly scrutinized market? This scenario necessitates strategic thinking and proactive engagement with compliance solutions to thrive in this evolving landscape.
AVS Life Sciences: Expert Guidance on Compliance with the Cosmetic Ingredient Disclosure Bill
AVS Life Sciences provides specialized consulting services designed to assist companies in navigating the complexities of the Cosmetic Ingredient Disclosure Bill. Their expert team formulates customized adherence strategies that align seamlessly with the new guidelines, ensuring clients meet their ingredient disclosure obligations effectively.
With a strong focus on quality management and regulatory compliance, AVS Life Sciences empowers clients to navigate the intricacies of the cosmetic ingredient disclosure bill with confidence. As the cosmetics industry faces heightened scrutiny—evident with over 13,000 registered establishments in the U.S. alone—the need for expert guidance has never been more critical.
Industry leaders assert that successfully navigating these regulations is vital for upholding consumer trust and safety. AVS Life Sciences distinguishes itself by providing comprehensive support across diverse sectors, including:
- Biopharmaceuticals
- Medical devices
- CQV services
This enables clients to comply and excel in a demanding regulatory environment.
Mandatory Ingredient Disclosure Requirements Under the Cosmetic Ingredient Disclosure Bill
The cosmetic ingredient disclosure bill mandates that all cosmetic products disclose a comprehensive list of components on their packaging and online platforms. This requirement extends beyond just the active ingredients to include fragrances and color additives. Companies are obligated to ensure that their component lists are accurate and updated regularly to reflect any formulation changes.
Failure to comply with these stipulations can lead to significant fines, with certain firms encountering penalties exceeding $100,000 for failing to disclose harmful substances. Regulatory authorities stress that adherence to the cosmetic ingredient disclosure bill's labeling requirements is essential for public safety and trust, highlighting the importance of transparency in the industry.
The push for compliance is further emphasized by increasing scrutiny from both consumers and regulatory bodies, with statistics indicating that a majority of clients expect full transparency regarding component listings. Additionally, upcoming regulatory changes, such as the Modernization of Cosmetics Regulation Act (MoCRA) and EU regulations on microplastics, are set to reshape the landscape of cosmetic component disclosure.
Businesses that neglect these responsibilities risk damaging their reputation and losing consumer trust, making it imperative for them to prioritize accurate component disclosure to avoid financial repercussions and maintain competitiveness in the market.
Enhancing Consumer Safety Through the Cosmetic Ingredient Disclosure Bill
The Cosmetic Ingredient Disclosure Bill is a crucial legislative initiative designed to improve safety for consumers by guaranteeing they are fully informed about the cosmetic products they use. By mandating complete ingredient transparency, the cosmetic ingredient disclosure bill empowers individuals, particularly those with allergies or sensitivities, to make informed choices. Such transparency is anticipated to cultivate a deeper trust between consumers and brands, ultimately resulting in safer cosmetic products within the marketplace. This legislative measure not only addresses compliance challenges but also sets a standard for accountability, encouraging brands to prioritize consumer safety and product integrity.
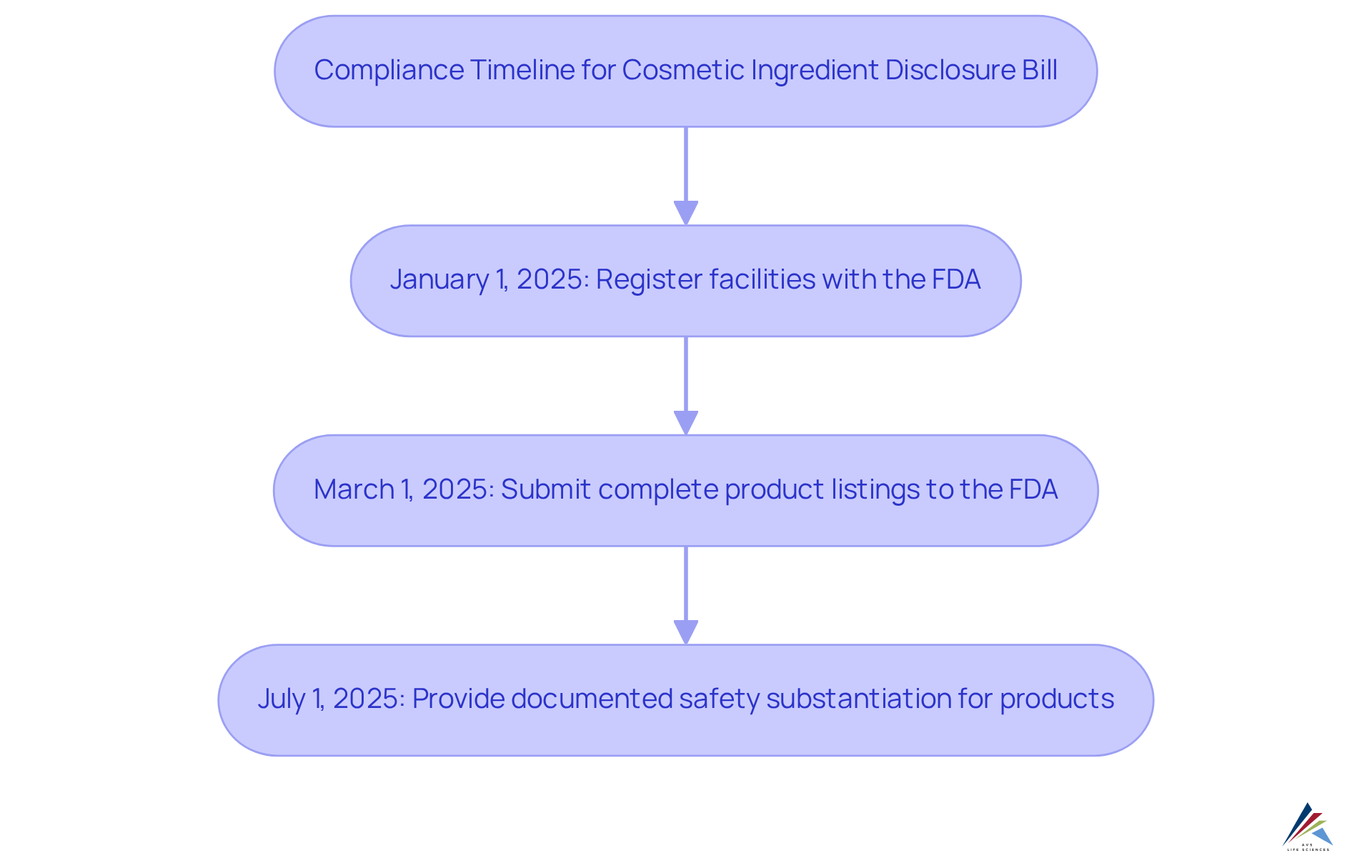
Timeline for Compliance: Key Dates for the Cosmetic Ingredient Disclosure Bill
The compliance timeline for the Cosmetic Ingredient Disclosure Bill delineates essential deadlines that cosmetic manufacturers must meet:
- January 1, 2025: All cosmetic manufacturers are required to register their facilities with the FDA.
- March 1, 2025: Complete product listings, including disclosures required by the cosmetic ingredient disclosure bill, must be submitted to the FDA.
- July 1, 2025: All products must have documented safety substantiation as required by the cosmetic ingredient disclosure bill before they can be sold to the public.
To avert penalties and ensure compliance, companies should initiate preparations well ahead of these deadlines. Recent surveys reveal that 42% of regulatory professionals report their organizations have missed at least one regulatory requirement, underscoring the urgency for proactive measures. Regulatory specialists emphasize that timely registration and comprehensive component disclosure are crucial for maintaining market access and consumer confidence in an increasingly scrutinized industry. As noted by RegASK, "Those who act swiftly to adopt AI will gain a competitive advantage, while those who lag will struggle to keep pace as the regulatory landscape evolves." Moreover, companies should recognize that product recalls frequently incur costs exceeding $10 million, highlighting the financial risks linked to non-compliance. With anticipated increases in restrictions by 2025, the necessity for vigilance and readiness is more critical than ever.
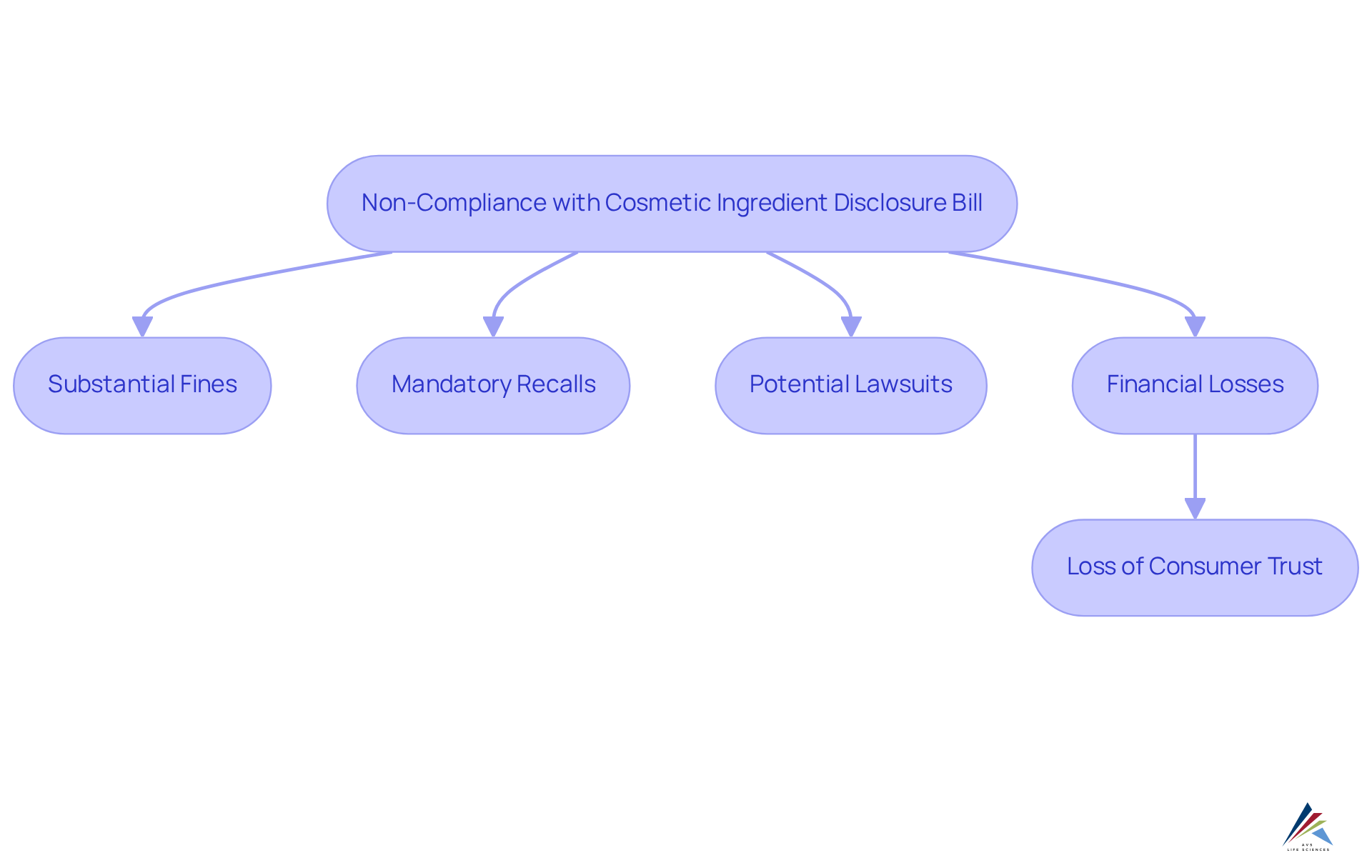
Consequences of Non-Compliance with the Cosmetic Ingredient Disclosure Bill
Non-compliance with the cosmetic ingredient disclosure bill poses significant repercussions for companies, including substantial fines, mandatory recalls of non-compliant products, and potential lawsuits. Brands that fail to disclose ingredients accurately may face recalls, disrupting operations and leading to financial losses and damage to brand reputation. The total cost of non-compliance can exceed $14 million, encompassing fines, penalties, and lost revenue due to erosion of consumer trust.
Regulatory bodies are poised to intensify scrutiny on cosmetic products, making adherence to the new regulations essential. Companies that neglect compliance risk facing enforcement actions, including warning letters and product seizures. High-profile cases illustrate the severe consequences of non-compliance with the cosmetic ingredient disclosure bill; for instance, several major brands have faced recalls due to ingredient disclosure failures, resulting in public backlash and diminished confidence among buyers.
Legal experts emphasize that the risks associated with non-compliance extend beyond immediate financial penalties. The long-term effects on brand reputation can be detrimental, as buyers increasingly prefer brands that emphasize transparency and ethical practices. As the regulatory landscape evolves, proactive compliance strategies will be essential for brands aiming to uphold their market position and public trust in 2025 and beyond.
The Role of Consumer Advocacy Groups in the Cosmetic Ingredient Disclosure Bill
Advocacy organizations have played a crucial role in shaping the cosmetic ingredient disclosure bill, underscoring the essential need for ingredient transparency and safety for consumers. Their lobbying efforts have effectively raised awareness about the potential health risks associated with toxic chemicals in cosmetics, leading to a movement for stricter regulations that prioritize public health. For instance, the Toxic-Free Cosmetics Act, which prohibits harmful substances such as PFAS and formaldehyde, exemplifies the direct outcomes of these advocacy efforts, reflecting a growing public demand for safer products.
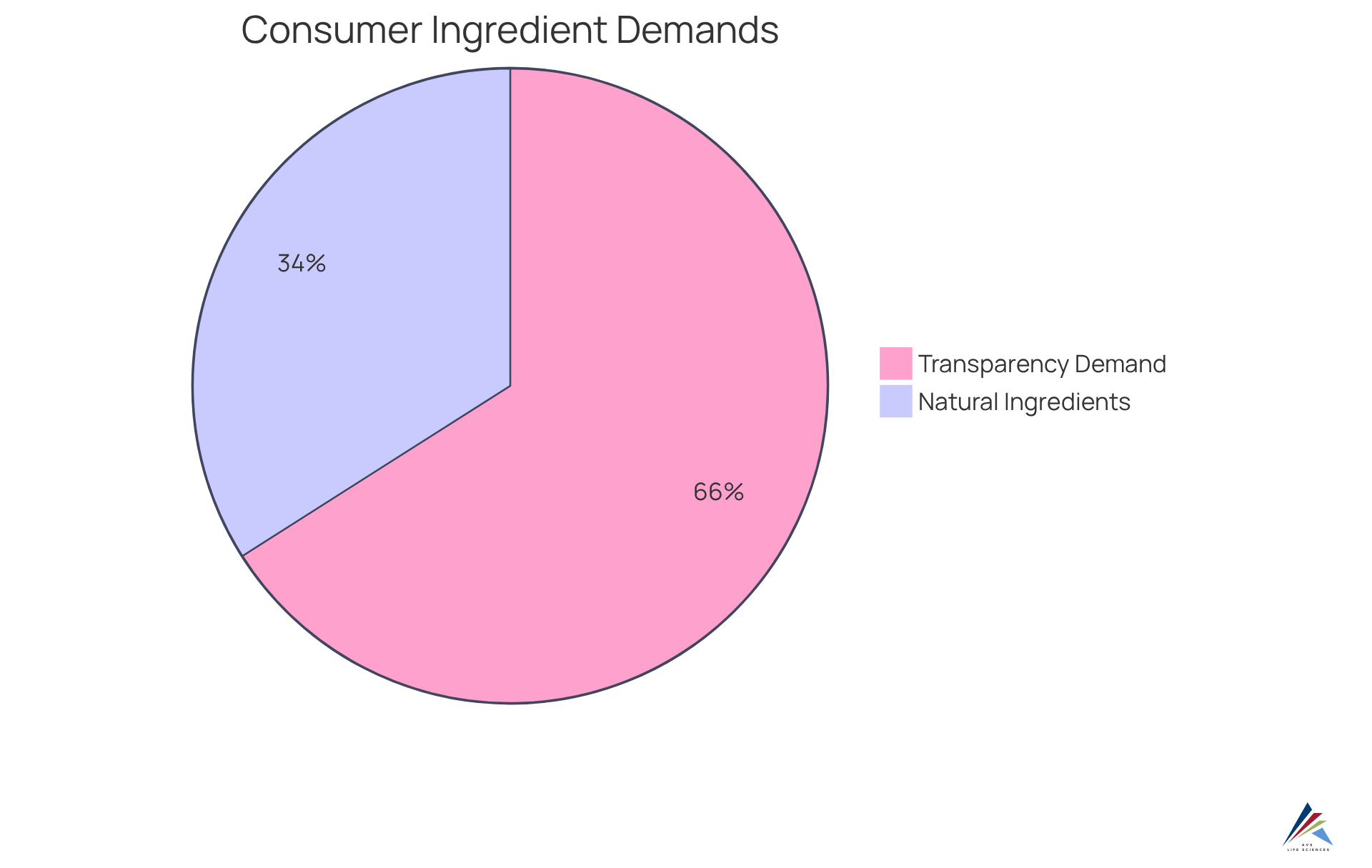
Experts agree that advocacy significantly enhances the transparency of components. As health concerns and brand skepticism rise, advocacy for consumers has emerged as a powerful influence on legislation. A substantial 78% of personal care buyers now demand transparency in components, illustrating the transformative impact of advocacy on industry standards. Furthermore, 40.2% of shoppers indicate that natural components are paramount when purchasing beauty items, indicating a clear market trend towards safer formulations.
The influence of advocacy on ingredient transparency legislation cannot be overstated. Organizations have mobilized communities, emphasizing the moral imperative of ensuring safe cosmetics, particularly for vulnerable populations. This grassroots movement has not only resulted in legislative changes but has also cultivated a culture of accountability within the beauty industry. For example, the advocacy for shielding children from harmful exposures has underscored the necessity for strict regulations, ensuring that young individuals are safeguarded from toxic substances.
Consequently, manufacturers are increasingly compelled to enhance their communication strategies and provide clear, transparent labeling to meet customer expectations.
In summary, the coordinated efforts of advocacy groups have profoundly impacted the development and promotion of the cosmetic ingredient disclosure bill, steering the industry towards improved transparency and safety for all individuals.
Global Context: How the Cosmetic Ingredient Disclosure Bill Aligns with International Standards
The cosmetic ingredient disclosure bill represents a significant advancement in aligning U.S. policies with global standards for cosmetic safety and transparency, particularly those established by the European Union. By 2025, many nations will have enacted stringent substance disclosure requirements, reflecting a global shift towards enhanced public safety and regulatory compliance. The cosmetic ingredient disclosure bill not only strengthens domestic safety protocols but also promotes international trade by ensuring that U.S. cosmetic brands adhere to the same rigorous standards as their international peers.
Experts emphasize that understanding and adapting to these evolving guidelines is crucial for brands aspiring to achieve global success. For instance, the FDA has recently underscored the importance of component transparency, stating, 'The FDA has placed a stronger emphasis on component transparency in recent updates,' necessitating more comprehensive labeling to empower consumers with knowledge about the products they use. This shift mirrors the EU's regulations, which mandate thorough ingredient disclosure, including potential allergens and nanomaterials.
Statistics reveal a rising trend in compliance, with a notable percentage of U.S. brands proactively aligning their practices with global standards. Significant examples include brands modifying products to meet EU requirements, which often encompass U.S. guidelines, thereby streamlining compliance processes. As the cosmetic industry continues to evolve, aligning U.S. regulations with international standards will be vital for maintaining competitiveness and ensuring public safety.

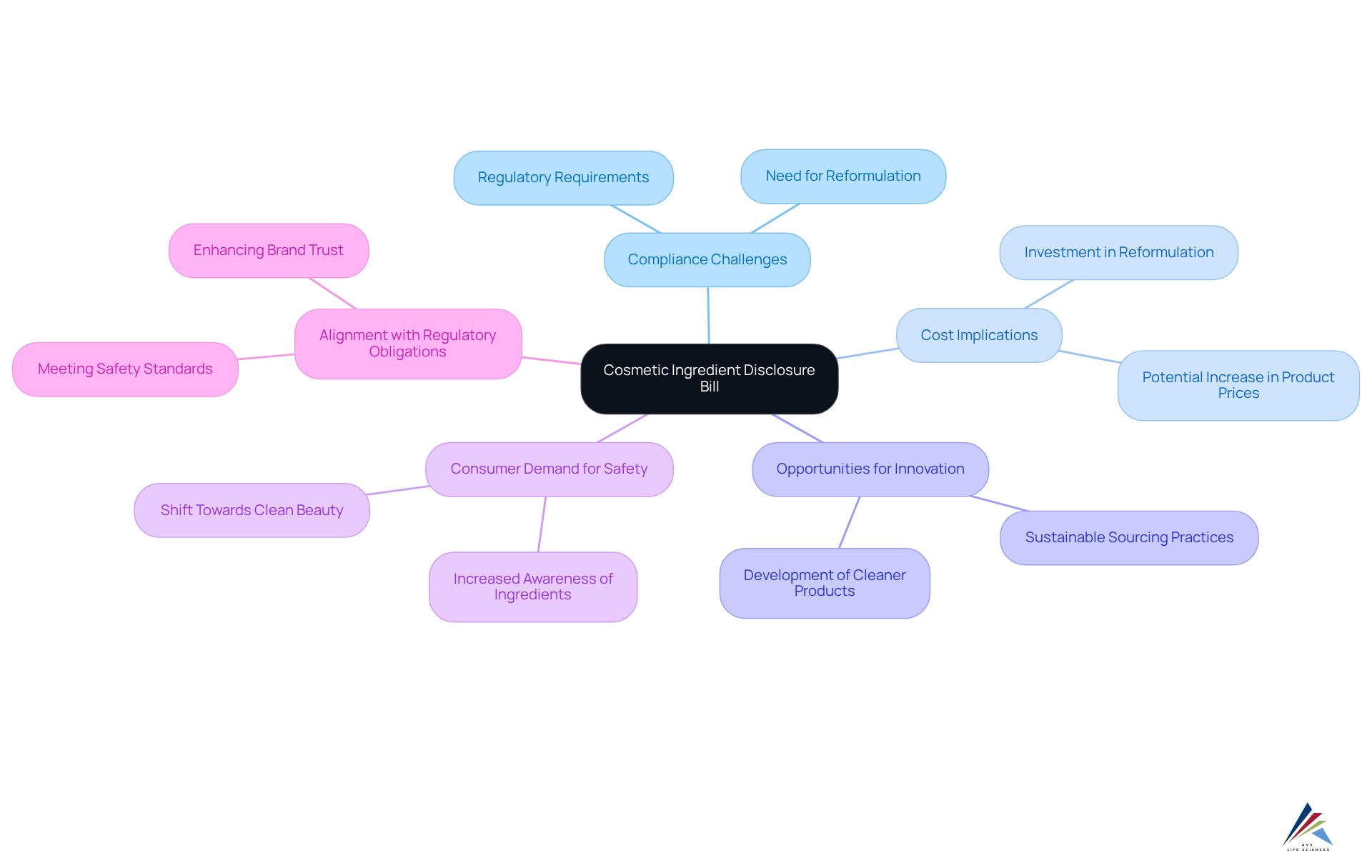
Impact on the Cosmetic Industry: Anticipated Changes Due to the Disclosure Bill
The cosmetic ingredient disclosure bill is set to revolutionize the cosmetic industry, ushering in a new era of transparency and accountability. Brands will face compliance challenges due to the cosmetic ingredient disclosure bill, necessitating significant investments in reformulating products to meet the new disclosure requirements. This shift, while potentially increasing costs, also presents opportunities for innovation; companies that prioritize the cosmetic ingredient disclosure bill alongside transparency and public safety stand to gain a competitive edge in the marketplace. Furthermore, the cosmetic ingredient disclosure bill is likely to spur consumer demand for cleaner, safer products, thereby shaping industry trends. As the industry adapts, the emphasis on compliance with the cosmetic ingredient disclosure bill will not only fulfill regulatory obligations but also align with evolving consumer expectations.
Resources for Understanding the Cosmetic Ingredient Disclosure Bill
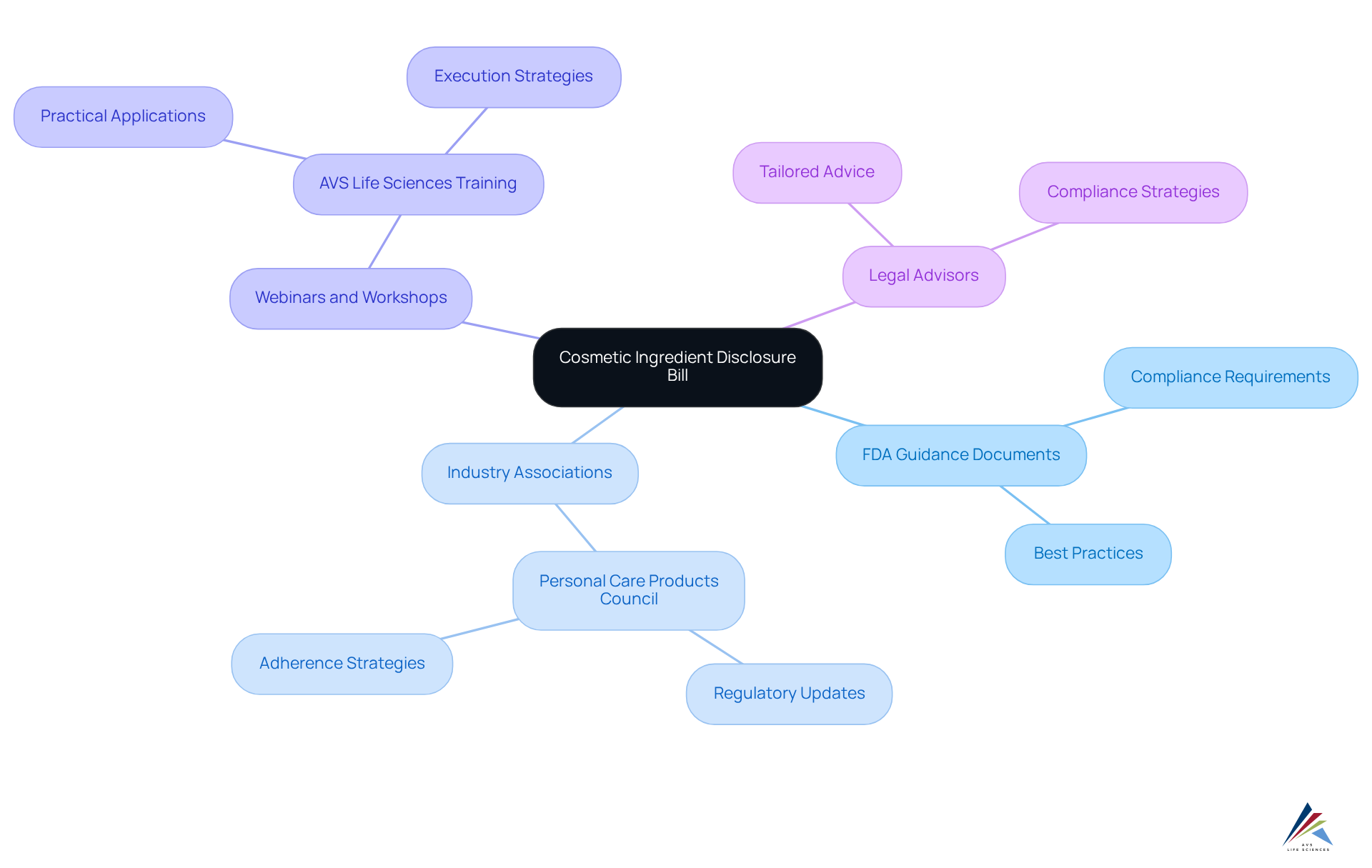
Navigating the complexities of the cosmetic ingredient disclosure bill presents significant challenges for companies. However, a variety of resources can enhance understanding and compliance, ensuring readiness for the regulations set to take effect in 2025.
- FDA Guidance Documents: The FDA provides comprehensive guidance on compliance requirements and best practices, which are essential for adherence to the new regulations.
- Industry Associations: Organizations such as the Personal Care Products Council offer vital resources and support, helping companies stay informed about regulatory changes and effective adherence strategies.
- Webinars and Workshops: Industry specialists and advisors, including those from AVS Life Sciences, conduct training sessions designed to equip companies with the necessary knowledge for compliance. These programs often cover practical applications of the rules and effective execution strategies.
- Legal Advisors: Partnering with legal experts specializing in cosmetic regulations can yield tailored advice and strategies, ensuring that companies are well-prepared to meet compliance requirements and avoid potential pitfalls.
By leveraging these resources, companies can not only enhance their compliance efforts but also position themselves as leaders in the industry.
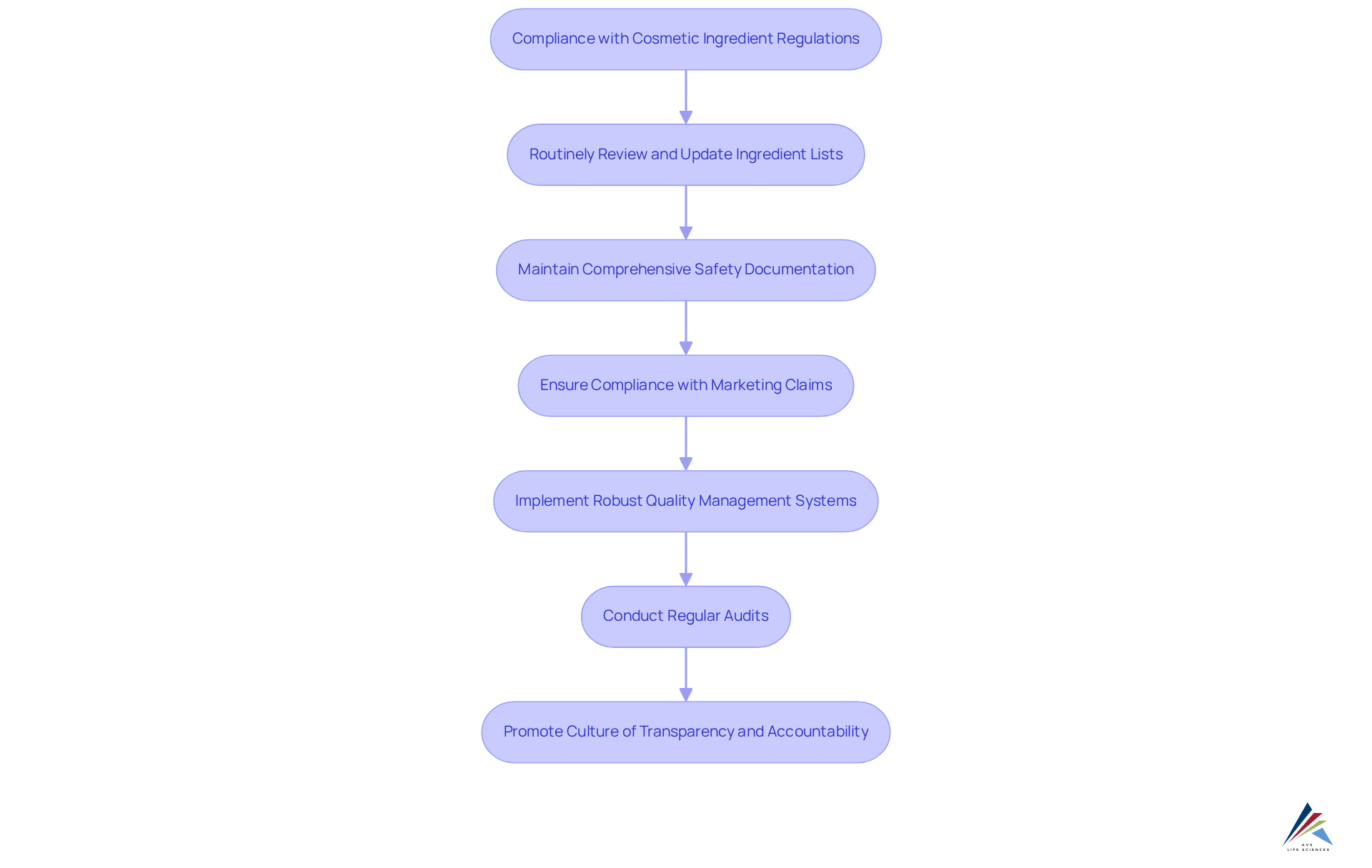
Ongoing Compliance: Adapting to Changes in Cosmetic Ingredient Regulations
In the dynamic landscape of the cosmetic industry, compliance with the cosmetic ingredient disclosure bill is of utmost importance. Companies must remain vigilant about the cosmetic ingredient disclosure bill and proactively adjust their practices. This involves:
- Routinely reviewing and updating ingredient lists
- Maintaining comprehensive safety documentation
- Ensuring that all marketing claims comply with regulatory standards
For instance, brands like Neutraderm and Glamour have faced recalls due to non-compliance, underscoring the critical need for rigorous oversight. Regulatory specialists stress that adapting to changes like the cosmetic ingredient disclosure bill is not just a legal obligation but a strategic advantage that can enhance brand reputation and customer loyalty.
To manage these intricacies effectively, companies should:
- Implement robust quality management systems
- Conduct regular audits for adherence
- Promote a culture of transparency and accountability
By cultivating a culture of compliance and transparency, brands not only fulfill regulatory obligations but also foster consumer trust in a market that is under increasing scrutiny.
Conclusion
The Cosmetic Ingredient Disclosure Bill represents a pivotal advancement in enhancing transparency and consumer safety within the cosmetics industry. By mandating comprehensive ingredient disclosure, this legislation empowers consumers to make informed choices while fostering a culture of accountability among brands. As the industry prepares for compliance by 2025, understanding and adhering to these new regulations is essential.
Key insights from the article underscore:
- The critical deadlines for compliance
- The significant consequences of non-compliance
- The vital role of consumer advocacy in shaping these regulations
Companies must:
- Prioritize accurate ingredient listings
- Engage with regulatory resources
- Adopt proactive compliance strategies to mitigate hefty penalties and reputational damage
Moreover, the alignment of U.S. standards with international regulations highlights a global shift towards safer cosmetic practices.
Ultimately, the Cosmetic Ingredient Disclosure Bill is not merely a regulatory challenge; it is an opportunity for brands to innovate and cultivate consumer trust. As the landscape evolves, embracing transparency and prioritizing consumer safety will be essential for success in an increasingly scrutinized market. Companies are urged to take proactive steps now to ensure compliance and position themselves as leaders in the pursuit of safer, more responsible cosmetics.
Frequently Asked Questions
What is the purpose of the Cosmetic Ingredient Disclosure Bill?
The Cosmetic Ingredient Disclosure Bill aims to improve consumer safety by ensuring that all cosmetic products disclose a complete list of ingredients, allowing consumers, especially those with allergies or sensitivities, to make informed choices.
What services does AVS Life Sciences provide regarding compliance with the Cosmetic Ingredient Disclosure Bill?
AVS Life Sciences offers specialized consulting services to assist companies in navigating the complexities of the Cosmetic Ingredient Disclosure Bill, including formulating customized adherence strategies and ensuring compliance with ingredient disclosure obligations.
What are the mandatory requirements for ingredient disclosure under the Cosmetic Ingredient Disclosure Bill?
The bill mandates that all cosmetic products disclose a comprehensive list of components on their packaging and online platforms, including active ingredients, fragrances, and color additives. Companies must ensure that these lists are accurate and regularly updated.
What are the consequences of failing to comply with the Cosmetic Ingredient Disclosure Bill?
Non-compliance can lead to significant fines, with penalties exceeding $100,000 for failing to disclose harmful substances. Companies may also risk damaging their reputation and losing consumer trust.
Why is transparency in ingredient disclosure important for the cosmetics industry?
Transparency is essential for public safety and trust, as consumers increasingly expect full disclosure regarding product components. It also helps brands maintain competitiveness and accountability in a demanding regulatory environment.
How does the Cosmetic Ingredient Disclosure Bill enhance consumer safety?
By mandating complete ingredient transparency, the bill empowers consumers to make informed choices about the products they use, fostering trust between consumers and brands and promoting safer cosmetic products in the marketplace.
What upcoming regulatory changes may impact cosmetic ingredient disclosure?
Upcoming changes include the Modernization of Cosmetics Regulation Act (MoCRA) and EU regulations on microplastics, which are expected to reshape the landscape of cosmetic component disclosure and compliance requirements.
