8 Key Insights on MoCRA Allergen Labeling Requirements

Overview
The article provides critical insights into the allergen labeling requirements mandated by the Modernization of Cosmetics Regulation Act (MoCRA). Compliance with these labeling standards is not merely a regulatory obligation; it is essential for safeguarding consumer safety and fostering trust. The new regulations require explicit disclosure of fragrance irritants, marking a pivotal transformation in the cosmetic industry's commitment to ingredient transparency. This shift underscores the importance of understanding and adhering to these requirements, as failure to comply could jeopardize both consumer safety and brand integrity.
Introduction
The landscape of cosmetic regulation is undergoing a significant transformation with the introduction of the Modernization of Cosmetics Regulation Act (MoCRA), particularly in the realm of allergen labeling. This pivotal legislation mandates enhanced transparency in ingredient disclosure and addresses the growing concerns surrounding allergic reactions associated with cosmetic products.
As companies prepare for compliance by 2025, they face the challenge of effectively navigating these new requirements while preserving consumer trust. What strategies can manufacturers implement to ensure they meet these stringent standards without compromising product integrity?
It is essential for companies to embrace innovative compliance solutions that not only align with regulatory expectations but also foster consumer confidence.
AVS Life Sciences: Expert Guidance on MoCRA Compliance and Allergen Labeling
AVS Life Sciences is dedicated to guiding businesses through the complexities of the Modernization of Cosmetics Regulation Act (MoCRA), particularly in allergenic substance identification. With the recent modifications to ingredient identification requirements under MoCRA, it is crucial for cosmetic producers to adapt their practices to comply with the . AVS Life Sciences provides comprehensive consulting services that encompass regulatory compliance, including GXP and FDA regulations, as well as quality management. This ensures clients not only understand these requirements but also implement effective adherence strategies tailored to their specific needs.
Industry leaders emphasize the critical nature of ingredient identification, asserting that clear and precise information is essential for consumer safety and trust. As the regulatory landscape shifts, AVS Life Sciences empowers its clients with the latest knowledge and tools necessary to uphold high quality and safety standards in their cosmetic products. The firm’s dedicated team of specialists employs strategies such as meticulous documentation practices and internal auditing techniques to develop holistic solutions that comply with current regulatory standards, fostering a proactive approach to meeting requirements.
In 2025, the impact of the MoCRA allergen labeling requirement on ingredient identification practices is expected to be profound, prompting companies to reassess their identification strategies. Effective labeling strategies adopted by cosmetics firms include:
- Thorough ingredient evaluations
- Transparent communication of potential irritants
- Continuous training for staff on regulatory requirements
By harnessing AVS Life Sciences' expertise in GXP and FDA compliance practices, companies can adeptly navigate these changes, ensuring they remain compliant while prioritizing consumer safety.
Understanding MoCRA: Key Allergen Labeling Requirements for Cosmetics
MoCRA mandates that cosmetic products must explicitly disclose any fragrance irritants in their formulations. This requirement includes specific substances recognized by the FDA, which must be clearly listed on product labels. The initiative aims to enhance user safety by providing transparent information about potential allergens, empowering individuals to make informed choices regarding the products they use. Notably, this marks the most significant expansion of the FDA’s authority over cosmetics since the FD&C Act of 1938, underscoring its vital role in public health protection.
Recent statistics reveal a growing concern regarding allergic reactions to cosmetics, with around 10% of consumers reporting adverse reactions linked to fragrance ingredients. This statistic underscores the critical necessity of the , which is aimed at mitigating risks associated with allergenic substances.
In response to these regulations, numerous cosmetic companies are proactively adjusting their labeling practices. Leading brands are revising their ingredient lists to meet the MoCRA allergen labeling requirement by including detailed disclosures of fragrance allergens instead of relying on generic terms like 'fragrance'. This shift not only aligns with the MoCRA allergen labeling requirement but also fosters brand trust and enhances public confidence.
The impact of MoCRA on cosmetic product identification is substantial, as it establishes a new standard for accountability and consumer protection within the industry. Companies must now ensure that their labels comply with these regulations, which necessitate comprehensive safety assessments for all cosmetic products. Furthermore, the NPRM for fragrance allergy identification requirements is scheduled for January 2025, emphasizing the urgency for companies to prepare for compliance. As the industry evolves, the focus on ingredient disclosure is poised to lead to improved safety measures and heightened consumer awareness regarding the components in their cosmetics.
Impact of MoCRA on Manufacturers: Adapting to New Allergen Labeling Standards
Producers must modify their packaging methods to meet the MoCRA allergen labeling requirement for disclosing allergens. This adaptation presents significant that necessitate a comprehensive approach. Companies may need to rework products to eliminate certain irritants or update packaging procedures to ensure adherence to these new standards. For instance, investing in advanced labeling technology and providing comprehensive training for staff will be essential for navigating these requirements effectively.
The financial implications of reformulation can be substantial. Brands may face increased production costs and potential delays in product launches as they strive to meet these standards. Furthermore, the challenges posed by the MoCRA allergen labeling requirement, particularly in 2025, include the necessity for transparent ingredient disclosures and the risk of penalties for non-compliance. These penalties could manifest as product recalls and fines, underscoring the importance of proactive measures.
As manufacturers work diligently to meet these requirements, successful reformulation examples will be vital in demonstrating compliance and preserving buyer trust. Engaging with AVS Life Sciences can provide the necessary support and expertise to navigate these complexities, ensuring that your brand not only complies but thrives in this evolving regulatory landscape.
Ingredient Transparency: The Role of Allergen Disclosure in Consumer Safety
Ingredient transparency is paramount in the cosmetics sector, especially concerning the disclosure of allergens. By clearly identifying harmful substances, producers comply with the MoCRA allergen labeling requirement and cultivate trust among consumers. This is essential for individuals with allergies or sensitivities, enabling informed decisions and enhancing overall safety and satisfaction.
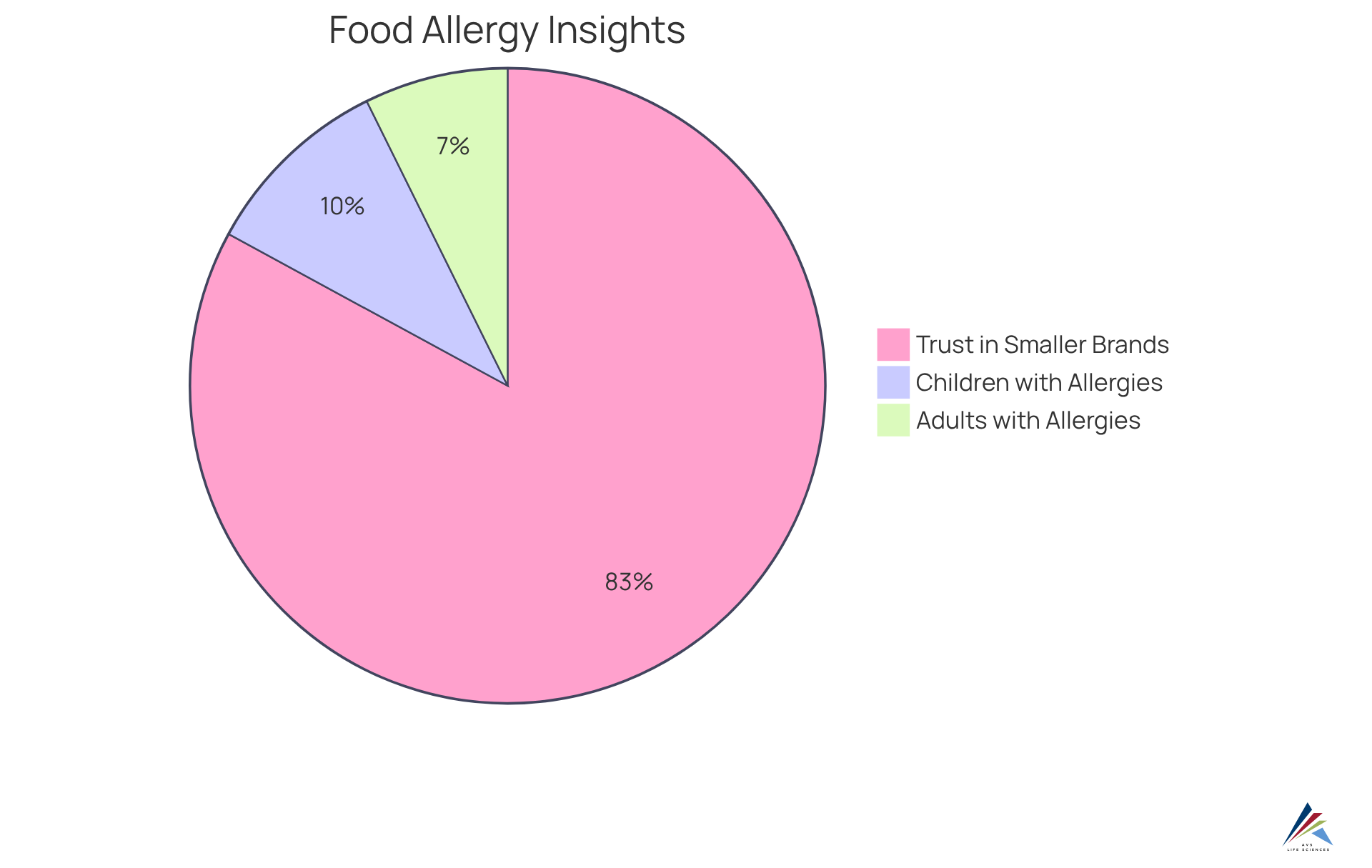
Approximately 6% of adults and 8% of children in the U.S. report being diagnosed with at least one food allergy, underscoring the critical need for clear allergen labeling. Businesses that prioritize ingredient clarity are more likely to develop stronger customer loyalty; indeed, 68% of individuals with food allergies express greater trust in smaller brands committed to managing allergens.
Furthermore, effective disclosure practices regarding food sensitivities can significantly bolster brand trust, as 71% of those with food allergies dedicate 3 to 5 minutes to reviewing labels for each food product. By implementing the MoCRA allergen labeling requirement, companies can effectively communicate their commitment to consumer welfare, which ultimately leads to increased trust and loyalty in a competitive marketplace.
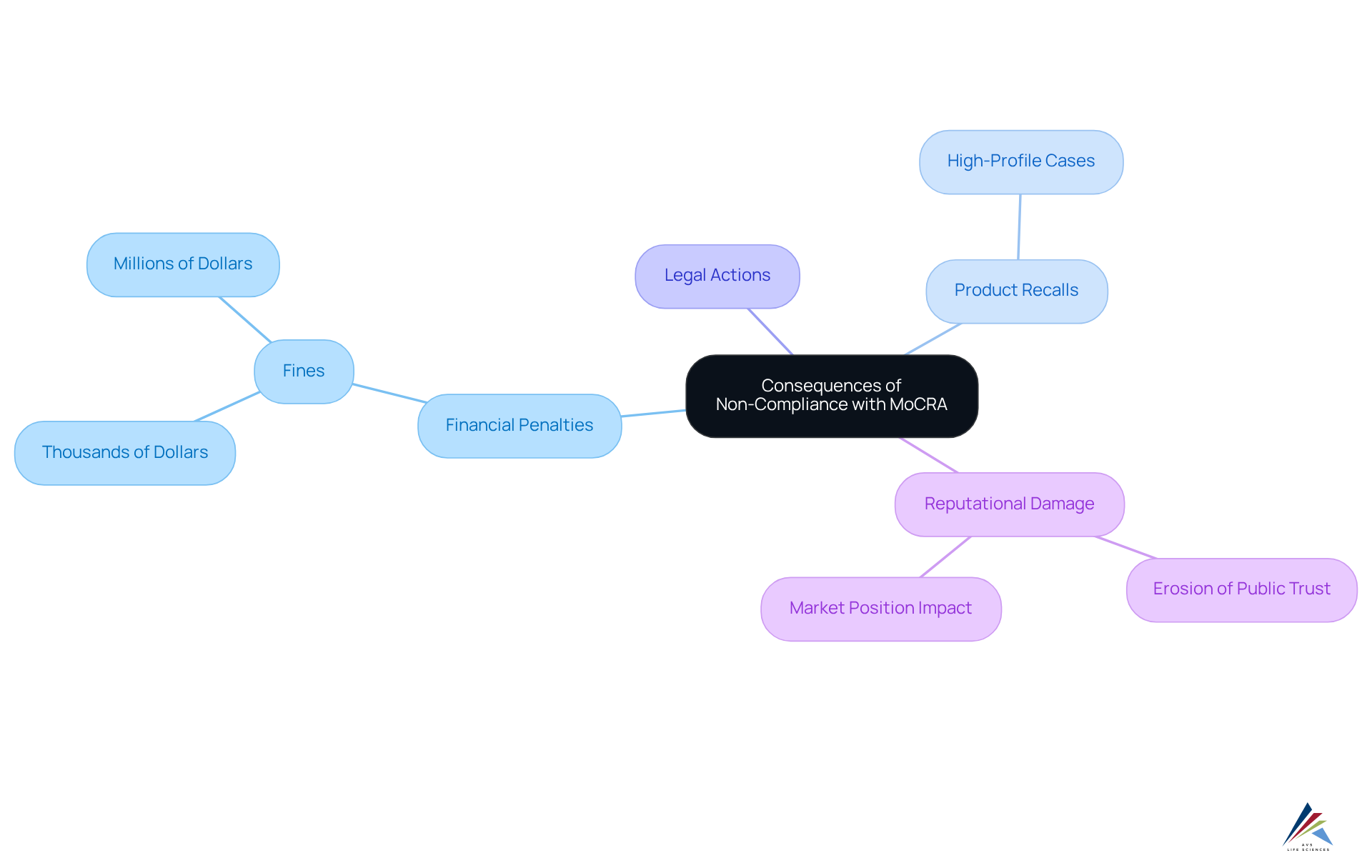
Consequences of Non-Compliance: Understanding Penalties Under MoCRA
Non-compliance with the Modernization of Cosmetics Regulation Act (MoCRA) presents significant challenges for cosmetic companies, leading to severe repercussions such as substantial fines, mandatory product recalls, and potential legal action. The FDA wields the authority to impose financial penalties that can escalate from thousands to millions of dollars, contingent upon the severity of the violation.
For instance, businesses failing to meet ingredient identification standards may face escalating penalties, reflecting the seriousness of their non-compliance. Legal experts warn that the fallout from neglecting these regulations extends beyond immediate financial penalties; it can inflict considerable reputational damage and erode public trust. Such consequences can have enduring effects on a company's market position and profitability.
Recent incidents underscore the urgency of compliance with the MoCRA allergen labeling requirement, as several high-profile product recalls have occurred due to allergen labeling violations, highlighting the imperative for manufacturers to maintain accurate and transparent labeling practices.
As the regulatory landscape evolves, the financial and operational stakes for non-compliance continue to rise, making adherence to the MoCRA allergen labeling requirement essential for maintaining customer trust and ensuring business viability.
To navigate these complexities, companies can leverage AVS Life Sciences' , specifically designed to ensure compliance with regulatory standards and enhance product safety. By adopting these solutions, businesses can mitigate risks associated with non-compliance and foster customer trust.
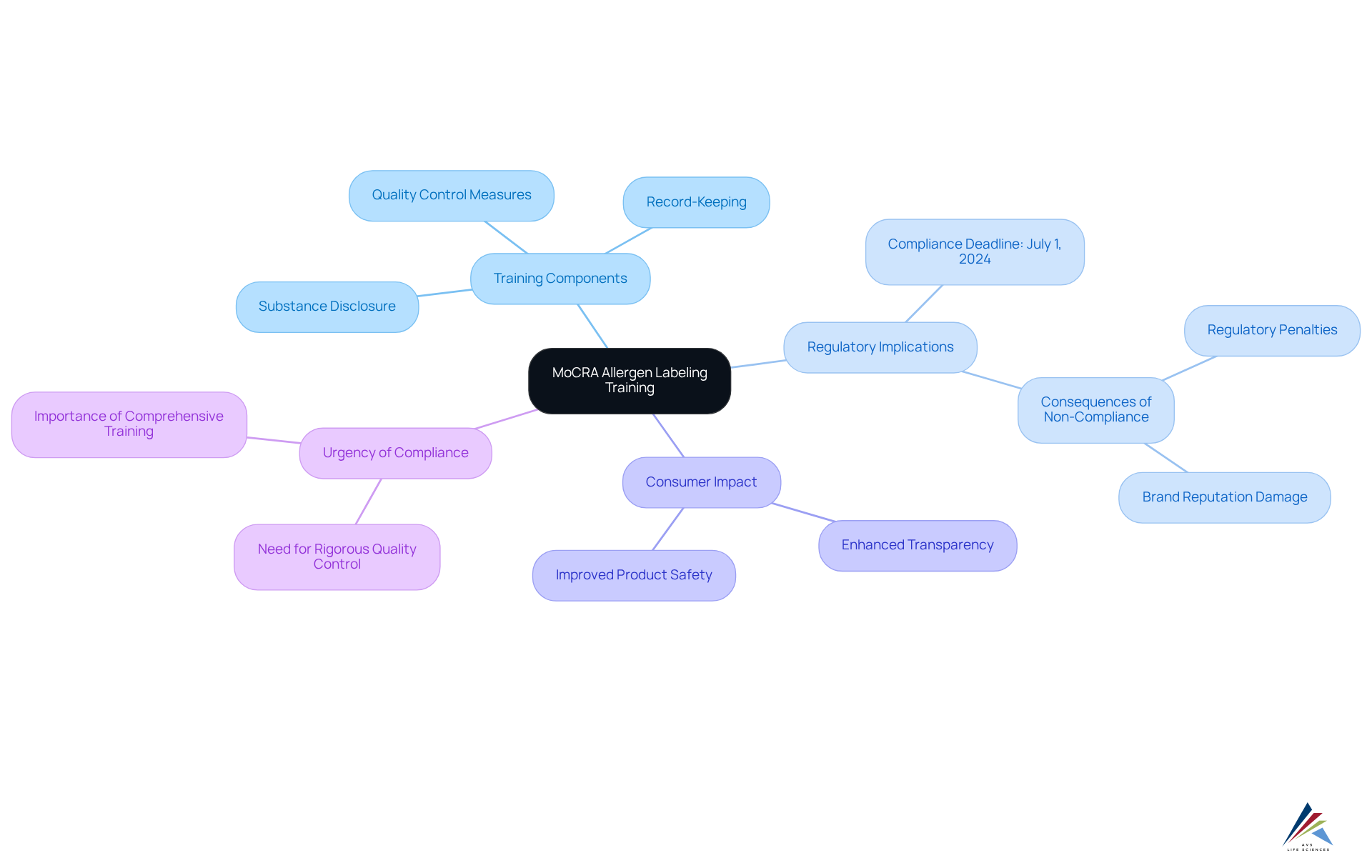
Training Compliance Officers: Essential Knowledge for MoCRA Allergen Labeling
Training compliance officers on the is essential for ensuring adherence to regulatory standards. This training should encompass the details of substance disclosure, emphasizing that cosmetic labels must comply with the MoCRA allergen labeling requirement by listing all fragrance irritants to enhance consumer transparency regarding potential sensitivities.
Given that individuals use an average of 6 to 12 cosmetic products daily, the implications of the MoCRA allergen labeling requirement are significant. Non-compliance can lead to serious consequences, including regulatory penalties and damage to brand reputation.
The MoCRA allergen labeling requirement, with a deadline of July 1, 2024, adds urgency to this training, highlighting the need for rigorous quality control measures and detailed record-keeping as part of regulations. By equipping compliance officers with comprehensive knowledge, companies can strengthen their compliance framework, mitigate risks, and foster a culture of safety and transparency.
Effective training programs demonstrate that knowledgeable staff are better equipped to handle the intricacies of ingredient identification, ultimately improving product safety and consumer confidence.
Leveraging Technology: Tools for Effective MoCRA Allergen Labeling Compliance
Utilizing technology is crucial for satisfying the MoCRA allergen labeling requirement, particularly in the . AVS Life Sciences offers software solutions designed to automate the tagging process, ensuring accurate allergen disclosure that meets the MoCRA allergen labeling requirement and significantly reducing the risk of misidentification. This automation not only streamlines the tagging workflow but also enhances traceability by meticulously tracking ingredient sourcing and managing essential documentation. Such a proactive strategy mitigates human error and accelerates compliance, empowering manufacturers to swiftly adapt to regulatory changes. By embracing these advanced tools, companies can achieve heightened operational efficiency while fostering consumer trust through transparent labeling practices.

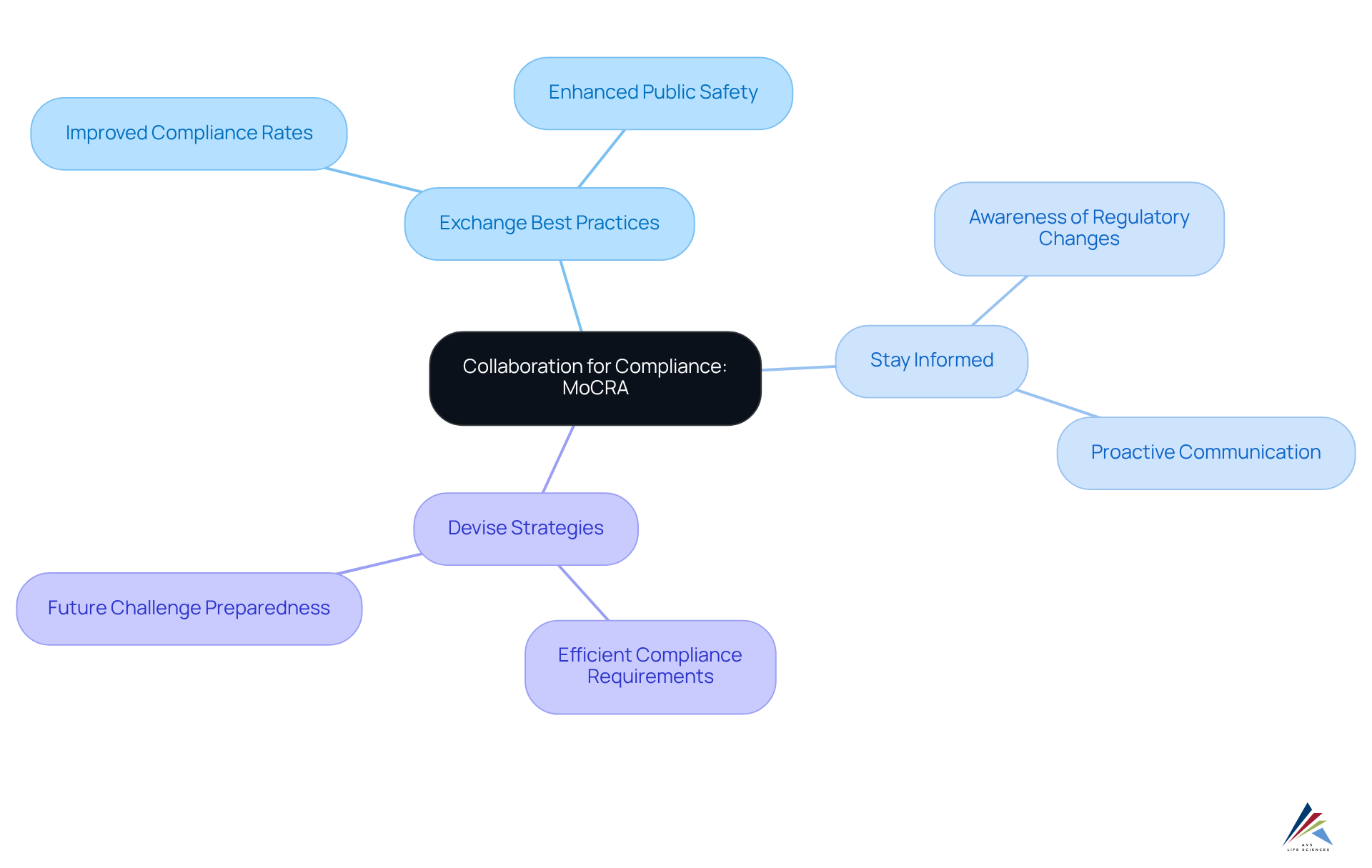
Collaboration for Compliance: Industry and Regulatory Body Partnerships Under MoCRA
Cooperation among industry participants and regulatory organizations is crucial for effective adherence to the Modernization of Cosmetics Regulation Act (MoCRA). By fostering strategic partnerships, companies can:
- Exchange best practices
- Remain informed about regulatory changes
- Devise strategies to meet compliance requirements efficiently
Engaging with regulatory bodies not only enhances understanding of forthcoming modifications but also equips firms to tackle future challenges in ingredient identification. Notably, effective partnerships demonstrate that proactive communication and shared knowledge significantly elevate , ultimately benefiting both the industry and public safety.
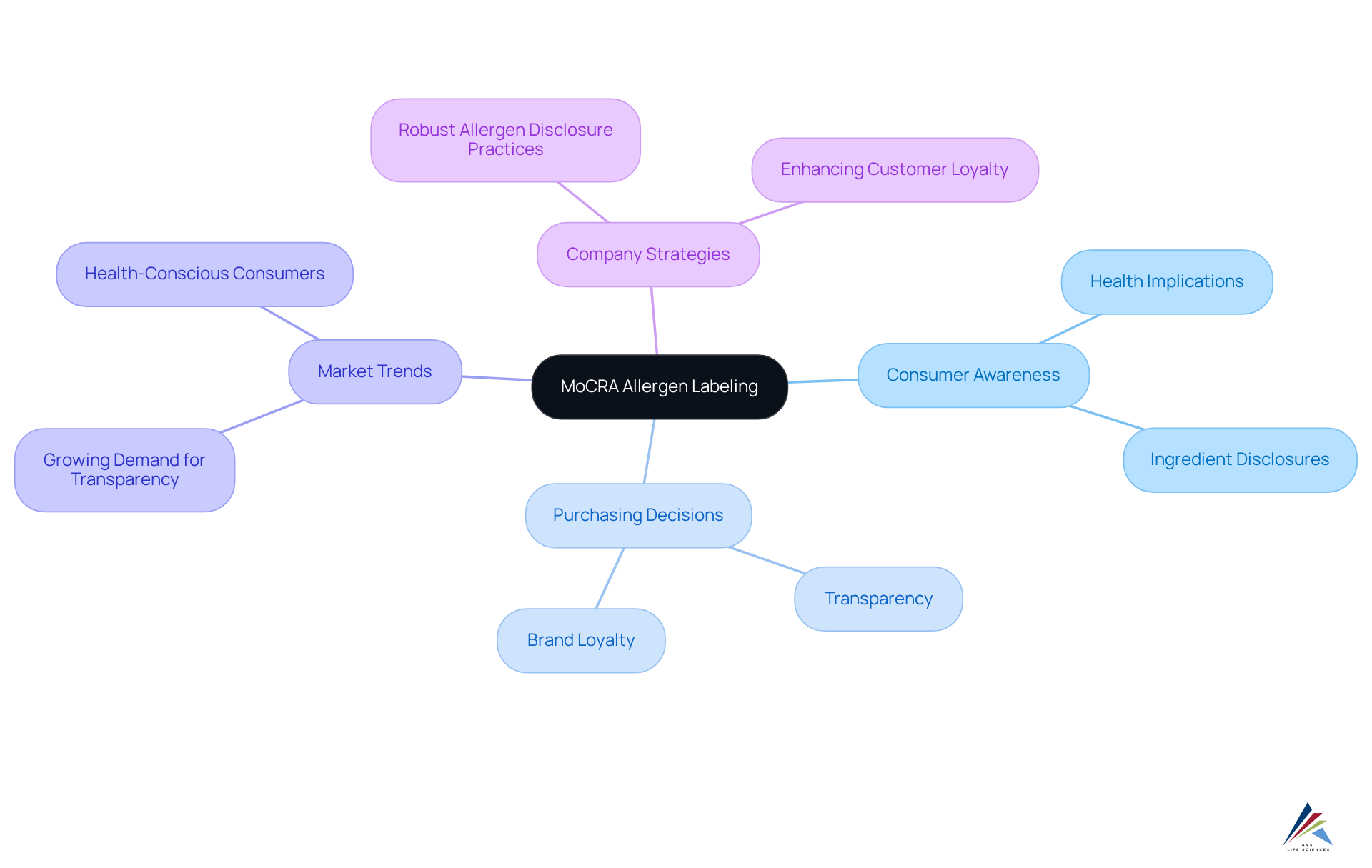
Consumer Impact: How MoCRA Allergen Labeling Influences Purchasing Decisions
The is set to significantly influence buyers' purchasing decisions. As awareness of allergenic triggers and their potential health implications expands, consumers are actively seeking products that provide transparent and accurate information.
Market analysis indicates that a considerable segment of shoppers prioritizes ingredient disclosures when making purchasing choices, highlighting a growing trend toward transparency in product labeling. Companies that emphasize allergy information are likely to attract health-conscious consumers and cultivate brand loyalty.
For instance, a case study involving a leading cosmetics brand revealed that the implementation of robust allergen disclosure practices led to a marked increase in customer loyalty and satisfaction.
As the industry adapts to the changes mandated by MoCRA, including the MoCRA allergen labeling requirement for fragrance irritants, manufacturers must acknowledge the critical importance of identifying irritants to enhance sales and maintain a competitive edge in the marketplace.
Future Trends: The Evolution of Allergen Labeling in the Cosmetics Industry
The cosmetics sector is poised for significant transformation in ingredient identification due to rising consumer awareness and increased regulatory scrutiny. Industry analysts forecast that future trends will likely encompass more stringent labeling requirements, specifically the mocra allergen labeling requirement for disclosing newly identified allergens. Furthermore, advancements in technology are expected to streamline regulatory processes, facilitating compliance for companies as regulations evolve.
Organizations that proactively embrace these changes will not only meet public demands for transparency but also align with regulatory expectations, ensuring their products remain safe and reliable. For instance, several leading brands have already begun revising their labeling practices to comply with the mocra allergen labeling requirement, demonstrating a commitment to consumer safety and regulatory compliance. This proactive stance positions them advantageously in a competitive market that increasingly prioritizes .
Conclusion
The introduction of the Modernization of Cosmetics Regulation Act (MoCRA) signifies a transformative shift in the cosmetics industry, particularly in allergen labeling. As companies gear up for compliance by 2025, grasping the implications of these new regulations is vital for ensuring consumer safety and preserving brand trust.
Key insights indicate that the MoCRA allergen labeling requirement underscores the necessity for transparency in ingredient disclosure, especially regarding fragrance irritants. This evolution is propelled by an increasing consumer awareness of allergic reactions, with many actively searching for products that offer clear and precise allergen information. Companies that implement comprehensive ingredient evaluations, foster transparent communication, and engage in ongoing staff training are strategically positioned to meet these new standards and cultivate consumer loyalty.
The shifting landscape of allergen labeling under MoCRA not only addresses regulatory requirements but also emphasizes the importance of proactive strategies for compliance. As the industry navigates these changes, it is essential for manufacturers to adopt innovative solutions, leverage technology, and collaborate with regulatory bodies. By prioritizing allergen transparency and compliance, companies can bolster consumer trust and secure their market positions within a competitive arena.
Frequently Asked Questions
What is the Modernization of Cosmetics Regulation Act (MoCRA)?
MoCRA is a regulatory framework that mandates cosmetic products to disclose any fragrance irritants in their formulations, enhancing user safety and providing transparent information about potential allergens.
Why is allergen labeling important under MoCRA?
Allergen labeling is crucial because it helps mitigate risks associated with allergic reactions to cosmetics, which affect around 10% of consumers. It ensures that consumers have clear information about the ingredients in the products they use.
What are the key requirements for allergen labeling under MoCRA?
Cosmetic products must explicitly list fragrance irritants recognized by the FDA on their labels, moving away from generic terms like 'fragrance' to detailed disclosures.
How can AVS Life Sciences assist businesses with MoCRA compliance?
AVS Life Sciences provides comprehensive consulting services that help businesses understand and implement effective strategies for regulatory compliance, including GXP and FDA regulations, tailored to their specific needs.
What strategies can cosmetic firms adopt to comply with MoCRA allergen labeling requirements?
Effective strategies include thorough ingredient evaluations, transparent communication of potential irritants, and continuous training for staff on regulatory requirements.
What challenges do manufacturers face in adapting to the MoCRA allergen labeling standards?
Manufacturers must modify packaging methods and may need to reformulate products to eliminate certain irritants, which can lead to increased production costs and potential delays in product launches.
What are the financial implications of reformulating products for MoCRA compliance?
Reformulating products can result in substantial costs and delays, as companies must invest in advanced labeling technology and training to ensure compliance with the new standards.
What are the potential penalties for non-compliance with MoCRA allergen labeling requirements?
Non-compliance can lead to product recalls and fines, emphasizing the importance of proactive measures to adhere to the regulations.
When is the NPRM for fragrance allergy identification requirements scheduled?
The NPRM for fragrance allergy identification requirements is scheduled for January 2025, highlighting the urgency for companies to prepare for compliance.
How does AVS Life Sciences support businesses in navigating MoCRA complexities?
AVS Life Sciences offers expertise and support to help brands not only comply with MoCRA but also thrive in the evolving regulatory landscape, ensuring high quality and safety standards in their products.
