8 Key Differences Between Autologous vs Allogeneic Stem Cell Therapies

Overview
The primary distinctions between autologous and allogeneic stem cell therapies stem from their sources and the associated risks.
- Autologous therapies utilize the patient's own cells, effectively minimizing immune rejection.
- In contrast, allogeneic therapies depend on donor cells, which can introduce greater risks of complications, such as graft-versus-host disease.
- Notably, autologous treatments typically exhibit a safer profile and lower costs, rendering them a preferred choice for many patients, particularly in pediatric cases.
- While allogeneic therapies entail complexities and higher expenses, they can provide significant advantages for conditions necessitating immune reconstitution.
Introduction
The landscape of stem cell therapies is rapidly evolving, with autologous and allogeneic approaches leading the charge in regenerative medicine. These two methodologies offer distinct advantages and challenges, significantly impacting patient safety, treatment efficacy, and ethical considerations. As healthcare professionals and patients navigate the complexities of these therapies, it becomes crucial to comprehend the key differences between autologous and allogeneic stem cell treatments.
What are the implications of selecting one approach over the other? Furthermore, how do recent advancements shape their future in clinical practice? Understanding these dynamics is essential for informed decision-making and optimizing patient outcomes.
AVS Life Sciences: Regulatory Compliance in Autologous and Allogeneic Stem Cell Therapies
AVS Life Sciences plays a crucial role in ensuring that treatments adhere to strict regulatory standards, whether they are autologous vs allogeneic. Compliance with [Good Manufacturing Practices (GMP)](https://stemcellres.biomedcentral.com/articles/10.1186/s13287-024-04065-9), [Quality System Regulations (QSR)](https://stemcellres.biomedcentral.com/articles/10.1186/s13287-024-04065-9), and ISO standards is paramount. By offering across multiple sectors—biopharmaceuticals, medical devices, in-vitro diagnostics, and nutraceuticals—AVS Life Sciences assists clients in navigating the complexities of [regulatory submissions and compliance](https://globalrph.com/2025/03/stem-cell-therapy-success-rates-hit-78-new-research-reveals-breakthrough-results). This guarantees that treatments are not only safe but also effective for patient use.
Their customized consulting services, spanning a broad spectrum of fields, are particularly beneficial in the rapidly evolving domain of regenerative treatments, where regulations can vary significantly across different regions. With a steadfast focus on ensuring quality and regulatory compliance throughout the drug development lifecycle, AVS Life Sciences positions itself as a trusted partner in the life sciences sector.
Source of Stem Cells: Autologous vs Allogeneic
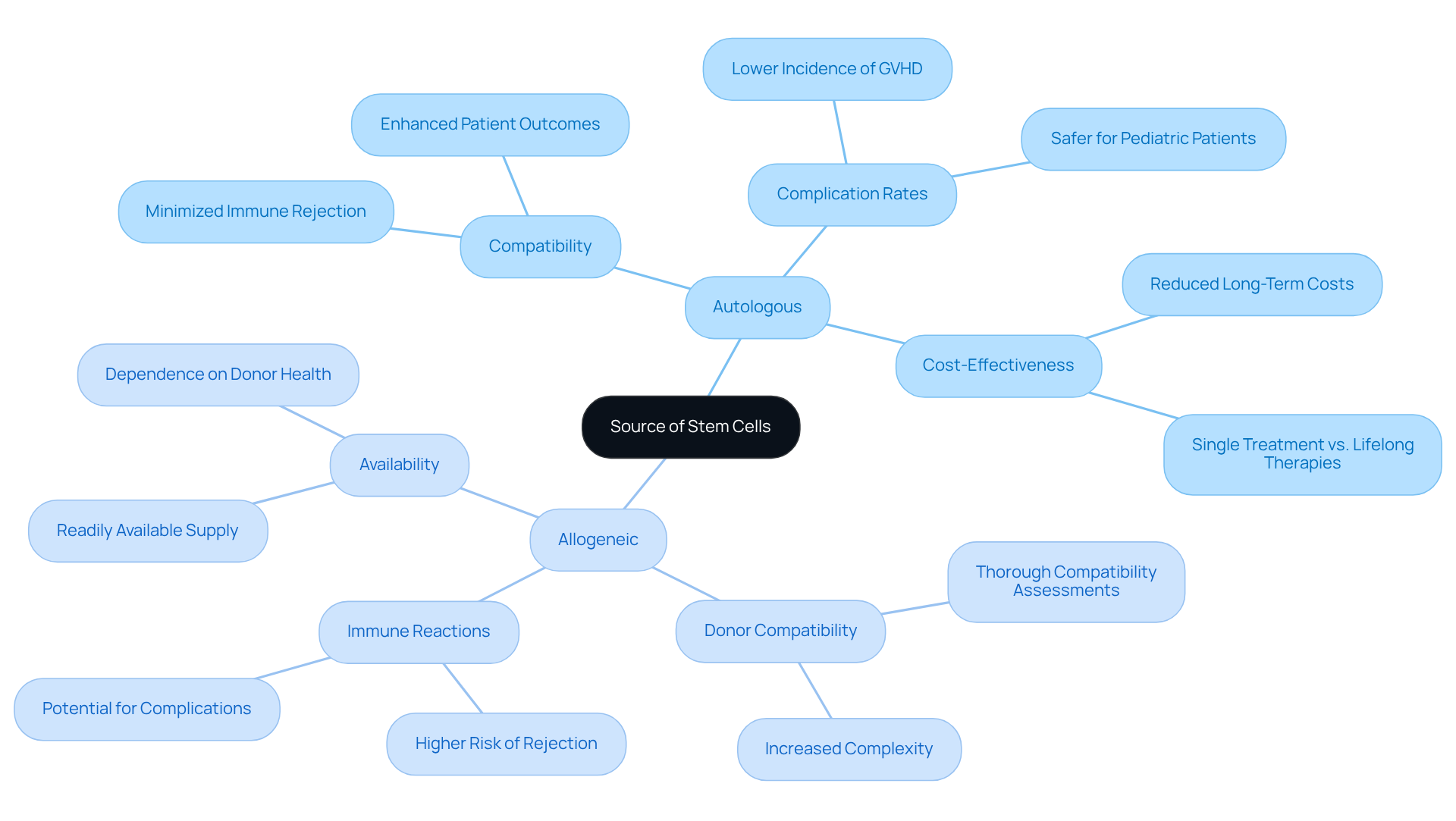
When discussing autologous vs allogeneic treatments, it is important to note that involve harvesting progenitor cells from the patient’s own body, significantly enhancing compatibility and minimizing the risk of immune rejection. This method results in a reduced incidence of complications, such as graft-versus-host disease (GVHD), thereby presenting a safer option for many patients, particularly in pediatric cases where lower complication rates are paramount.
Conversely, donor therapies utilize progenitor types from healthy contributors, which, while providing a more readily available supply, may introduce risks of immune reactions, highlighting the differences in outcomes between autologous vs allogeneic treatments. Research indicates that immune rejection rates are considerably elevated in allogeneic transplants compared to autologous transplants, necessitating thorough donor compatibility assessments to mitigate these risks.
Furthermore, the financial implications of autologous tissue transplants are substantial, as they can be more cost-effective compared to lifelong therapies for chronic conditions. Clinicians must carefully evaluate these factors when recommending treatment options, as they directly influence both therapeutic strategies and the likelihood of successful outcomes.
Recent studies underscore the importance of understanding the nuances between these two types of progenitor tissues, autologous vs allogeneic, particularly concerning patient well-being and the specific ailments being addressed. Notably, the success rate of regenerative treatments can vary based on the condition, with blood cancers demonstrating a success rate of 60-70%. The growing number of clinical trials for autologous biological treatments further highlights the strong interest and investment in this area, emphasizing its potential in modern medicine.
Manufacturing Processes: Key Differences in Stem Cell Therapies
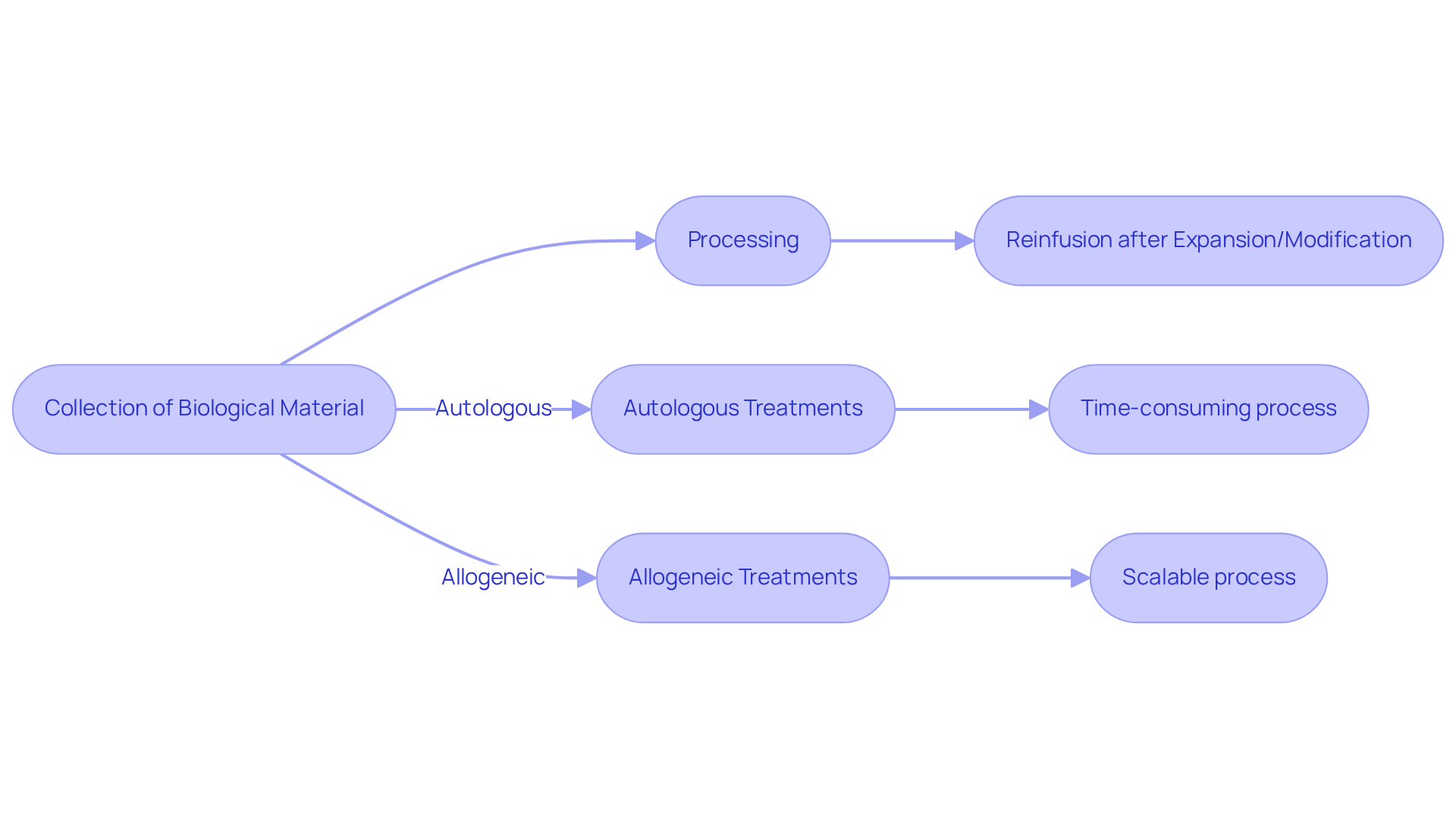
The production method for treatments can be categorized into autologous vs allogeneic progenitor treatments, which typically involves the collection of biological material from the patient, its subsequent processing, and the reinfusion of the material after expansion or modification. This method is often time-consuming and resource-intensive, frequently requiring several weeks for completion.
In contrast, the distinction between autologous vs allogeneic stem cell treatments is that those derived from donors can be produced in larger quantities, facilitating quicker access and scalability.
Nonetheless, the process of autologous vs allogeneic transplantation necessitates rigorous matching protocols and quality controls to mitigate the risk of immune rejection. Understanding these is essential for improving treatment delivery and optimizing patient outcomes.
Clinical Applications: Where Autologous and Allogeneic Therapies Are Used
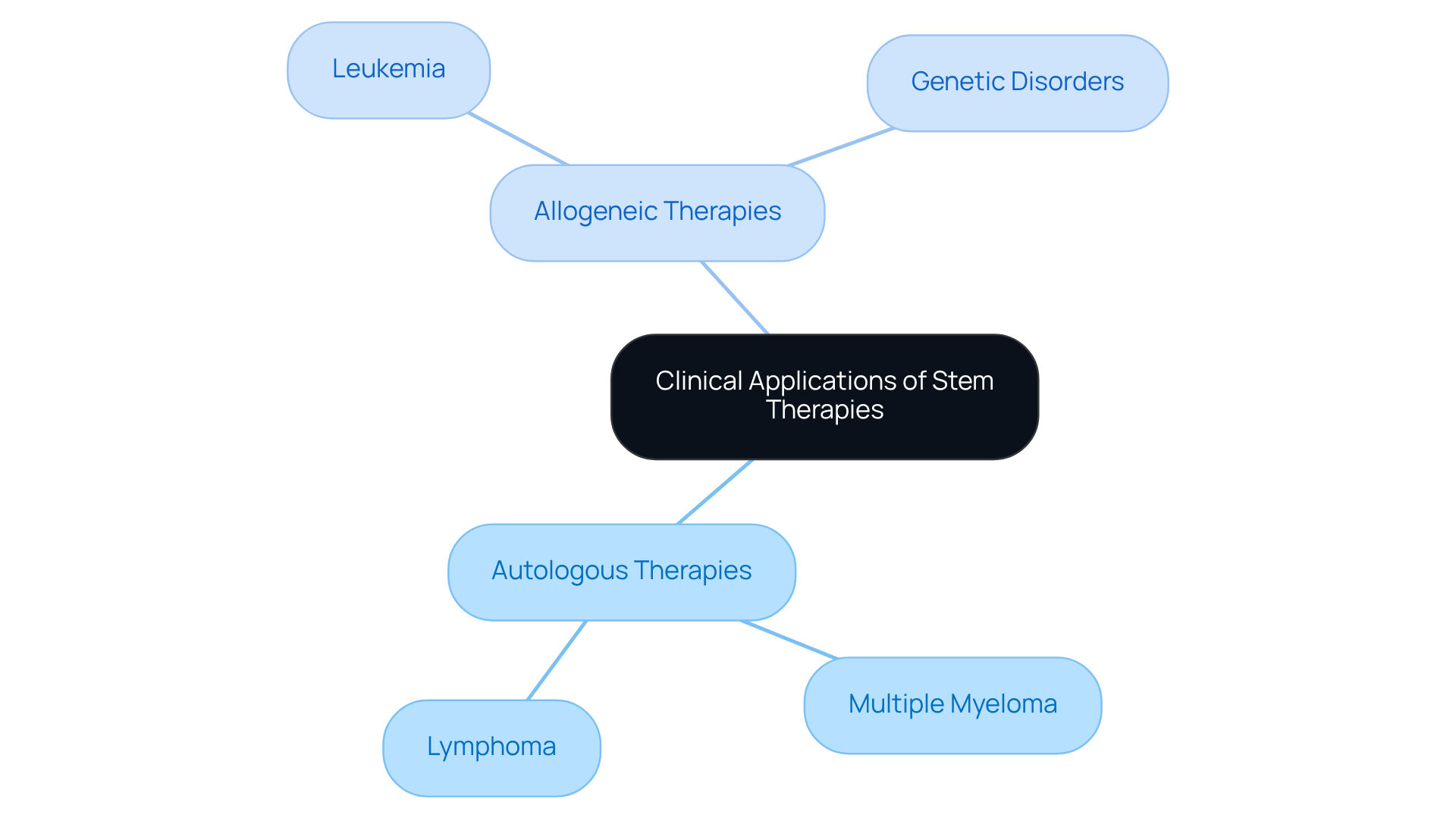
The management of blood cancers, including multiple myeloma and lymphoma, involves a pivotal role for autologous vs allogeneic stem treatments. In these cases, the individual’s own components are meticulously collected and subsequently reinfused following high-dose chemotherapy, optimizing recovery outcomes.
In contrast, treatments can be categorized as autologous vs allogeneic, with the latter often employed for conditions such as leukemia and certain genetic disorders. Here, donor components present a curative potential through immune reconstitution, significantly enhancing treatment efficacy.
Understanding the clinical applications of these treatment modalities is crucial for making informed care decisions, ultimately empowering patients and healthcare providers alike.
Safety Profiles: Evaluating Risks in Autologous and Allogeneic Therapies
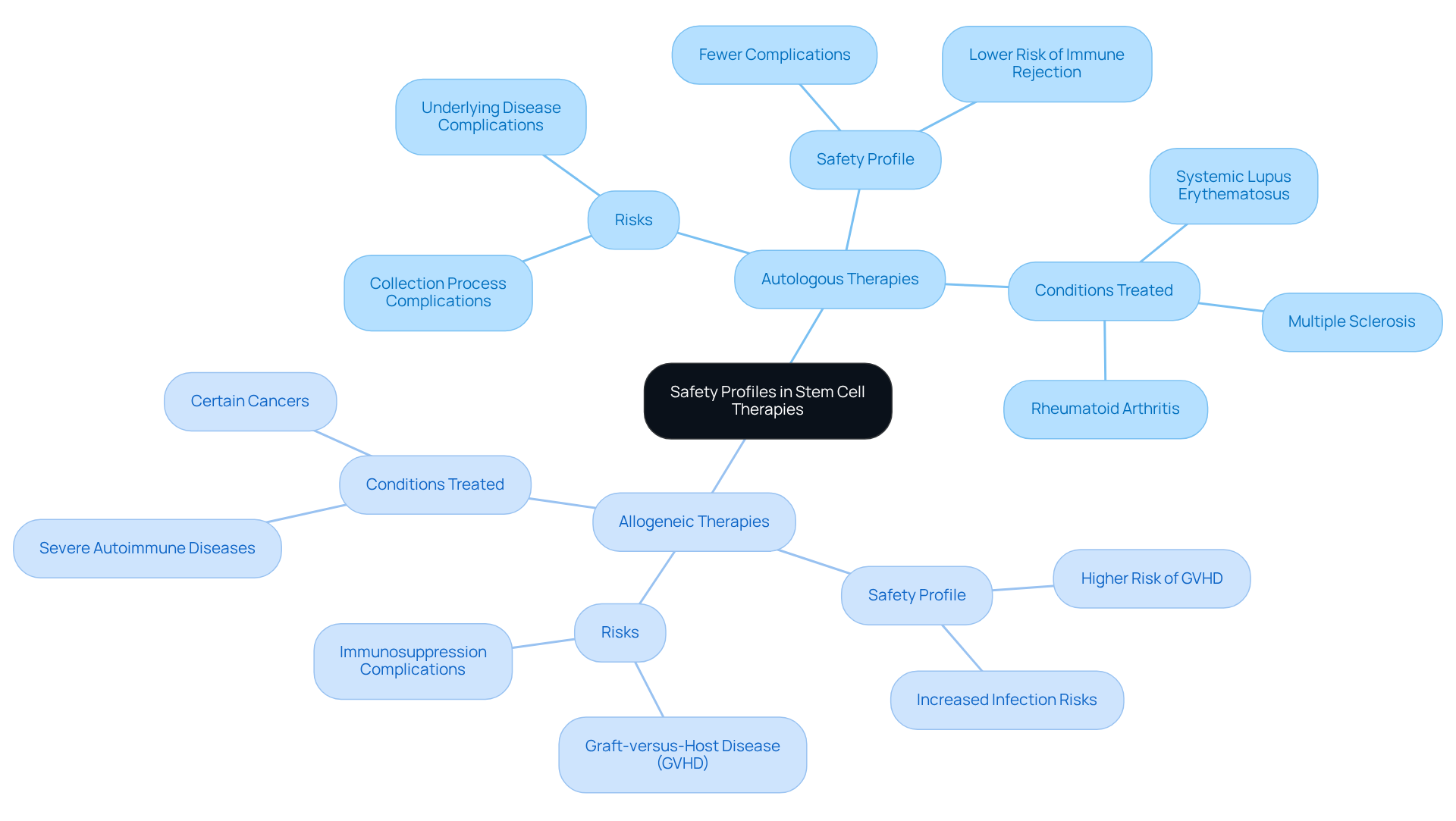
When considering autologous vs allogeneic stem cell treatments, typically exhibit a beneficial safety profile, as the cells are sourced from the patient, which significantly reduces the risk of immune rejection. Nevertheless, risks persist, particularly concerning the collection process and potential complications arising from the underlying disease.
In contrast, the comparison of autologous vs allogeneic donor-based treatments expands care options but introduces heightened risks, including a greater incidence of graft-versus-host disease (GVHD) and infections due to the necessary immunosuppression. For example, GVHD occurrence rates can soar to 50% in donor transplants, necessitating vigilant monitoring and management.
Evaluating these safety profiles is essential for clinicians when recommending treatment options and for patients as they consider their choices. Recent case studies underscore the importance of thorough risk evaluations, indicating that while autologous vs allogeneic interventions generally lead to fewer complications, donor-derived treatments can offer significant benefits for conditions such as systemic lupus erythematosus and rheumatoid arthritis, despite their associated risks.
Furthermore, there exists a risk of developing new autoimmune diseases post-HSCT, complicating the decision-making process for both healthcare professionals and patients. As healthcare professionals continue to refine treatment protocols, a comprehensive understanding of these dynamics will be crucial for optimizing patient outcomes.
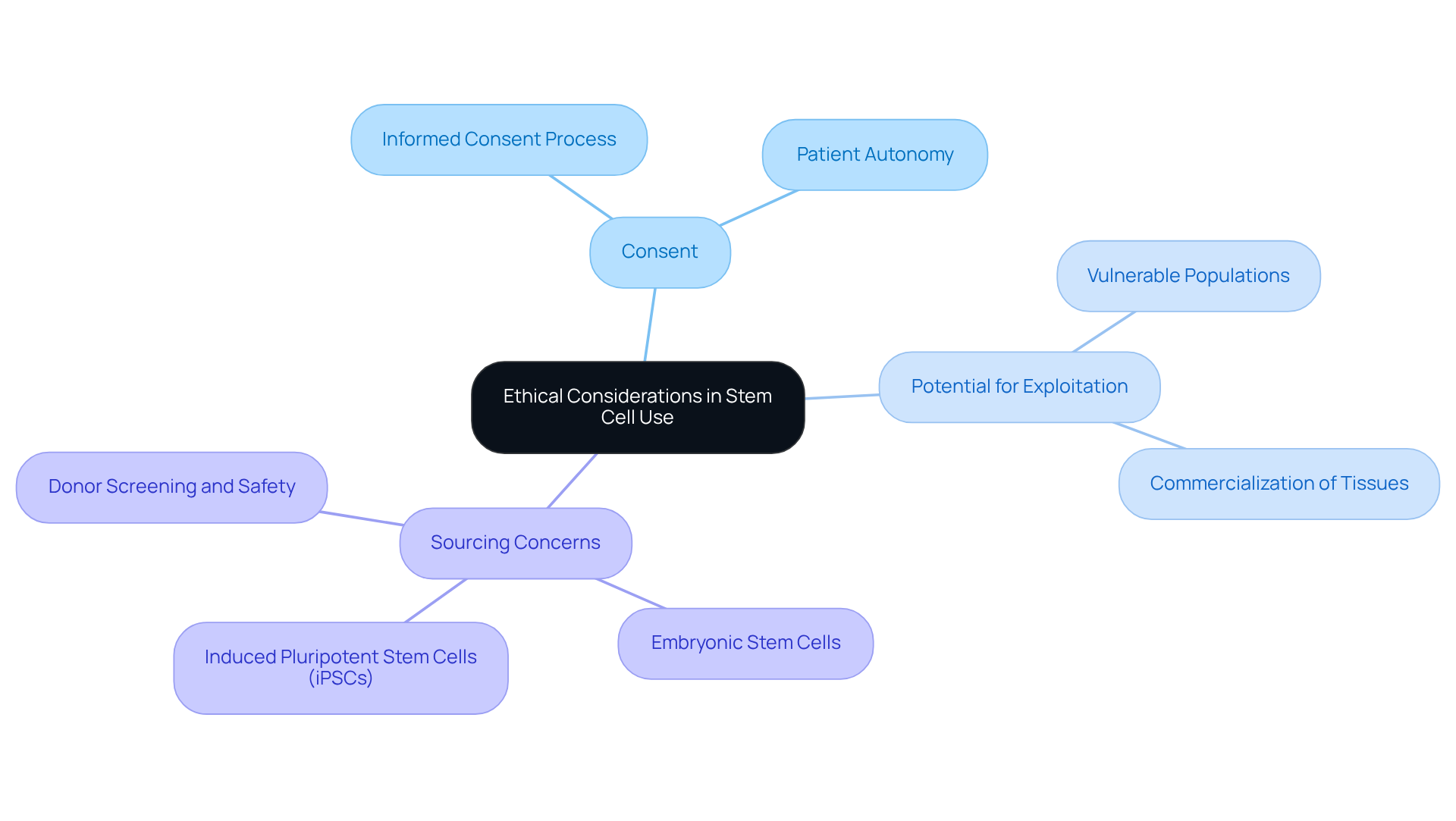
Ethical Considerations: Autologous vs Allogeneic Stem Cell Use
The application of autologous vs allogeneic tissues is generally perceived as ethically straightforward, since individuals are utilizing their own biological materials. However, transplant procedures that involve autologous vs allogeneic donor sources introduce significant ethical concerns, particularly regarding:
- Consent
- The potential for exploitation
- The ramifications of employing embryonic or induced pluripotent sources
These demand careful navigation to ensure that patient rights and welfare are at the forefront of clinical practice.
Cost Analysis: Comparing Autologous and Allogeneic Stem Cell Therapies
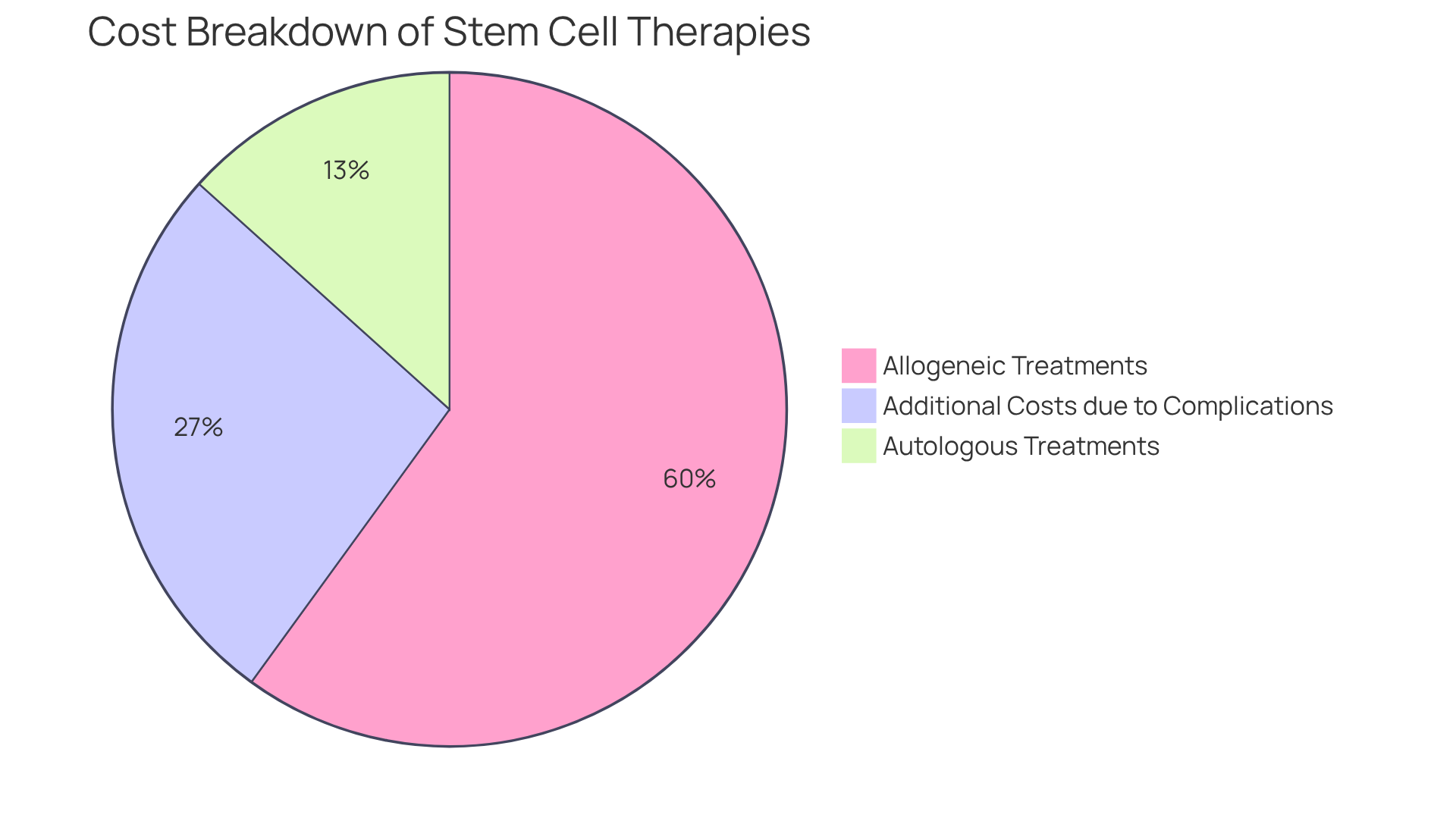
When considering autologous vs allogeneic stem cell treatments, autologous options generally incur lower expenses compared to donor-derived approaches, primarily due to the simpler production process and the lack of donor compatibility requirements. Costs for autologous treatments typically range from $5,000 to $15,000, whereas donor therapies can surpass $50,000. This substantial price discrepancy arises from the rigorous donor screening, matching procedures, and comprehensive post-transplant care associated with related treatments.
For instance, a study indicated that the median costs for T-cell depleted grafts were reported at $113,000, in contrast to $155,000 for non-depleted grafts, highlighting the financial implications of donor-related complexities. Furthermore, Lee et al. observed that complications such as to transplant expenses, further intensifying the financial burden.
Understanding the cost disparities in autologous vs allogeneic treatments is crucial for both patients and healthcare providers when assessing treatment options, as financial considerations significantly influence clinical decision-making. Moreover, the economic impact of post-transplant complications, including infections and GVHD, accentuates the costs associated with donor transplants, making it essential to evaluate these factors meticulously.
Additionally, Saito et al. noted that patients undergoing reduced intensity conditioning experienced lower median costs and fewer hospital days compared to those on high-dose regimens, underscoring the variability within donor-derived treatments. The broader societal burden of cancer care, projected to increase considerably, emphasizes the necessity of comprehending these costs within the framework of healthcare expenditures.
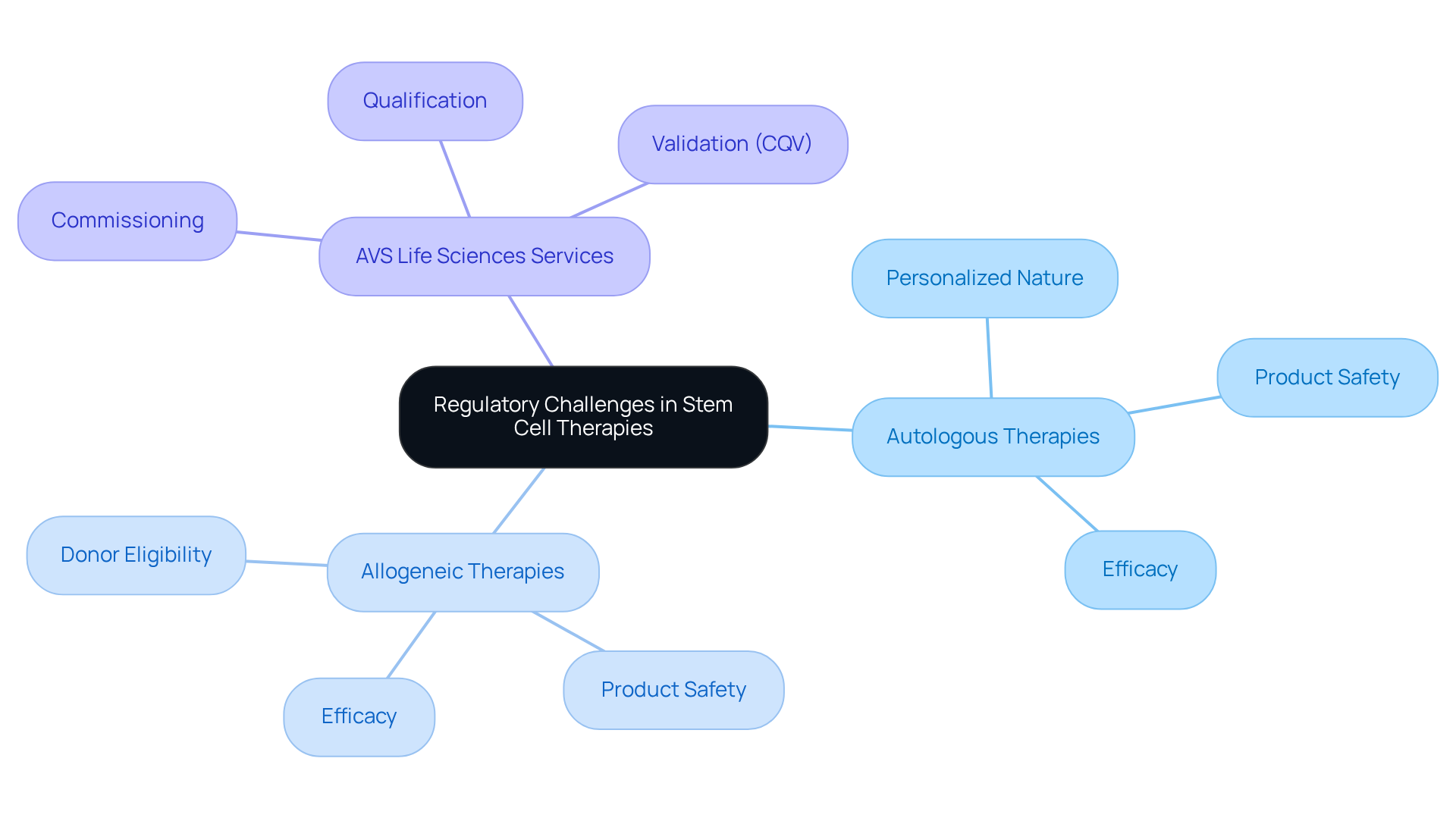
Regulatory Challenges: Navigating Compliance in Stem Cell Therapies
The regulatory challenges encountered by treatments can be differentiated as autologous vs allogeneic stem cell therapies. The comparison of autologous vs allogeneic approaches often reveals hurdles related to their personalized nature, complicating adherence to current regulations. Conversely, in the context of autologous vs allogeneic treatments, donor-derived options, while potentially more scalable, must navigate stringent regulations concerning donor eligibility, product safety, and efficacy.
AVS Life Sciences stands as your dedicated partner for comprehensive regulatory and quality solutions, offering services such as:
- Commissioning
- Qualification
- Validation (CQV)
alongside quality management to assist clients in overcoming these challenges. With extensive knowledge in biopharmaceuticals, medical devices, and nutraceuticals, AVS Life Sciences ensures that treatments meet the for successful market entry.
For more information on how we can support your regulatory needs, contact us today.
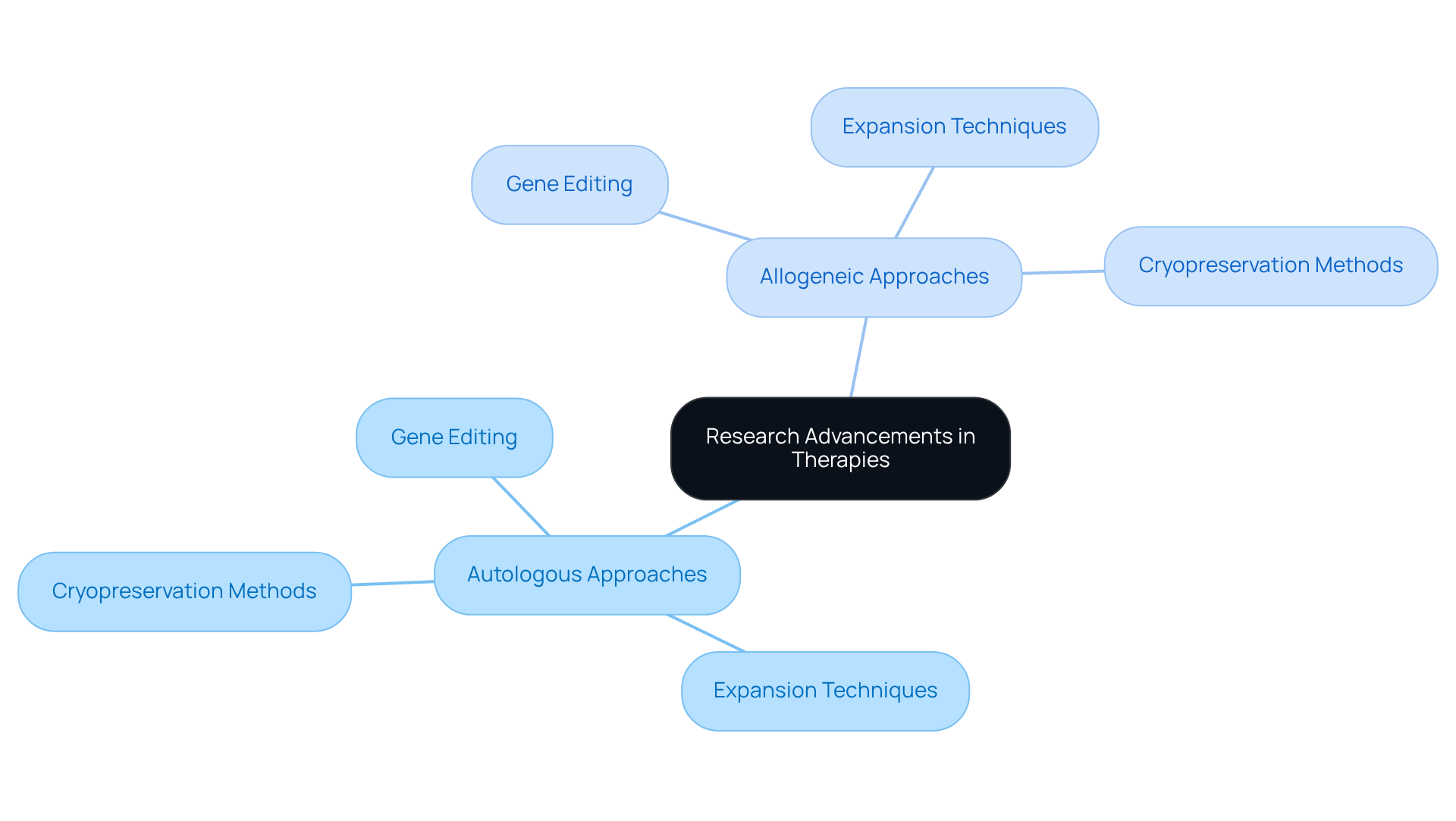
Research Advancements: Innovations in Autologous and Allogeneic Therapies
Recent advancements in therapies, particularly in the context of autologous vs allogeneic approaches involving progenitor units, have significantly enhanced treatment effectiveness and safety. Notably, innovations such as gene editing, which enables precise alterations of foundational units, have shown considerable potential in improving therapeutic outcomes.
Furthermore, advanced expansion techniques are facilitating the generation of increased quantities of viable units, while enhanced cryopreservation methods ensure improved preservation of progenitor integrity during storage and transport.
These advancements not only augment the but also address critical challenges related to production and regulatory compliance. It is imperative for healthcare professionals and researchers to remain knowledgeable about these developments as they navigate the evolving landscape of regenerative treatments.
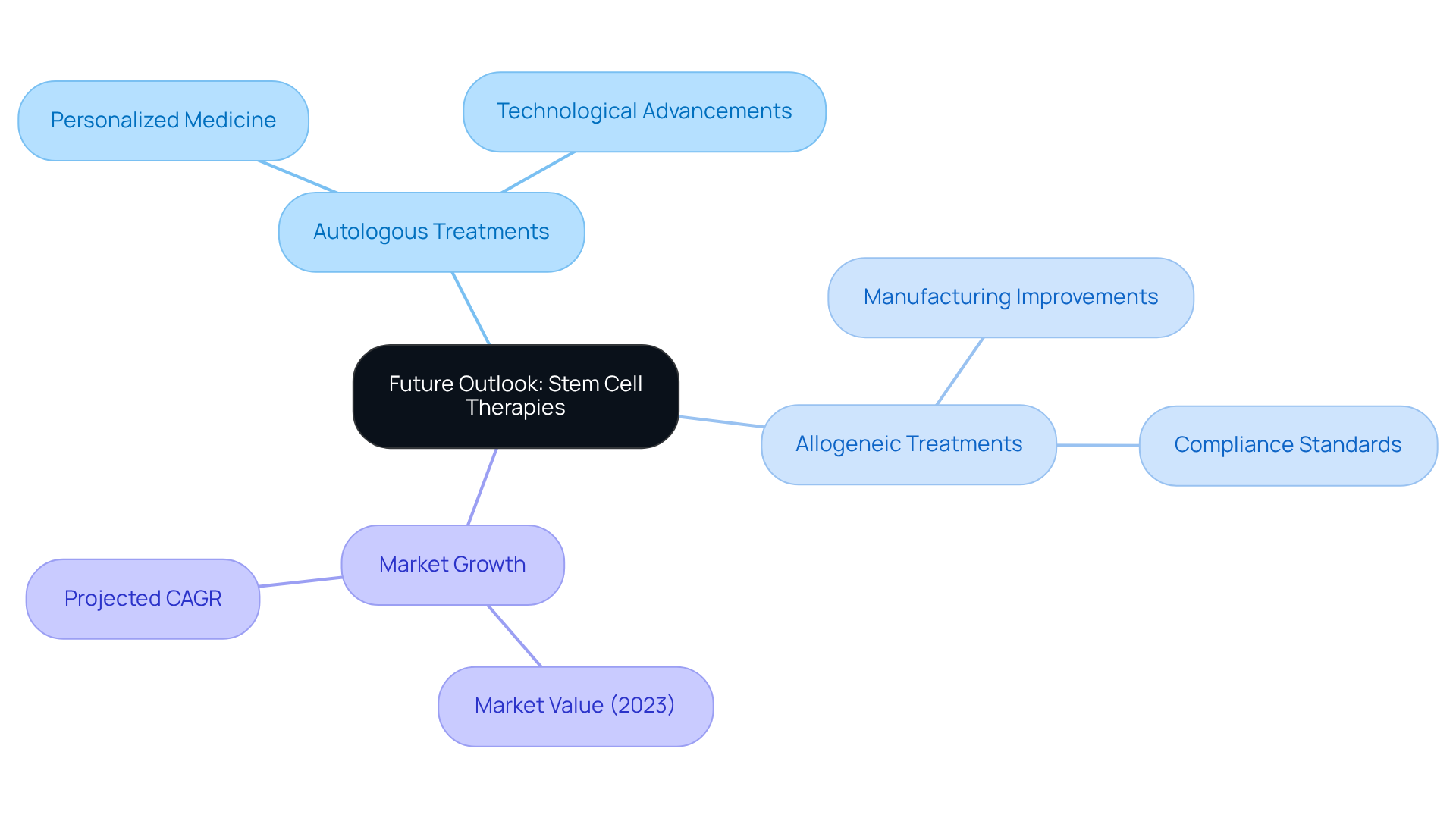
Future Outlook: The Evolution of Autologous and Allogeneic Stem Cell Therapies
The future of autologous vs allogeneic treatments is exceptionally promising, driven by ongoing research and technological advancements poised to enhance both their effectiveness and accessibility. As the field progresses, we foresee a significant integration of personalized medicine approaches, tailoring treatments to meet individual patient needs. This transformative shift will be bolstered by , exemplified by AVS Life Sciences, which specializes in upgrading GMP facilities to adhere to stringent compliance standards. Their recent case study showcases how AVS adeptly guided a pharmaceutical manufacturer in transitioning from a Biosafety Level 1 to a Level 2 GMP facility, thereby enabling the client to manufacture lentivirus vector material.
Such advancements in facility compliance are crucial for developing safer and more efficient treatments, ultimately broadening the applications of regenerative medicine across various medical conditions. This evolution is expected to yield enhanced patient outcomes and an expansion of available treatment options. Notably, the global stem cell therapy market, valued at USD 286 million in 2023, is projected to grow at a compound annual growth rate (CAGR) of 16.5%, underscoring the rising demand for innovative therapies that address complex health challenges.
Conclusion
The exploration of autologous versus allogeneic stem cell therapies reveals significant distinctions that are crucial for informed decision-making in regenerative medicine. Autologous therapies utilize a patient's own cells, enhancing compatibility and minimizing rejection risks, whereas allogeneic therapies involve donor cells, which can introduce additional complications. Understanding these differences is vital for healthcare providers and patients alike as they navigate treatment options tailored to individual needs.
Key insights from this comparison emphasize the varying safety profiles, manufacturing processes, clinical applications, and cost implications associated with each therapy type.
- Autologous treatments generally present a safer profile with fewer complications.
- Allogeneic therapies may offer broader applications for certain conditions but come with increased risks and costs.
- Ethical considerations and regulatory challenges further complicate the landscape, underscoring the need for meticulous evaluation in treatment planning.
As advancements in research and technology continue to evolve, the future of both autologous and allogeneic stem cell therapies looks promising. Embracing personalized medicine and improving manufacturing processes will enhance treatment efficacy and accessibility. Stakeholders in the healthcare sector must remain vigilant in understanding these dynamics, as the landscape of stem cell therapies is poised for significant growth and innovation, ultimately leading to better patient outcomes and expanded treatment options.
Frequently Asked Questions
What is the role of AVS Life Sciences in stem cell therapies?
AVS Life Sciences ensures that stem cell treatments comply with regulatory standards, including Good Manufacturing Practices (GMP), Quality System Regulations (QSR), and ISO standards. They provide regulatory and quality solutions across various sectors to help clients navigate regulatory submissions and compliance, ensuring treatments are safe and effective.
What are the differences between autologous and allogeneic stem cell treatments?
Autologous treatments involve harvesting progenitor cells from the patient's own body, enhancing compatibility and reducing the risk of immune rejection. Allogeneic treatments use progenitor cells from healthy donors, which may introduce risks of immune reactions. Autologous treatments generally have lower complication rates, making them safer for many patients.
What are the risks associated with allogeneic stem cell therapies?
Allogeneic stem cell therapies have elevated immune rejection rates compared to autologous therapies, necessitating thorough donor compatibility assessments to mitigate these risks. This can lead to complications such as graft-versus-host disease (GVHD).
How do financial implications differ between autologous and allogeneic treatments?
Autologous tissue transplants can be more cost-effective compared to lifelong therapies for chronic conditions. Clinicians must evaluate these financial factors when recommending treatment options, as they impact therapeutic strategies and the likelihood of successful outcomes.
What are the manufacturing processes for autologous and allogeneic stem cell therapies?
Autologous stem cell therapies involve collecting biological material from the patient, processing it, and reinfusing it after expansion or modification, which is often time-consuming. Allogeneic treatments can be produced in larger quantities, allowing for quicker access and scalability.
Why is understanding the differences between autologous and allogeneic treatments important?
Understanding these differences is crucial for patient well-being and the specific ailments being addressed. Success rates can vary based on the condition, and recent studies indicate a 60-70% success rate for regenerative treatments in blood cancers, highlighting the significance of this knowledge in modern medicine.
What is the focus of AVS Life Sciences regarding regulatory compliance?
AVS Life Sciences focuses on ensuring quality and regulatory compliance throughout the drug development lifecycle, positioning itself as a trusted partner in the life sciences sector, especially in the rapidly evolving field of regenerative treatments.
