8 Key Benefits of Decentralized Trials for Compliance Officers

Overview
The article delineates eight pivotal benefits of decentralized trials specifically for compliance officers. It emphasizes enhanced patient engagement, streamlined data collection, and improved adherence to regulatory standards. Evidence substantiates these benefits, demonstrating that decentralized trials significantly boost participant retention rates, mitigate logistical challenges, and leverage innovative technologies. Ultimately, these factors contribute to superior data integrity and operational efficiency in clinical research, underscoring the necessity for compliance officers to embrace these advancements.
Introduction
Decentralized trials are reshaping the landscape of clinical research, presenting a myriad of advantages that compliance officers can leverage amidst the complexities of regulatory adherence. These innovative methodologies significantly enhance patient engagement, streamline data collection, and yield substantial cost savings along with operational efficiencies.
However, as organizations adopt these advancements, they must confront the challenges of maintaining compliance and ensuring data integrity within an evolving regulatory environment.
What key benefits can compliance officers harness from decentralized trials to optimize their processes and elevate research outcomes?
AVS Life Sciences: Enhanced Compliance and Quality Assurance in Decentralized Trials
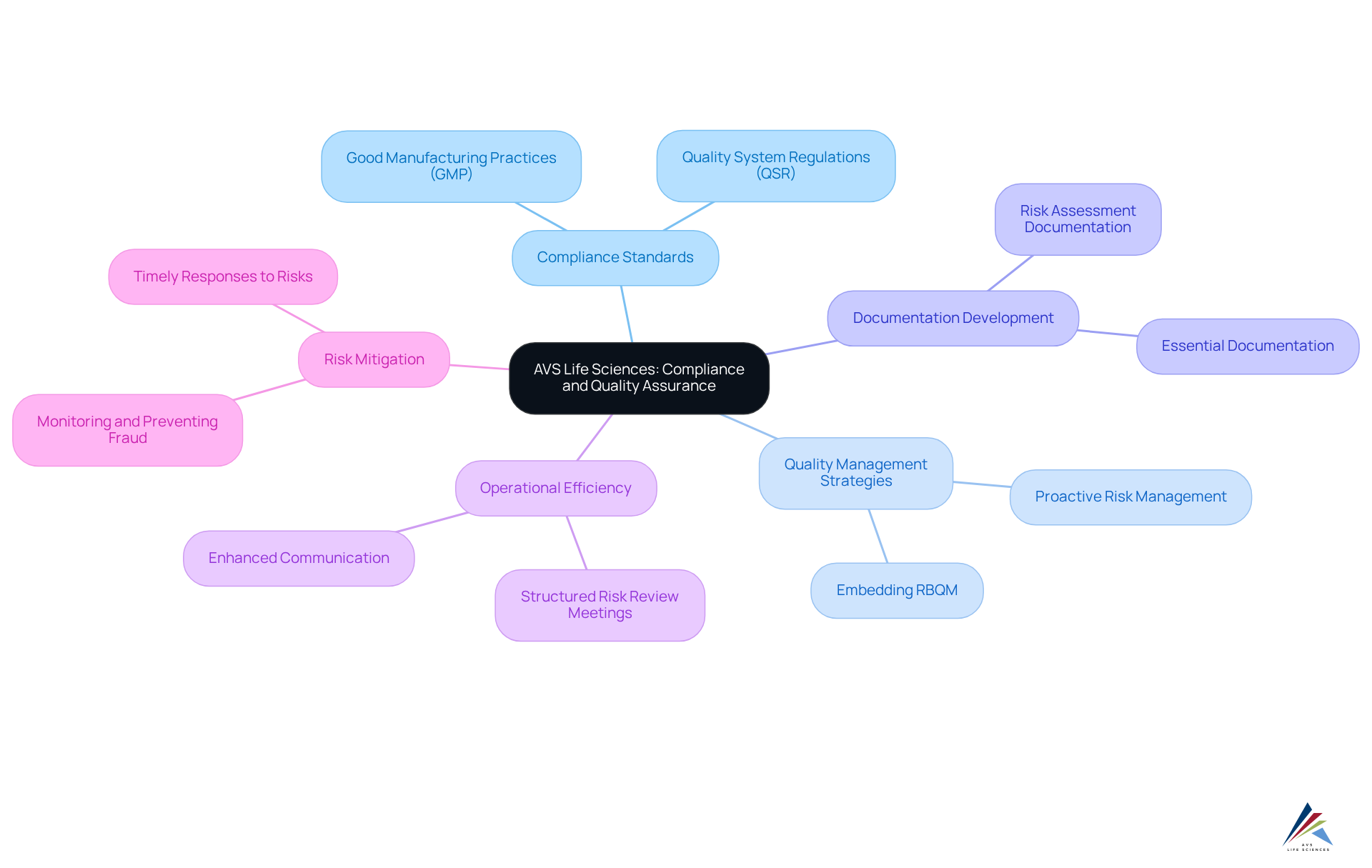
AVS Life Sciences is pivotal in ensuring that decentralized trials meet stringent compliance standards. By implementing phase-appropriate quality and regulatory strategies, the company adeptly navigates clients through the complexities of adherence in these innovative environments. Their proficiency in developing essential documentation and systems guarantees that all experimental activities align with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR). This unwavering commitment to quality assurance not only mitigates risks but also bolsters the overall integrity of the testing process, positioning AVS as a trusted partner for regulatory officers within the life sciences sector.
Recent advancements in quality assurance underscore the importance of these practices in decentralized trials, enhancing operational efficiency and ensuring compliance with evolving regulatory demands. Effective compliance strategies are rooted in a proactive approach to quality management, which is vital for tackling the unique challenges posed by decentralized trials. With AVS Life Sciences, organizations can confidently engage in that elevate their research integrity and operational success.
Improved Patient Engagement Through Decentralized Trials
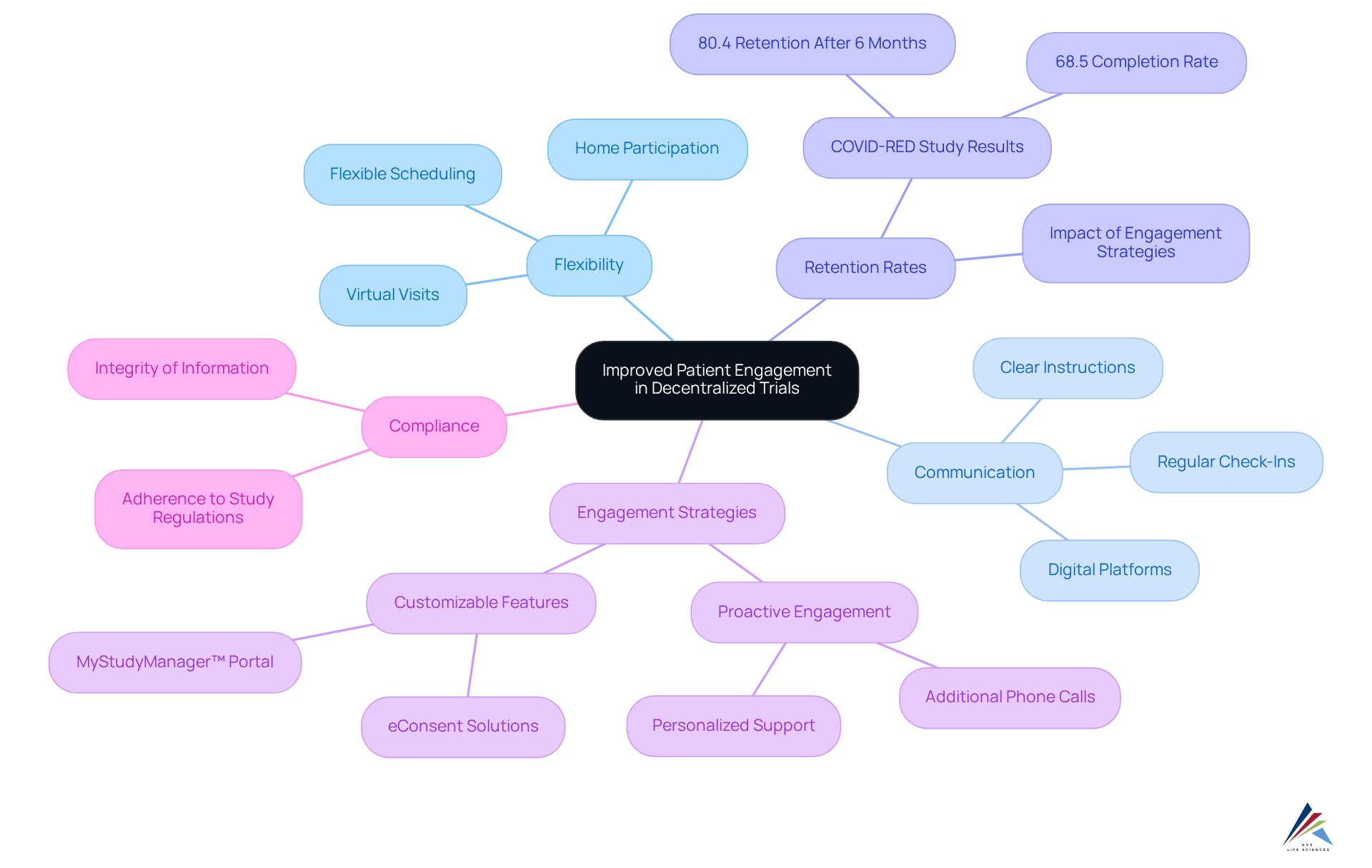
Decentralized trials significantly enhance patient involvement by allowing participants to engage in research from the comfort of their homes. This flexibility alleviates the burden of travel and time commitment, making . Improved communication through digital platforms fosters a sense of connection between patients and study coordinators, resulting in higher retention rates.
For instance, in the COVID-RED study, which enrolled 17,825 participants, 80.4% remained engaged after six months, demonstrating the effectiveness of these strategies. Furthermore, the dropout rate for vaccinated pensioners was reduced to 11.4% after additional phone calls, underscoring the impact of proactive engagement efforts.
Compliance officers can leverage these engagement strategies to ensure that participants remain dedicated to the study, ultimately enhancing information integrity and adherence to regulatory requirements. Engaged patients in decentralized trials are less likely to withdraw and tend to comply with study regulations, which is essential for the success of these studies.
Platforms like ENGAGE! offer customizable features that empower sites to manage participant interactions and streamline consent procedures, thereby further improving patient engagement.
Streamlined Data Collection and Real-Time Monitoring
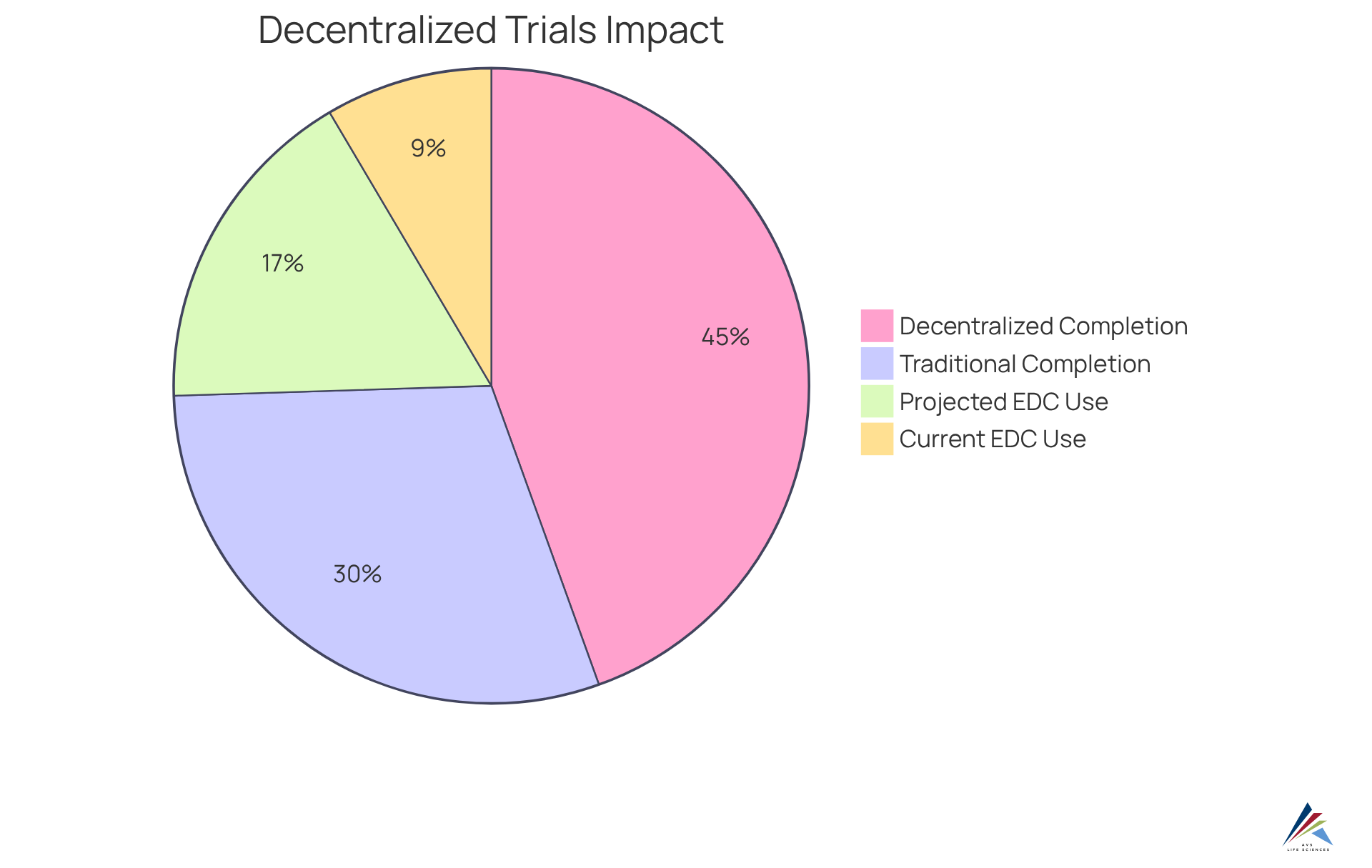
Decentralized trials harness advanced technologies to enhance information collection and facilitate real-time oversight, adhering to GXP and FDA standards. Electronic information capture (EDC) systems, alongside mobile health applications, enable continuous gathering of participant information, facilitating immediate analysis and timely interventions. This capability significantly enhances information quality, empowering oversight personnel to effectively monitor adherence to research protocols, thereby ensuring robust documentation practices and alignment with Standard Operating Procedures (SOPs). By minimizing delays in information reporting, organizations can swiftly address any compliance issues that may arise, fostering a more efficient and responsive process.
Current trends indicate that the integration of real-time monitoring technologies is set to expand, with forecasts suggesting that the average percentage of EDC studies collecting patient information from sensors and wearables is anticipated to double from 17% to 34% within the next two years. Furthermore, an impressive 89% of patients who chose the decentralized trials completed the study, compared to just 60% of those in traditional studies, underscoring the effectiveness of decentralized approaches. This shift not only enhances compliance but also fosters a proactive stance on and information integrity.
Compliance officers are encouraged to consider the incorporation of EDC systems into their study designs to elevate data quality and participant engagement while ensuring strict adherence to regulatory standards.
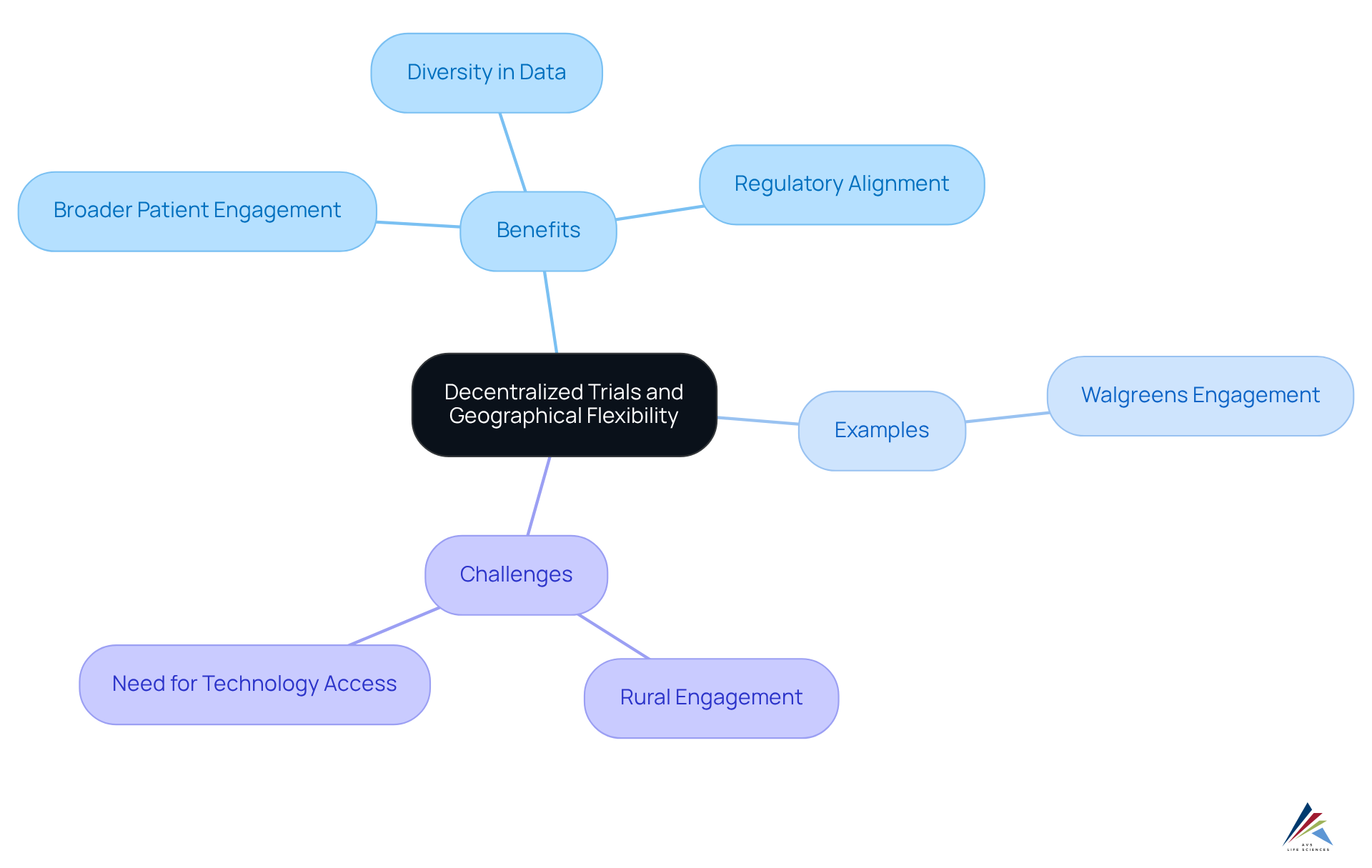
Geographical Flexibility and Access to Diverse Patient Populations
Decentralized trials present significant geographical flexibility, enabling researchers to engage a broader and more diverse patient population. By eliminating the necessity for participants to travel to centralized locations, these decentralized trials can effectively include individuals from varied demographics and regions. This inclusivity not only enhances the representativeness of study data but also aligns with in clinical research.
AVS Life Sciences empowers life sciences businesses through expert knowledge and oversight, fostering a community that champions diversity, equity, and inclusion. For instance, Walgreens has successfully engaged over 4 million patients for potential enrollment in clinical studies, with more than 60% being women, underscoring the opportunity for diverse participation.
Compliance officers can leverage this geographical flexibility to ensure their studies meet critical diversity benchmarks, ultimately enhancing the robustness and validity of outcomes. Decentralized trials demonstrate an ability to recruit a wider range of patients, which is vital for developing treatments that are effective across various populations.
Nevertheless, challenges persist in maintaining engagement from rural participants, emphasizing the necessity for ongoing investment in education and technology access to sustain these diversity gains.
Cost-Effectiveness and Resource Optimization in Trial Management
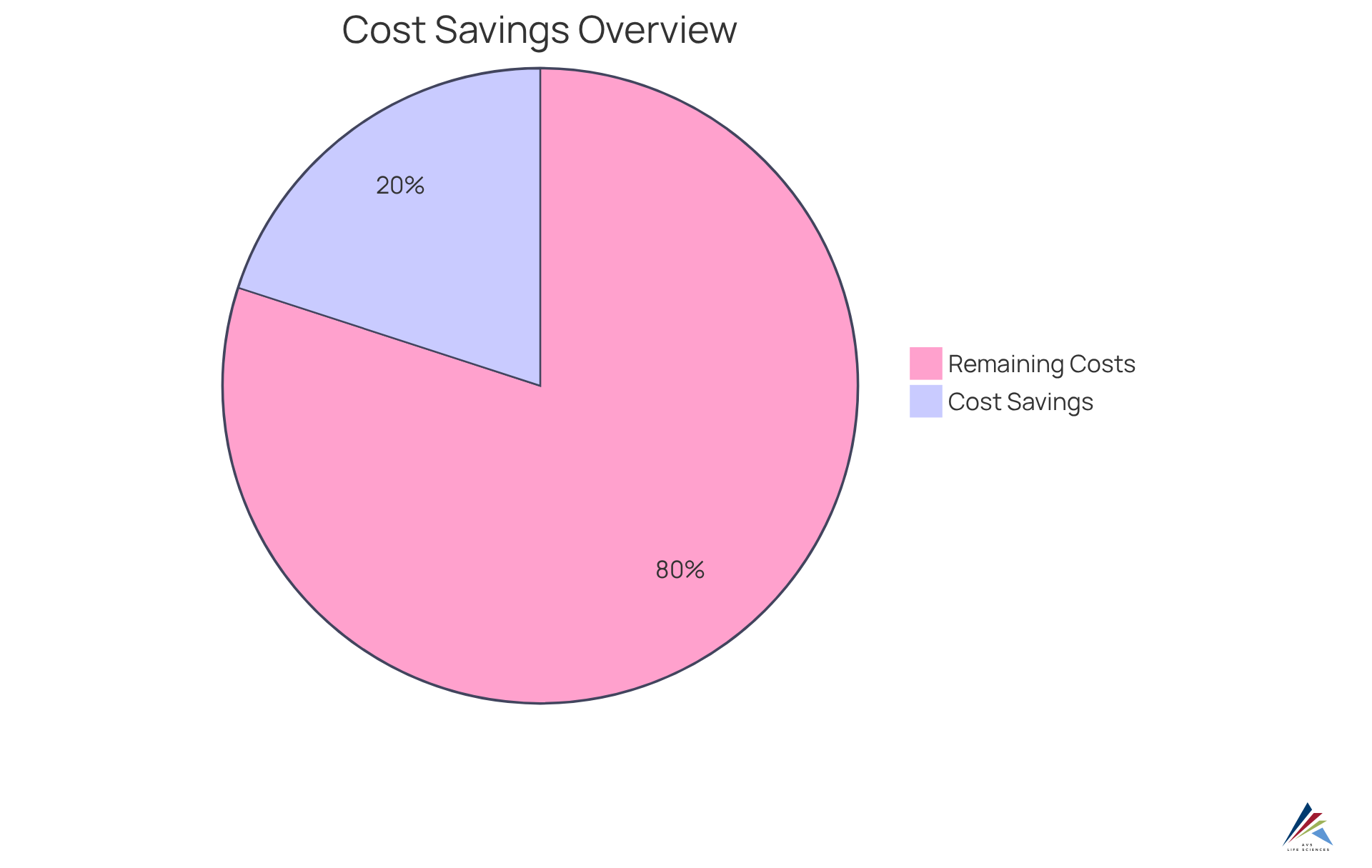
Decentralized trials present a compelling solution to the pressing challenges of compliance and resource management. By significantly reducing the need for physical sites and the associated overhead costs, organizations can allocate resources with remarkable efficiency. Furthermore, the integration of telemedicine and remote monitoring technologies minimizes travel expenses for both participants and staff, streamlining operations. Compliance officers can leverage these cost-effective strategies to enhance budget allocations while ensuring that all regulatory requirements are meticulously met. This approach not only fosters a more but also aligns with the evolving landscape of clinical research. Notably, research indicates that decentralized trials can yield a 10-25% reduction in costs, making them an attractive option for organizations keen on optimizing resources while maintaining compliance. The evidence is clear: embracing decentralized trials is not merely an operational shift; it is a strategic imperative for forward-thinking organizations.
Accelerated Participant Recruitment and Retention
Decentralized trials significantly simplify participant recruitment and retention by eliminating the logistical challenges associated with conventional site visits. By providing remote involvement options, potential participants can engage with studies more conveniently, broadening the candidate pool and enhancing retention rates.
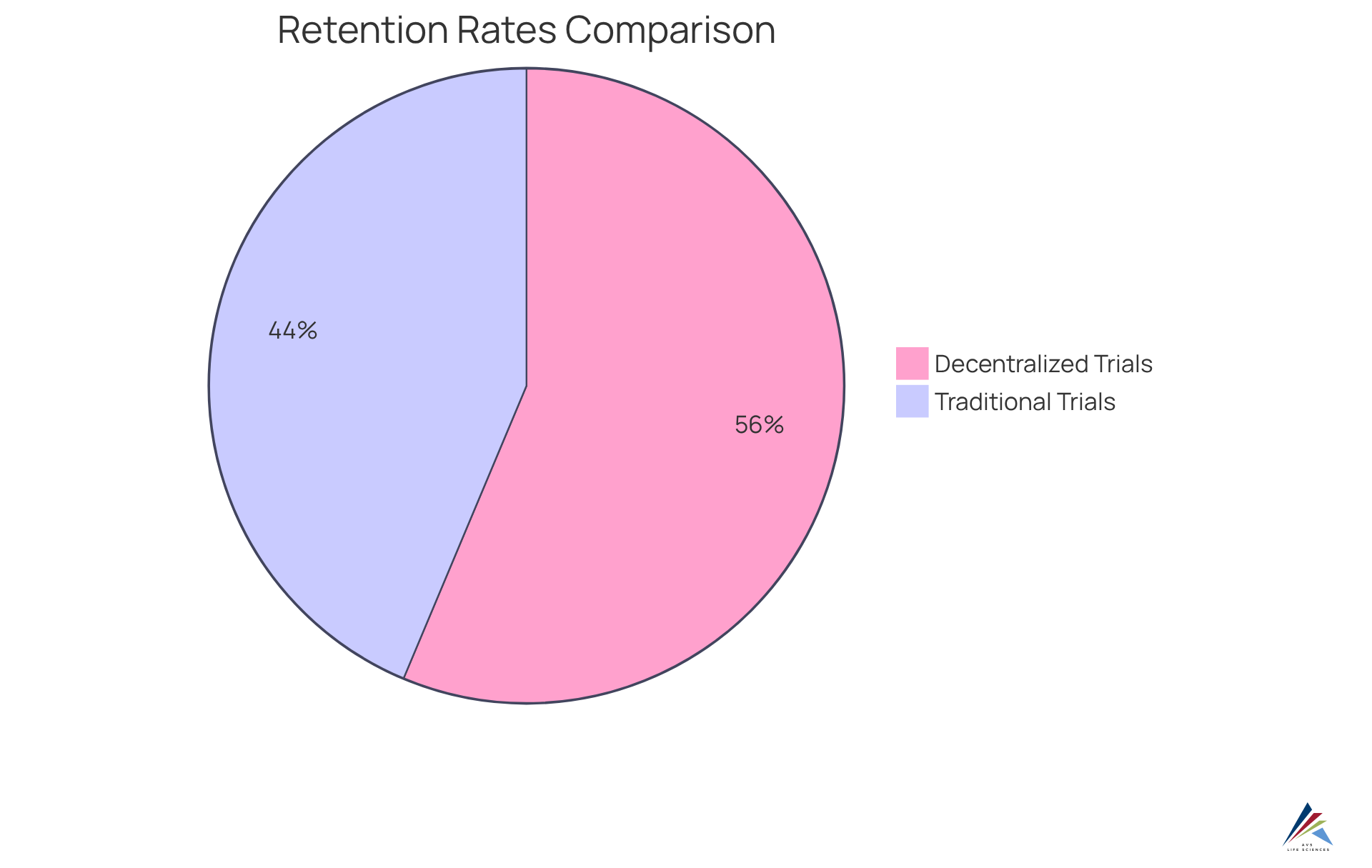
Research indicates that retention rates in decentralized trials can reach up to 89%, compared to just 69% in traditional studies, showcasing the effectiveness of these models. Compliance officers can leverage these innovative recruitment strategies to meet enrollment targets while ensuring adherence to regulatory timelines.
As Amanda McDowell, a recruitment expert, states, 'Decentralized patient recruitment refers to locating individuals not enrolled at your clinical research location, but who reside nearby and inviting them for screening.'
Furthermore, the integration of telehealth and local lab services allows for a more patient-centric approach, addressing common barriers such as travel distance—where studies show that for every additional 30 miles a patient must travel, enrollment and retention rates decrease by 10 percent.
This shift towards decentralized trials not only enhances participant commitment but also fosters a more , ultimately leading to more robust data and improved outcomes.
Reduced Logistical Challenges and Increased Operational Efficiency
Decentralized trials effectively alleviate the logistical challenges inherent in conventional clinical research. By enabling remote testing activities, organizations can significantly lessen the difficulties associated with coordinating multiple site visits and managing transportation logistics. This shift not only streamlines study management but also , as all study activities can be executed in a timely and organized manner.
In fact, 75% of respondents who have participated in decentralized or virtual studies reported achieving cost savings, underscoring the financial advantages of this method. Moreover, 79% of research team members observed that decentralized trials improve data quality, which is essential for maintaining compliance standards. Compliance officers stand to gain immensely from these efficiencies, allowing them to focus on monitoring and ensuring adherence to legal requirements rather than grappling with logistical hurdles.
As logistics specialists emphasize, selecting a single logistics partner capable of scaling services worldwide can further optimize operations, ensuring that tests proceed seamlessly and effectively in 2025 and beyond. Furthermore, AVS Life Sciences offers comprehensive quality management and regulatory adherence solutions that can enhance the effectiveness of decentralized trials.
As noted by Amos J de Jong, 'Data collected in DCTs are furthermore expected to be more representative of the real-world,' highlighting the critical importance of real-world applicability in compliance efforts. Additionally, 74% of respondents believe that decentralized trials are superior for patient retention compared to conventional studies, further supporting the advantages of this approach.
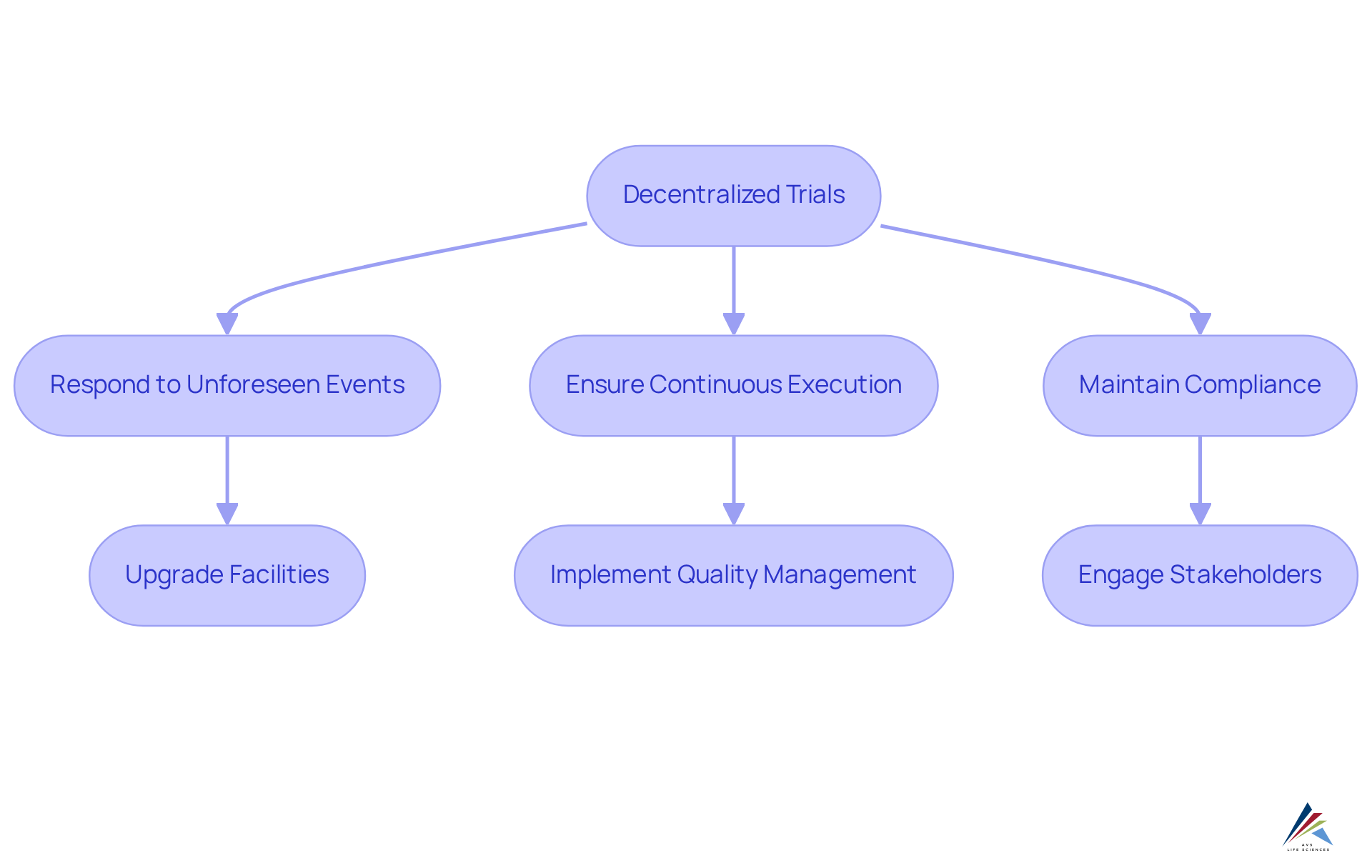
Adaptability to Changing Conditions and Public Health Needs
Decentralized trials provide exceptional flexibility in response to evolving circumstances and public health demands. In the face of unforeseen events, such as pandemics or natural disasters, these methodologies allow trials to continue without interruption. This adaptability is crucial for oversight personnel, facilitating ongoing compliance with legal obligations while meeting the dynamic needs of participants and stakeholders.
For instance, AVS Life Sciences successfully upgraded a biotechnology GMP facility from a Biosafety Level 1 to a Level 2 GMP facility, ensuring comprehensive quality management and strict adherence to regulations. This transformation exemplifies how decentralized trials support the continuous execution of clinical studies, highlighting their effectiveness in and compliance.
Consequently, organizations can ensure their assessments remain both pertinent and compliant in an ever-changing landscape.
Enhanced Data Integrity and Security Measures
Decentralized trials significantly improve information integrity and security by utilizing advanced technologies and stringent protocols. Organizations employ encrypted information transmission and secure storage methods to protect sensitive participant details, ensuring adherence to legal standards. Frequent audits and ongoing surveillance of information access improve security protocols, enabling compliance officers to uphold oversight and confirm that all information handling practices conform to regulatory standards.
AVS Life Sciences exemplifies this commitment through its successful upgrade of a biotechnology GMP facility, transitioning from a Level 1 to a Level 2 GMP facility. This project not only ensured adherence to stringent but also enhanced the client's ability to manufacture medication with lentivirus vector material, allowing them to focus on developing life-saving medicines.
By prioritizing information integrity, organizations enhance the credibility and reliability of their testing results while aligning with the evolving landscape of cybersecurity, where safeguarding participant information is essential. Moreover, the incorporation of Personal Health Records (PHRs) can improve information accuracy and reliability, addressing deficiencies in information completeness in decentralized trials.
However, challenges persist in ensuring information quality and integrity, which regulators such as the FDA and EMA stress as essential elements of successful clinical studies.
Utilization of Innovative Technologies in Decentralized Trials
The incorporation of cutting-edge technologies in decentralized trials is revolutionizing clinical research, ensuring strict adherence to legal standards and quality management practices. Tools such as , mobile health applications, and telemedicine platforms facilitate remote monitoring and data collection, significantly enhancing participant engagement and adherence. These technologies not only optimize testing processes but also provide compliance officers with real-time insights into testing activities, enabling proactive management of regulatory adherence, including GXP and FDA regulations.
Moreover, the implementation of Standard Operating Procedures (SOPs) alongside effective Technical Writing practices is crucial for upholding comprehensive quality management standards. By embracing these innovations, organizations can significantly improve the efficiency and effectiveness of their decentralized trials, ultimately resulting in better health outcomes and enhanced patient experiences.
Conclusion
Decentralized trials represent a transformative approach in clinical research, offering substantial benefits for compliance officers and organizations alike. By integrating innovative technologies and flexible methodologies, these trials not only enhance participant engagement but also streamline compliance processes. The shift towards decentralized models is not merely a trend; it is a strategic evolution that supports the integrity and reliability of clinical outcomes.
Key insights from the discussion highlight the multifaceted advantages of decentralized trials. These include:
- Improved patient engagement through remote participation
- Streamlined data collection and real-time monitoring
- Geographical flexibility that promotes diversity
- Significant cost savings
- Accelerated participant recruitment and retention
- Reduced logistical challenges
Furthermore, these trials ultimately foster a more efficient and compliant research environment.
The implications of embracing decentralized trials extend beyond immediate operational benefits. Organizations are encouraged to adopt these innovative practices to ensure they remain competitive in a rapidly evolving landscape. By prioritizing compliance and quality assurance, compliance officers can not only enhance the integrity of their studies but also contribute to the advancement of clinical research that is inclusive, efficient, and responsive to the needs of diverse populations. The future of clinical trials lies in the hands of those willing to innovate and adapt, making decentralized trials a crucial element in the journey towards more effective healthcare solutions.
Frequently Asked Questions
What role does AVS Life Sciences play in decentralized trials?
AVS Life Sciences ensures that decentralized trials meet compliance standards by implementing phase-appropriate quality and regulatory strategies, developing essential documentation, and systems that align with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR).
How does AVS Life Sciences enhance compliance in decentralized trials?
The company adopts a proactive approach to quality management, which helps tackle the unique challenges of decentralized trials, ultimately enhancing operational efficiency and ensuring compliance with evolving regulatory demands.
What are the benefits of decentralized trials for patient engagement?
Decentralized trials allow participants to engage in research from home, reducing travel burdens and time commitments, which makes participation more appealing and fosters improved communication, leading to higher retention rates.
Can you provide an example of successful patient engagement in a decentralized trial?
In the COVID-RED study, 80.4% of the 17,825 enrolled participants remained engaged after six months, demonstrating the effectiveness of engagement strategies. Additionally, the dropout rate for vaccinated pensioners was reduced to 11.4% with proactive phone calls.
How do decentralized trials improve data collection and monitoring?
They utilize advanced technologies such as electronic information capture (EDC) systems and mobile health applications to gather participant information continuously, enabling real-time analysis and timely interventions, thus enhancing information quality and compliance.
What trends are expected in real-time monitoring technologies for decentralized trials?
The integration of real-time monitoring technologies is expected to expand, with forecasts indicating that the percentage of EDC studies collecting patient information from sensors and wearables will double from 17% to 34% within the next two years.
What is the completion rate for patients in decentralized trials compared to traditional studies?
An impressive 89% of patients who chose decentralized trials completed the study, compared to only 60% of those in traditional studies, highlighting the effectiveness of decentralized approaches.
What should compliance officers consider when designing studies?
Compliance officers are encouraged to incorporate EDC systems into their study designs to enhance data quality, participant engagement, and ensure strict adherence to regulatory standards.
