8 Essential Steps for Effective Cleaning and Process Validation

Overview
The article outlines eight essential steps for effective cleaning and process validation in the pharmaceutical and biotechnology sectors. It emphasizes the critical importance of regulatory compliance and quality management. A structured approach is detailed, encompassing:
- An understanding of regulatory expectations
- The implementation of a lifecycle methodology
- The definition of key components
- The establishment of acceptance criteria
Each of these elements is necessary to ensure thorough sanitation and safety in manufacturing processes. By addressing compliance challenges head-on, the article not only presents solutions but also equips readers with actionable insights that foster engagement with AVS Life Sciences.
Introduction
In the highly regulated pharmaceutical and biotechnology sectors, the significance of effective cleaning and process validation is paramount. Organizations are compelled to meet stringent regulatory requirements while ensuring product safety, all the while navigating complex validation protocols that must evolve with industry standards.
This article explores eight essential steps that not only streamline the cleaning and validation process but also enhance compliance and operational efficiency, ultimately safeguarding patient health.
How can companies leverage these best practices to remain competitive in a landscape characterized by increasing scrutiny and rapid technological advancements?
AVS Life Sciences: Comprehensive Solutions for Cleaning and Process Validation
AVS Life Sciences provides a comprehensive array of services meticulously designed for cleaning/process validation (stage 1-3) in the pharmaceutical and biotechnology sectors. By prioritizing regulatory compliance and quality management, AVS Life Sciences empowers clients to adeptly navigate the complexities of approval processes. Their extensive solutions encompass:
- Verification and commissioning
- Quality assurance consulting
- Engineering support
All tailored to meet the rigorous standards of the life sciences industry, including compliance with GXP and FDA regulations.
As the pharmaceutical landscape evolves, particularly with the rise of high-potency active pharmaceutical ingredients (HPAPIs) and the shift towards continuous production, effective hygiene assurance programs are becoming increasingly vital. These developments necessitate robust cleaning/process validation (stage 1-3) protocols that ensure the thorough removal of contaminants while adhering to (GMP).
AVS Life Sciences is at the forefront of market trends in service approval processes, particularly emphasizing cleaning/process validation (stage 1-3) to respond to the growing demand for scientifically justified, risk-based methodologies, as underscored by the FDA's guidance. The integration of advanced technologies, such as real-time monitoring and predictive analytics, is revolutionizing hygiene verification practices, boosting efficiency, and ensuring patient safety.
Industry leaders recognize the critical role of efficient sanitation verification in maintaining product integrity and regulatory compliance. AVS Life Sciences' commitment to excellence and innovation, paired with its extensive GXP regulatory services, positions it as a trusted partner for organizations seeking to implement effective sanitation verification strategies in an increasingly complex regulatory environment.
Understand Regulatory Expectations and Global Guidelines
Organizations must thoroughly understand the regulatory expectations established by agencies such as the FDA and EMA to ensure compliance in the cleaning/process validation (stage 1-3) of sanitation. The FDA's guidance on '' highlights the importance of cleaning/process validation (stage 1-3) to demonstrate effective sanitation protocols, which includes the necessity for scientific rationale and comprehensive documentation. In fact, the FDA has criticized companies for failing to demonstrate that contaminants could be effectively eliminated using their sanitation procedures. Similarly, the EMA mandates a Validation Master Plan (VMP) that outlines strategies for cleaning/process validation (stage 1-3) to maintain cleanliness, including worst-case scenario assessments for highly toxic products. A 2021 EMA report emphasized that hygiene shortcomings were among the primary findings during GMP inspections, highlighting the essential need for organizations to align their cleaning/process validation (stage 1-3) practices with both local and global standards.
Adhering to ISO standards enhances sanitization verification programs through cleaning/process validation (stage 1-3), as these standards provide a framework for essential documentation and verification protocols. Organizations that invest in cleaning/process validation (stage 1-3) as part of robust sanitation protocols not only meet regulatory requirements but also experience fewer deviations and significantly reduced risk profiles. Research indicates that such investments lead to improved adherence outcomes. By incorporating these guidelines, organizations can establish efficient cleaning/process validation (stage 1-3) programs that enhance product safety and compliance.
Implement a Cleaning Validation Lifecycle Approach
Implementing a cleaning/process validation (stage 1-3) approach encompasses three critical phases: design, qualification, and continued verification. This ensures that cleaning/process validation (stage 1-3) is not only verified at the outset but is also consistently assessed and enhanced over time. By embracing this lifecycle approach, organizations can adeptly manage risks associated with contamination, particularly during the cleaning/process validation (stage 1-3), as insufficient sanitation can pose serious threats in pharmaceutical manufacturing. For instance, cleaning/process validation (stage 1-3) is essential for confirming that traces of active pharmaceutical ingredients are eradicated to specified levels, thereby upholding quality and compliance with Good Manufacturing Practices (GMP).
Organizations that have effectively adopted this lifecycle approach report noteworthy enhancements in operational efficiency and compliance. A significant case study illustrates how a leading pharmaceutical company implemented a digital sanitation verification solution, resulting in improved monitoring capabilities and a reduction in cross-contamination risks during product changeovers. This aligns with the FDA's emphasis on risk-oriented decision-making in sanitization assessment processes, where safety-related metrics such as Acceptable Daily Exposure (ADE) and Permitted Daily Exposure (PDE) values play a crucial role.
Moreover, continuous verification during the cleaning/process validation (stage 1-3) is indispensable in this lifecycle, enabling organizations to adjust to evolving conditions while upholding high-quality standards. By incorporating cleaning/process validation (stage 1-3) into their sanitation verification protocols through a quality risk management (QRM) system, companies can proactively identify and mitigate potential threats, ensuring ongoing compliance and safety of their products. This comprehensive approach not only protects against contamination but also enhances overall operational effectiveness, establishing it as a cornerstone of contemporary pharmaceutical manufacturing.

Define Key Components of a Cleaning Validation Program
A successful sanitization verification program must include several crucial elements to guarantee compliance and improve quality, particularly in accordance with GXP and FDA regulations. These components are essential:
- Clear Scope and Objectives: Establishing specific goals for the cleaning validation process is crucial. This clarity aligns the program with regulatory requirements and organizational standards, reflecting AVS Life Sciences' .
- Detailed Sanitizing Procedures: Comprehensive procedures should outline the necessary steps for effective sanitation, including the use of appropriate agents and techniques tailored to the equipment and products involved. For instance, integrating automated sanitation practices and clean-in-place modules can significantly enhance the efficiency of these procedures, ensuring adherence to established SOPs.
- Risk Evaluations: Performing comprehensive risk evaluations enables organizations to recognize potential contamination threats and prioritize sanitation efforts based on the severity of these threats. The case study of a continuous flow system for producing energetic compounds illustrates this point, as it was designed to mitigate explosion risks through stringent validation processes, showcasing AVS Life Sciences' expertise in managing quality in complex environments.
- Sampling Plans: A well-structured sampling plan is essential for identifying where and how samples will be gathered to evaluate sanitation effectiveness, thus ensuring compliance with regulatory standards.
- Analytical Approaches: Employing robust analytical methods, such as HPLC and LC/MS, guarantees precise quantification of residues, instilling confidence in the sanitization process and aiding adherence to FDA regulations.
- Acceptance Criteria: Defining clear acceptance criteria assists in assessing whether the sanitation process meets the necessary standards for safety and effectiveness, a fundamental aspect of AVS Life Sciences' verification strategies.
Effective cleaning/process validation (stage 1-3) programs in the pharmaceutical industry have demonstrated high success rates, particularly when these components are meticulously outlined and executed. For example, the previously referenced continuous flow system, capable of producing compounds at a scale of 30 g/hr, emphasizes the significance of thorough sanitation checks in preserving operational efficiency and compliance. By concentrating on these essential elements, organizations can optimize their verification efforts, ensuring that purification processes are not only efficient but also adhere to stringent regulatory standards.
Select Worst-Case Product and Equipment for Validation
When selecting worst-case products and equipment for assessment, organizations must consider critical factors such as solubility, potency, and toxicity. The worst-case scenario should illustrate the most challenging cleaning/process validation (stage 1-3) conditions, ensuring that the assessment process is sufficiently thorough to address any potential contamination risks. This strategic decision enables organizations to focus their verification efforts where they are most needed, thus enhancing overall adherence and safety.
A compelling case study from AVS Life Sciences exemplifies this approach effectively. By assisting a leading biotechnology firm in upgrading their production area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, AVS Life Sciences demonstrated the importance of comprehensive verification processes. Throughout this upgrade, challenges emerged, including the identification of anomalies in test results due to improperly installed barcode scanner cameras. Such issues underscored the necessity for more rigorous testing protocols. Their meticulous documentation and quality assurance measures ensured full traceability, allowing the client to concentrate on developing essential medicines.
This case underscores the significance of evaluating sanitation conditions and the cleaning/process validation (stage 1-3) strategies to ensure within the pharmaceutical sector.

Utilize Effective Sampling Methods: Swab and Rinse Techniques
In the realm of sanitation validation, two primary sampling methods stand out: swab and rinse techniques. Swab sampling entails the physical act of wiping a defined area of equipment to gather residues, while rinse sampling is designed to collect contaminants from the entirety of the rinsed surface area. It is imperative that both methods undergo thorough verification as part of the cleaning/process validation (stage 1-3) to ensure they accurately reflect the effectiveness of the sanitization process. Organizations must judiciously select the method that aligns best with their equipment and maintenance protocols to secure reliable results.
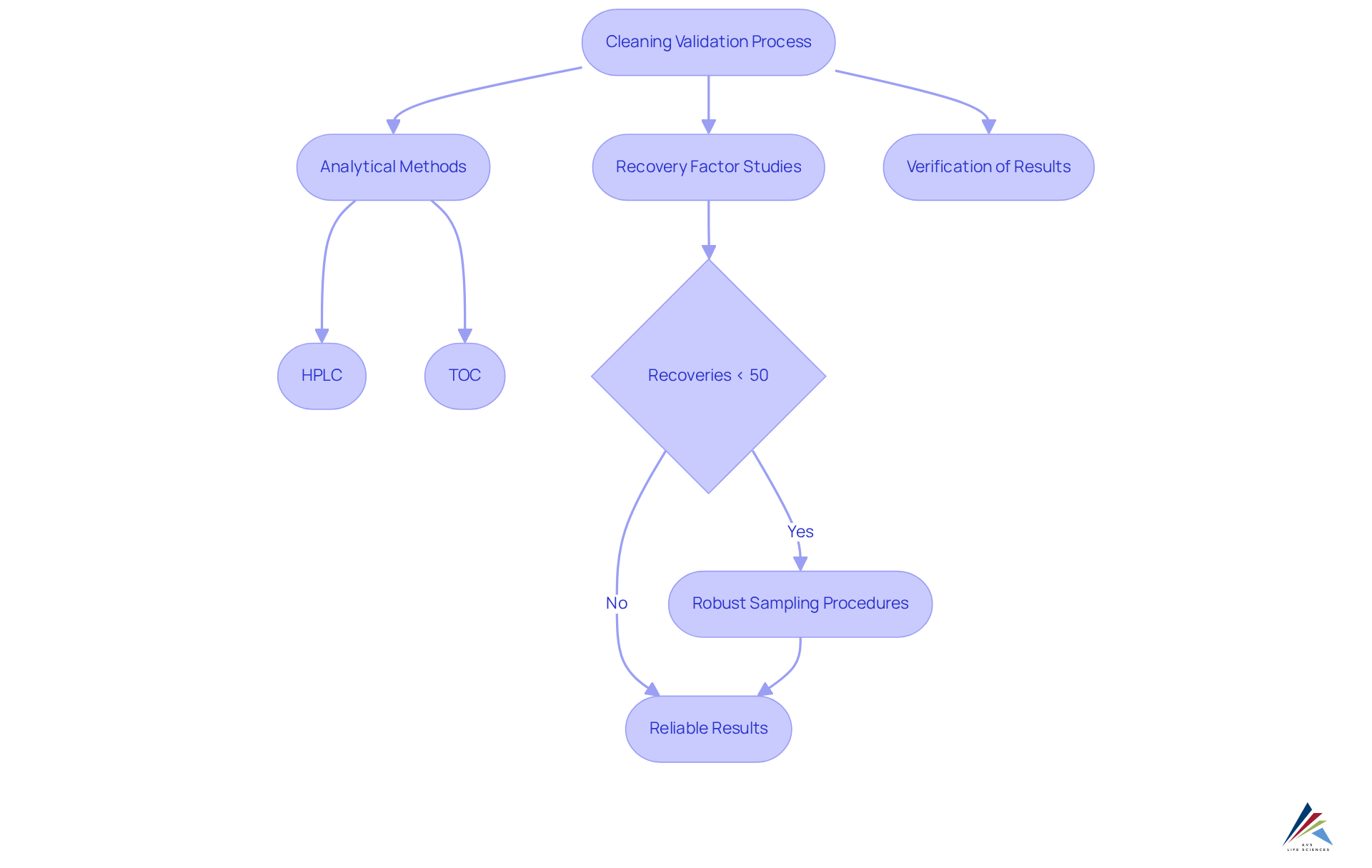
Employ Analytical Methods and Recovery Factor Studies
Analytical methods, such as High-Performance Liquid Chromatography (HPLC) and Total Organic Carbon (TOC) analysis, are indispensable for measuring residues on cleaned surfaces. These techniques yield precise measurements essential for ensuring that cleaning/process validation (stage 1-3) complies with regulatory standards.
Furthermore, recovery factor studies play a critical role in assessing the effectiveness of sampling techniques employed during verification. For example, recoveries below 50% are generally deemed 'questionable,' underscoring the necessity for robust sampling procedures.
Verifying these analytical methods within the (stage 1-3) not only facilitates adherence to regulations but also enhances the overall reliability of sterilization assessment outcomes, thereby ensuring organizations maintain high operational standards.
A recent case study by AVS Life Sciences exemplifies the transformative impact of upgrading a biotech facility to GMP Level 2, where improved quality management and compliance measures were instituted. This transition underscores the vital importance of efficient hygiene verification and the significance of reliable sampling methods.
As Mohammad Ovais emphasizes, the reliability of sanitation verification outcomes hinges on the integrity of these processes. Such comprehensive approaches are crucial for achieving effective sanitation results, ultimately safeguarding patient safety and enhancing the quality of life for target patients.
Establish Acceptance Criteria for Cleaning Validation
Establishing acceptance standards for sanitization assessment is crucial for ensuring safety and compliance with regulations. These standards must be rooted in scientifically justified limits, particularly health-based exposure limits (HBELs) and permissible daily exposure (PDE) thresholds. Organizations are required to define clear residue limits that must not be exceeded, which is vital for evaluating the effectiveness of cleaning/process validation (stage 1-3).
For instance, the European Medicines Agency (EMA) mandates that companies establish HBELs for all pharmaceutical products, thereby ensuring that sanitation assessments meet safety standards. Similarly, the FDA underscores the necessity for equipment to be cleaned adequately to prevent contamination that could jeopardize the safety, identity, strength, quality, or purity of drug products. This regulatory focus has resulted in a heightened demand for rigorous cleaning/process validation (stage 1-3) procedures, particularly concerning high-potency active pharmaceutical ingredients (HPAPIs).
Recent trends indicate that thresholds for traditional actives can be significantly raised based on specific toxicological assessments, allowing for more flexible and scientifically sound practices to ensure efficacy. For example, limits may be adjusted to as high as 2% of the active ingredient in certain scenarios, reflecting a move towards risk-based strategies within the industry.
Moreover, organizations are encouraged to employ professional judgment when determining acceptable daily exposure (ADE) and permitted daily exposure (PDE) values. This ensures that are not only scientifically valid but also practical for the specific active ingredients in question. Such a strategy is imperative for maintaining quality and safeguarding patient safety, particularly in the context of cleaning/process validation (stage 1-3), especially in light of historical challenges faced by the industry, such as the significant recall of 15 million doses of a vaccine due to contamination issues.
In conclusion, by establishing scientifically justified acceptance standards for sanitation verification, organizations can enhance their compliance efforts and ensure the safety of their pharmaceutical products.

Create a Cleaning Validation Protocol and Report
Creating a cleaning/process validation (stage 1-3) assessment protocol necessitates a clear outline of objectives, scope, methods, and acceptance criteria. This protocol must also specify the sampling plan and analytical techniques to be employed during the cleaning/process validation (stage 1-3).
Upon completing the verification activities, a detailed report is essential, documenting results and conclusions. Such documentation is crucial for demonstrating adherence during regulatory inspections and audits, as emphasize the importance of thorough and precise records. In practice, adherence rates during examinations can significantly depend on the quality of sanitation verification documentation.
Comprehensive reports not only serve as proof of compliance with established standards but also facilitate smoother audits, minimizing the risk of regulatory deviations. Efficient sanitation verification documentation is fundamental to the cleaning/process validation (stage 1-3), which is essential for maintaining product integrity and ensuring patient safety.
As noted, "This isn’t just regulatory theater. It’s strategic." Furthermore, the FDA issued 42 warning letters to manufacturers in 2022 regarding sanitation validation deficiencies, underscoring the essential requirement for thorough documentation.
AVS Life Sciences exemplifies this commitment through its successful enhancement of a biotechnology GMP facility, where meticulous documentation and quality assurance practices were pivotal in achieving regulatory standards. During this upgrade, challenges such as anomalies in test results due to improperly installed barcode scanner cameras were identified, leading to valuable lessons learned.
Routine monitoring is also crucial to verify that sanitation processes are functioning correctly, ensuring ongoing adherence and safety.

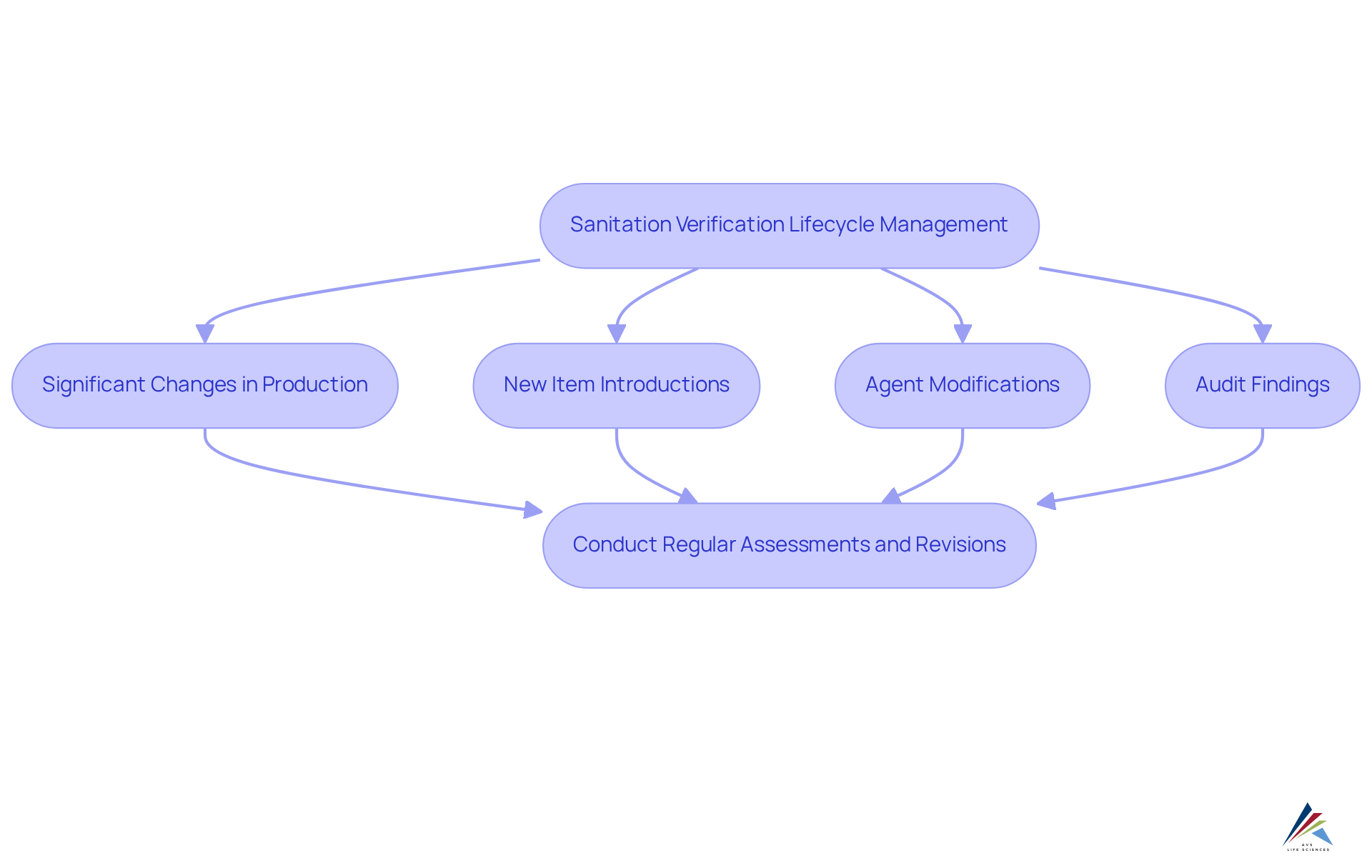
Manage Lifecycle and Identify Revalidation Triggers
To ensure continuous adherence, organizations must efficiently oversee the lifecycle of their sanitation verification processes. This involves conducting regular assessments and revisions to verification protocols in response to changes in manufacturing processes, equipment, or items. Key triggers for revalidation include:
- Significant changes in production
- New item introductions
- Agent modifications
- Findings from audits
The FDA underscores that serves as recorded proof that an authorized procedure will ensure equipment is suitable for handling pharmaceutical items. Furthermore, a well-structured sanitation verification plan is crucial for safeguarding item integrity and guaranteeing patient safety.
AVS Life Sciences exemplifies this by successfully upgrading a biotechnology GMP facility, where thorough quality management and regulatory adherence solutions were implemented, resulting in enhanced operational efficiency and improved product safety metrics. By proactively overseeing these aspects, organizations can sustain the efficiency of their sanitation verification efforts and ensure ongoing adherence to regulatory standards. Regular revalidation is essential for preventing cross-contamination and ensuring that sanitation methods remain effective.
Implementing a risk-based approach enables organizations to prioritize resources and focus on critical areas, thereby enhancing the overall effectiveness of cleaning validation. Organizations must recognize that maintaining compliance is not merely a regulatory obligation but a commitment to quality and safety that ultimately protects patients and upholds the integrity of the pharmaceutical industry.
Conclusion
AVS Life Sciences underscores the critical importance of effective cleaning and process validation within the pharmaceutical and biotechnology industries. By adhering to stringent regulatory standards and implementing best practices, organizations can ensure product safety and compliance, ultimately safeguarding patient health. The comprehensive solutions offered by AVS Life Sciences, spanning from verification to quality assurance consulting, are essential for navigating the complexities of sanitation processes.
The article delineates several key steps necessary for successful cleaning and process validation. It emphasizes the significance of:
- Understanding regulatory expectations
- Implementing a structured lifecycle approach
- Defining crucial components of a cleaning validation program
- Selecting appropriate sampling methods
- Establishing scientifically justified acceptance criteria
Each of these elements contributes to a robust framework that minimizes contamination risks and enhances operational efficiency.
In light of evolving industry standards and the increasing complexity of pharmaceutical production, organizations must prioritize the implementation of effective cleaning and process validation practices. By investing in comprehensive sanitation verification programs and staying informed about regulatory requirements, companies can not only achieve compliance but also enhance the quality of their products. This commitment to quality and safety transcends mere regulatory obligation; it is a fundamental aspect of protecting patients and maintaining the integrity of the pharmaceutical industry.
Frequently Asked Questions
What services does AVS Life Sciences provide for cleaning/process validation?
AVS Life Sciences offers services such as verification and commissioning, quality assurance consulting, and engineering support, all tailored to meet the rigorous standards of the life sciences industry.
Why is cleaning/process validation important in the pharmaceutical industry?
Cleaning/process validation is crucial for ensuring the thorough removal of contaminants and compliance with Good Manufacturing Practices (GMP), particularly in light of evolving pharmaceutical practices and the rise of high-potency active pharmaceutical ingredients (HPAPIs).
What are the stages involved in cleaning/process validation?
The cleaning/process validation approach encompasses three critical phases: design, qualification, and continued verification.
How does AVS Life Sciences ensure compliance with regulatory expectations?
AVS Life Sciences aligns its cleaning/process validation practices with regulatory guidelines from agencies like the FDA and EMA, emphasizing the need for scientific rationale, comprehensive documentation, and a Validation Master Plan (VMP).
What role do ISO standards play in cleaning/process validation?
Adhering to ISO standards enhances sanitization verification programs by providing a framework for essential documentation and verification protocols, helping organizations meet regulatory requirements and reduce risk profiles.
What is the significance of continuous verification in the cleaning validation lifecycle?
Continuous verification allows organizations to consistently assess and enhance their cleaning/process validation efforts, adapting to evolving conditions while maintaining high-quality standards and compliance.
How can organizations benefit from implementing a cleaning validation lifecycle approach?
Organizations adopting a cleaning validation lifecycle approach report improvements in operational efficiency and compliance, as it helps manage contamination risks and ensures effective sanitation protocols.
What are some key metrics emphasized by the FDA in sanitization assessment processes?
The FDA emphasizes safety-related metrics such as Acceptable Daily Exposure (ADE) and Permitted Daily Exposure (PDE) values in sanitization assessment processes.
