8 Benefits of CTMS Software for Clinical Trial Success

Overview
The article delineates eight pivotal benefits of Clinical Trial Management System (CTMS) software, underscoring its critical role in bolstering clinical trial success through enhanced data management, regulatory compliance, and overall operational efficiency. Each benefit is substantiated by compelling evidence, including:
- Increased data accuracy
- Streamlined processes
- Significant cost savings
This demonstrates how CTMS software is instrumental in facilitating effective clinical study management and achieving superior research outcomes.
Introduction
The landscape of clinical trials is evolving rapidly, presenting organizations with mounting pressure to streamline processes and ensure adherence to stringent regulations. In the face of these challenges, Clinical Trial Management Systems (CTMS) have emerged as indispensable tools, providing a multitude of benefits that can significantly elevate the success of clinical studies.
This article explores eight key advantages of CTMS software, illustrating how it not only optimizes data management and regulatory compliance but also fosters collaboration and enhances efficiency.
As organizations navigate the myriad of options available, the question remains: how can they identify the right CTMS solution that aligns with their unique needs and drives successful outcomes?
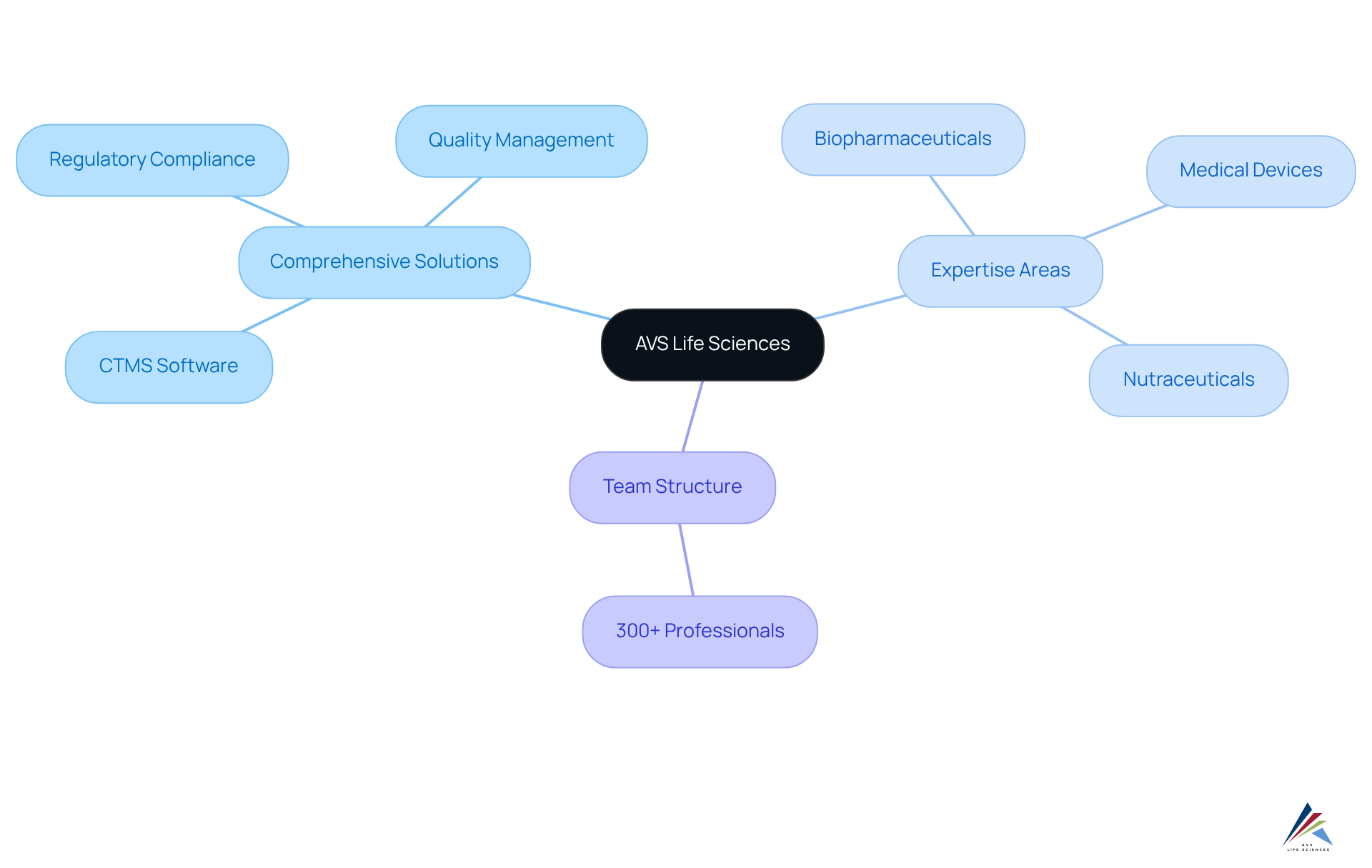
AVS Life Sciences: Streamlined Clinical Trial Management with Comprehensive Solutions
AVS Life Sciences presents a robust framework for research management, addressing the critical compliance challenges faced in the industry through its comprehensive . By integrating into its offerings, AVS empowers clients to navigate the intricacies of with precision.
The firm's profound expertise in biopharmaceuticals, medical devices, and nutraceuticals, coupled with a steadfast commitment to , facilitates streamlined processes that lead to .
With a dedicated team of over 300 adept professionals, AVS Life Sciences equips organizations to leverage ctms software for , ensuring that every aspect of study administration—from planning to execution—is meticulously managed in accordance with GxP, ISO, and QSR standards.
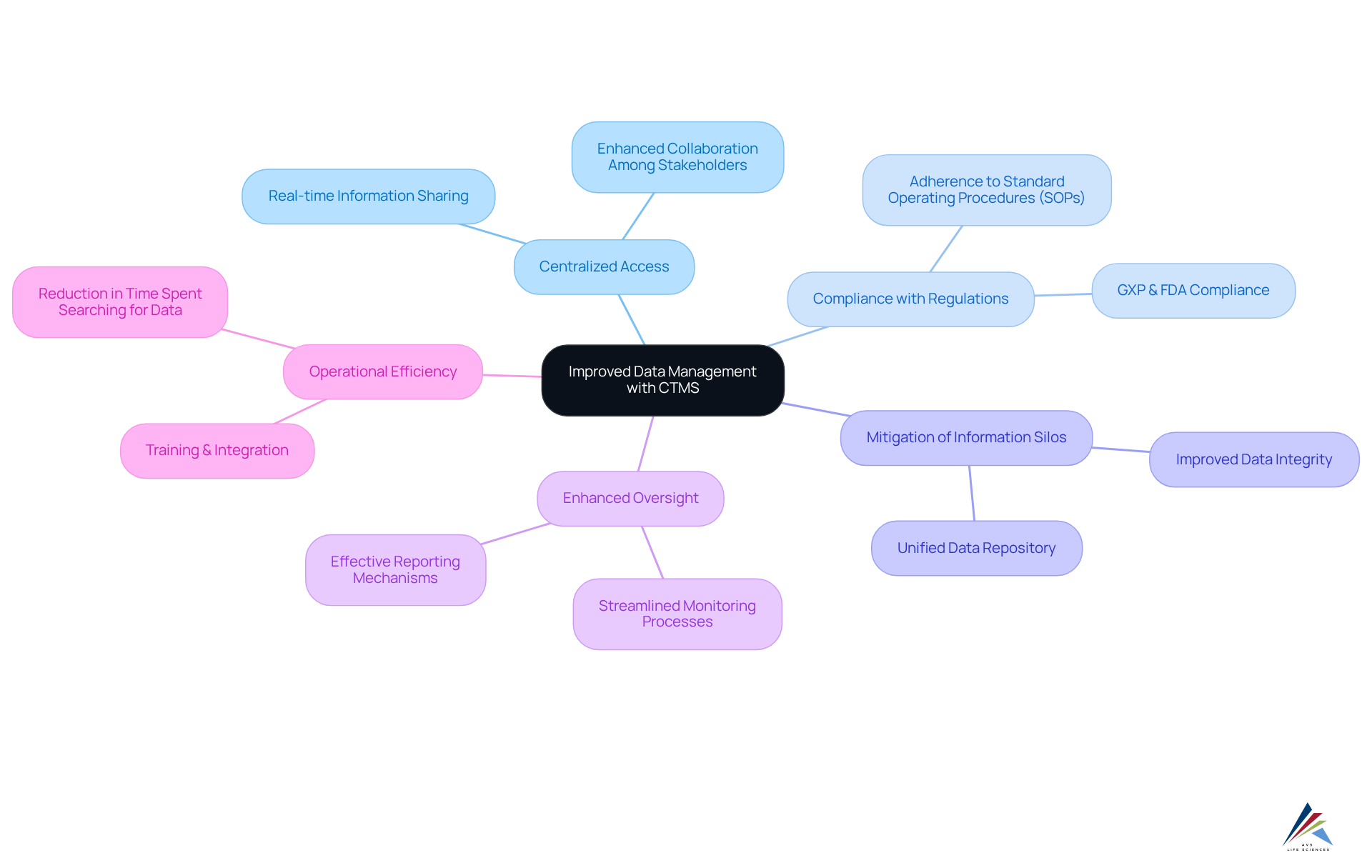
Improved Data Management: Centralized Access to Clinical Trial Information
CTMS software serves as an essential resource for centralizing clinical trial information, granting stakeholders while ensuring . This centralized framework significantly mitigates the risk of information silos, enabling all team members to operate with the most up-to-date information, which is critical for preserving information integrity.
Industry insights reveal that individuals spend 60% to 80% of their time searching for information, underscoring the efficiency gains achievable through the implementation of a . By , CTMS enhances the accuracy and reliability of study information, aligning with stringent regulatory compliance standards, including adherence to Standard Operating Procedures (SOPs).
Improved information management capabilities facilitate more effective oversight and reporting, ultimately resulting in superior study outcomes. As Geoffrey Moore aptly notes, "."
For instance, pharmaceutical companies utilizing CTMS software have reported substantial improvements in , showcasing the software's transformative impact on clinical trial management. To fully realize the , organizations must prioritize proper training and seamless integration of the software into their existing workflows.
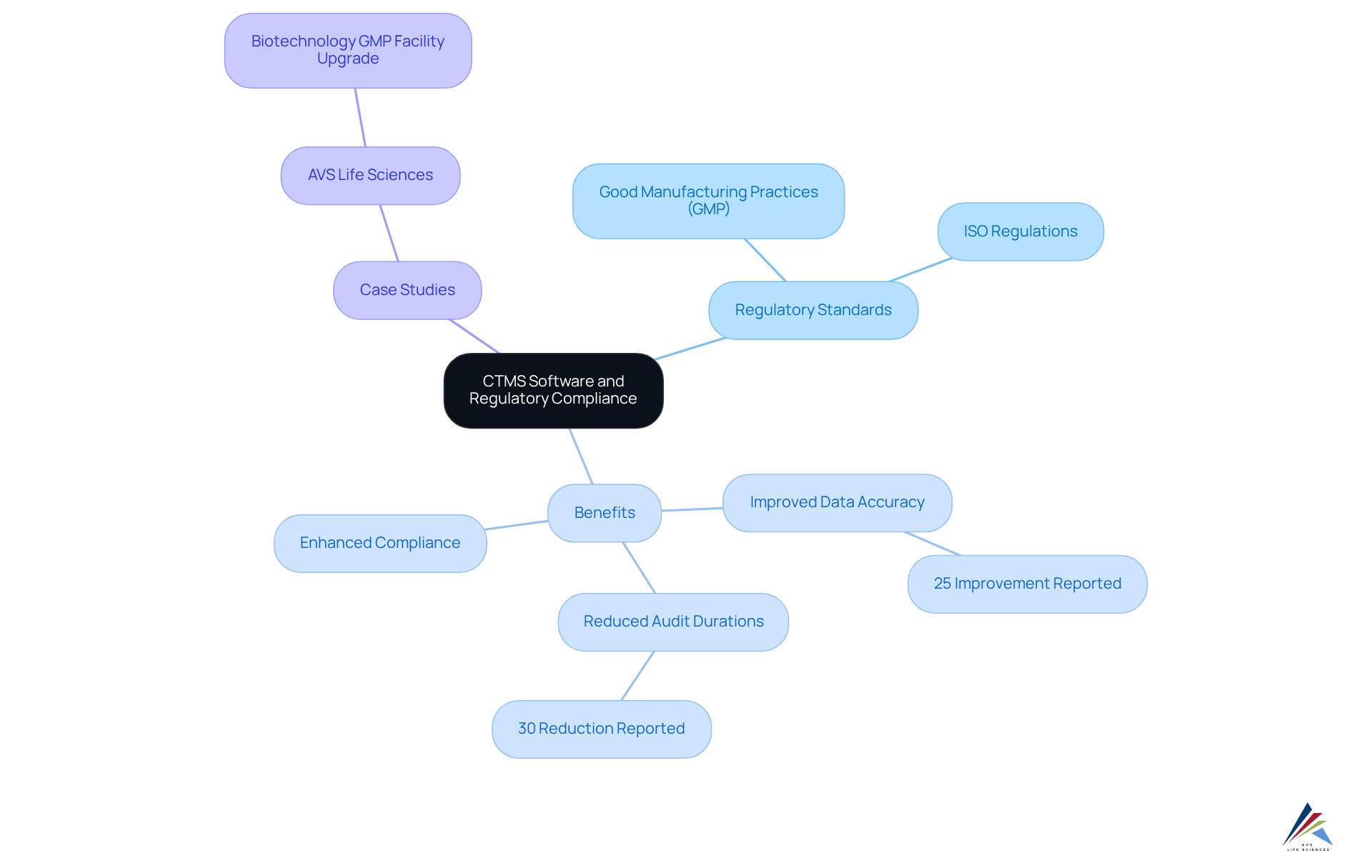
Regulatory Compliance Assurance: Meeting Industry Standards with CTMS
CTMS software is essential for organizations striving to meet regulatory standards such as and . By automating compliance tracking and documentation processes, this system alleviates the administrative burden on . Such automation ensures that all critical documentation, including Standard Operating Procedures (SOPs) and integrity records, is readily accessible for audits and inspections, significantly mitigating the risk of non-compliance.
Organizations utilizing CTMS software have reported a 25% improvement in data accuracy and a 30% reduction in audit durations, underscoring the effectiveness of CTMS software in ensuring compliance. Furthermore, bolster the overall integrity of research trials, fostering trust among stakeholders. Compliance officers have noted that while providing a robust framework for adhering to GMP and ISO standards, ultimately leading to more efficient and reliable clinical research outcomes.
AVS Life Sciences exemplifies this commitment to , as illustrated in a transformative case study where they successfully upgraded a biotechnology GMP facility. This upgrade prioritized and quality assurance, demonstrating AVS's proficiency in navigating complex compliance landscapes. Their dedication to ensuring and adherence to FDA regulations further emphasizes the critical role of in enhancing quality control and compliance within the biotech industry.
To fully leverage the benefits of , compliance officers should consider integrating into their workflows to improve documentation accuracy and streamline compliance efforts.
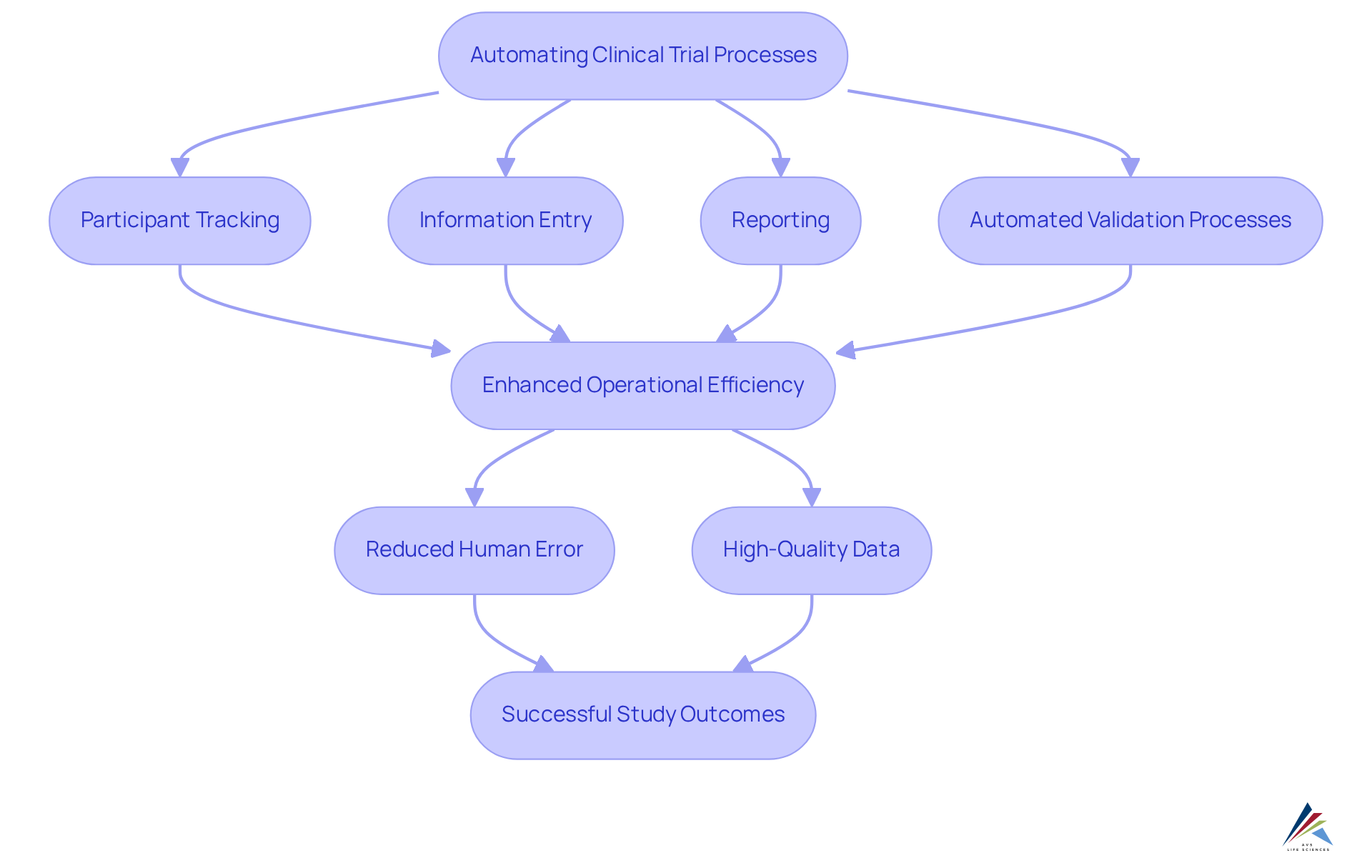
Increased Efficiency: Automating Clinical Trial Processes with CTMS
A key benefit of is their capacity to while ensuring compliance with . This optimization of tasks—including , information entry, and reporting—significantly reduces the time and effort required for study management. Such automation not only enhances operational efficiency but also allows clinical research teams to concentrate on critical activities like patient engagement and information analysis, all while adhering to excellent documentation practices and standard operating procedures (SOPs).
Furthermore, the integration of automated validation processes within clinical management systems minimizes the risk of human error, ensuring that study information remains accurate and reliable throughout the research. Consequently, organizations can maintain across various tests, ultimately leading to more successful outcomes.
Notably, 70% of healthcare organizations are actively considering the adoption of generative AI, reflecting a . As Michael Young, Co-CEO, asserts, ''
Moreover, can identify bottlenecks in study operations, further enhancing efficiency. , plays a crucial role in ensuring the effective implementation and maintenance of these systems.
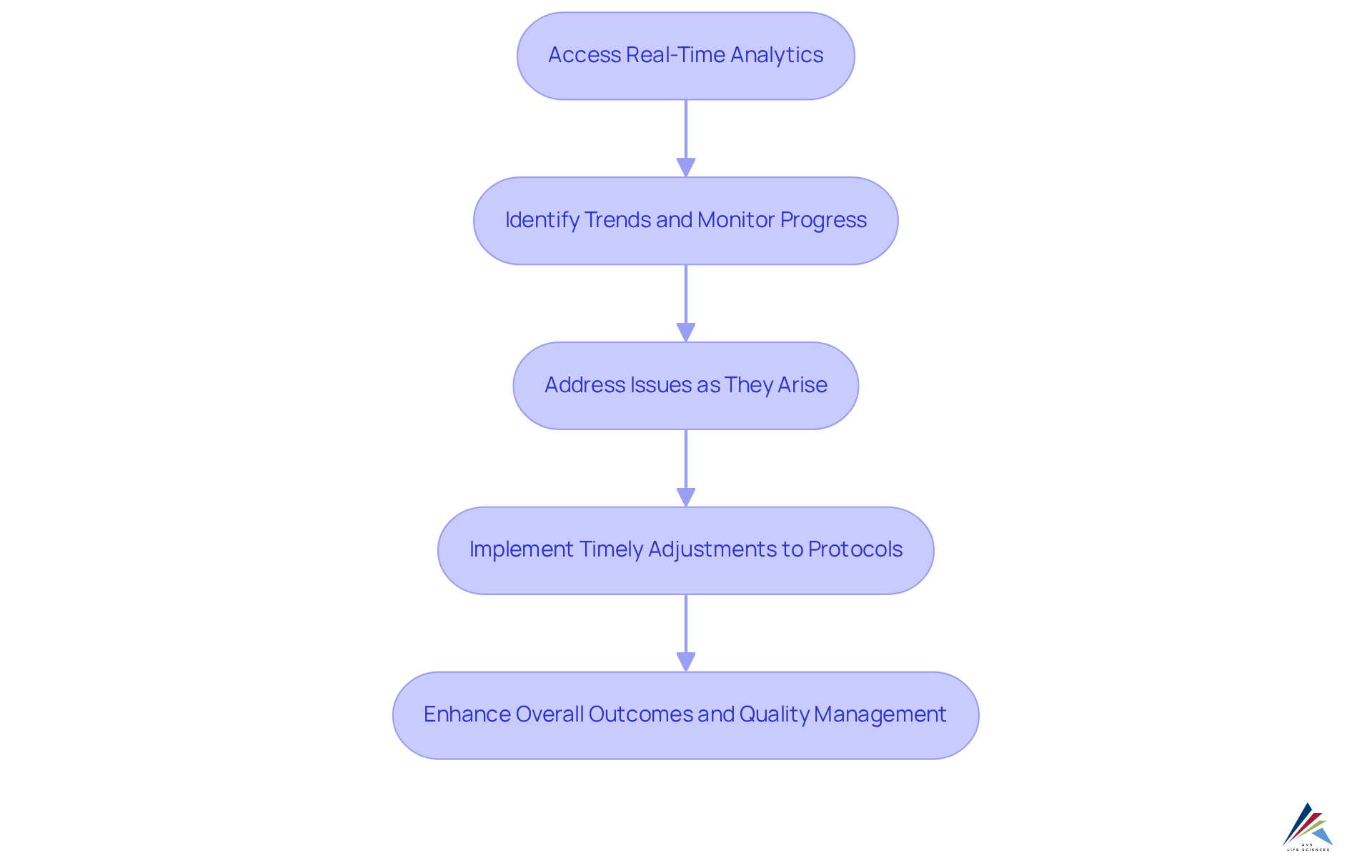
Real-Time Reporting: Enhanced Decision-Making with CTMS Analytics
The system delivers robust analytics and , empowering research teams to make informed decisions swiftly, while ensuring compliance with , , , and CFR Part 11. By providing immediate access to and examination metrics, stakeholders can identify trends, monitor progress, and address issues as they arise, all while adhering to exemplary documentation practices. This proactive approach to data analysis and supports essential alongside GXP sponsor responsibilities. As a result, can be implemented, ultimately fostering improved outcomes and ensuring comprehensive within the life sciences sector.
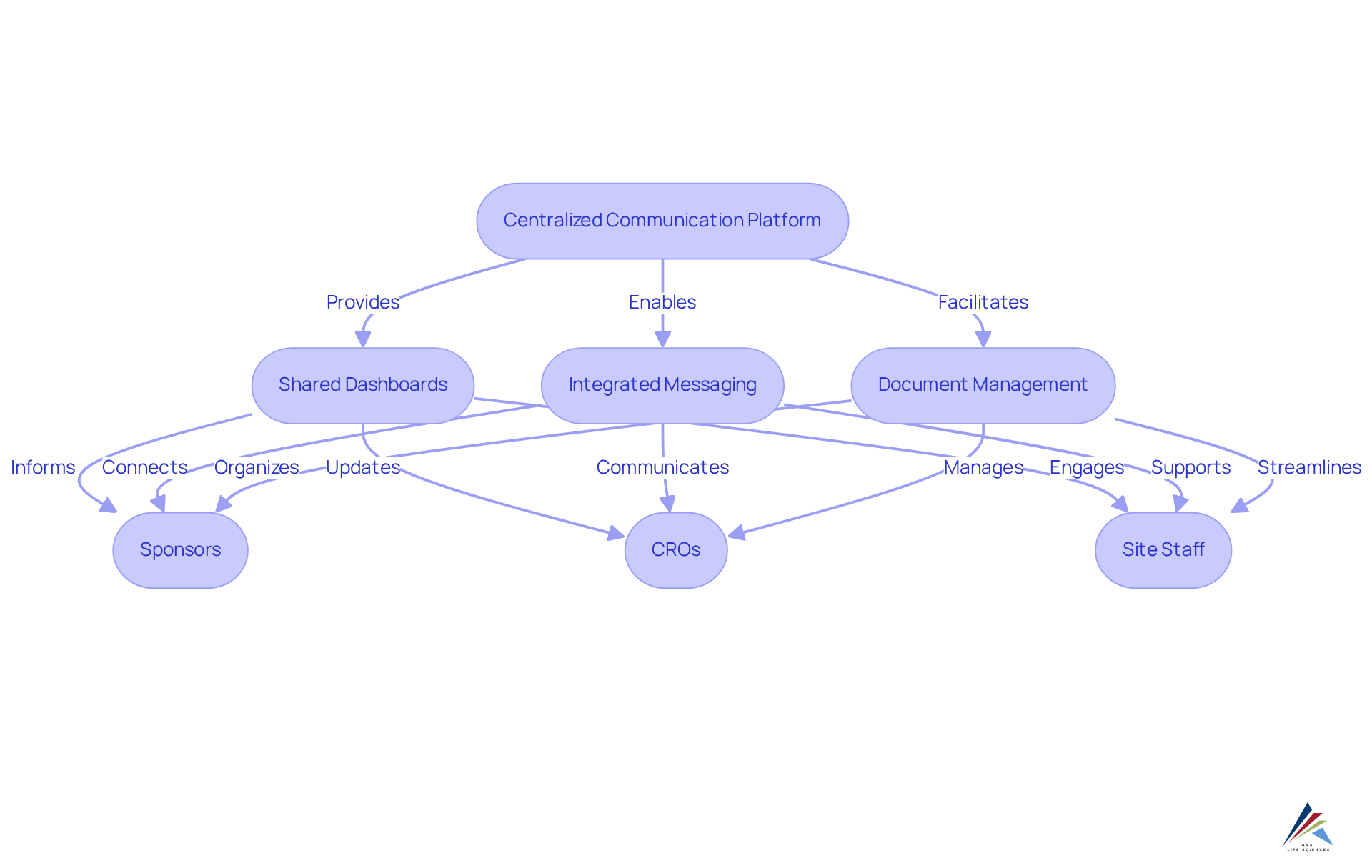
Enhanced Collaboration: Facilitating Communication Among Clinical Trial Stakeholders
The system significantly enhances cooperation among clinical research participants by offering a and information exchange. Key features, including , and comprehensive document management, streamline interactions between sponsors, CROs, and site staff. This ensures that all parties remain aligned on objectives and progress, effectively reducing misunderstandings and increasing overall efficiency.
For instance, ctms software dashboards provide real-time insights into , facilitating proactive management and . By fostering a collaborative environment, the organization not only supports the but also underscores the importance of , which is crucial for maintaining participant trust and engagement.
As highlighted by industry stakeholders, is not merely an option; it is a fundamental necessity that directly impacts study outcomes and participant safety.
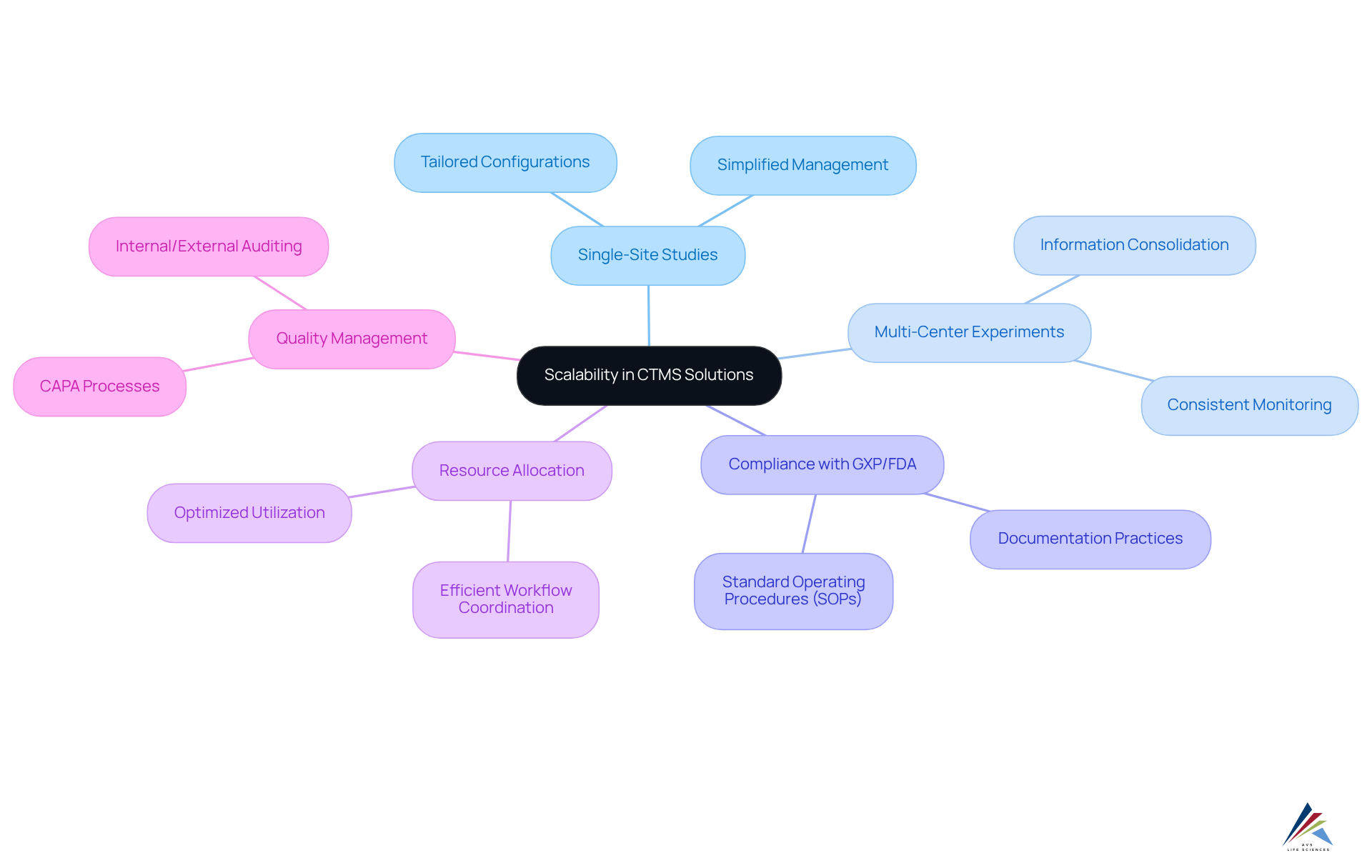
Scalability: Adapting CTMS Solutions to Varying Clinical Trial Needs
CTMS software solutions are inherently designed for scalability, empowering organizations to tailor their systems to the diverse demands of while ensuring . Whether overseeing a single-site study or a large multi-center experiment, these systems can be configured to adeptly manage varying scales and complexities, upholding and standard operating procedures (SOPs). This flexibility not only optimizes but also enhances overall efficiency and information integrity.
As the landscape of medical research continues to evolve, become essential for fostering growth and facilitating seamless adjustments to shifting demands. For instance, in multi-center studies, the system can consolidate information from various sites, ensuring consistent monitoring and compliance across all locations, which is crucial for the .
Moreover, organizations should implement to strengthen compliance and . According to industry reports, the market for CTMS software is projected to grow significantly, driven by the increasing complexity of studies and the demand for .
Industry leaders emphasize that the ability to adapt management systems based on study size is vital for improving outcomes and ensuring , ultimately advancing the success of research initiatives. To maximize the benefits of clinical study management systems, organizations should routinely assess their research needs and adjust their systems accordingly, ensuring they remain agile in a rapidly changing environment.
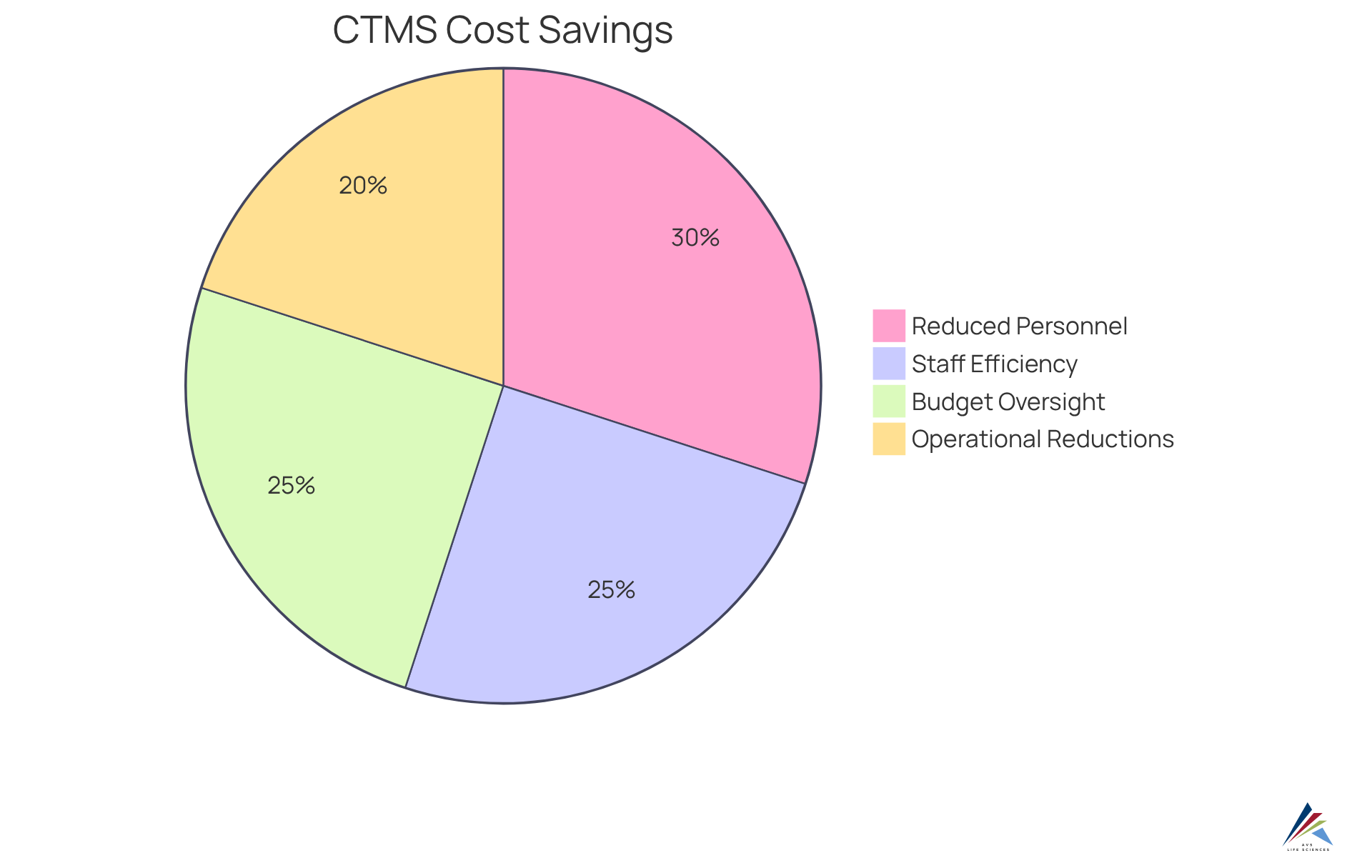
Cost-Effectiveness: Reducing Clinical Trial Management Expenses with CTMS
Implementing ctms software can yield substantial . By , this system significantly reduces administrative burdens and minimizes errors. Consequently, organizations can optimize their resources and budgets effectively. The automation of tasks not only but also decreases the necessity for additional personnel, leading to lower operational costs. Moreover, enables organizations to make informed , which is essential for maintaining a cost-effective management process.
Statistics suggest that regular can uncover and resolve financial inefficiencies, ultimately enhancing the economic viability of clinical trials. Notably, Deloitte states that the adoption of ctms software can result in up to 20% reductions in operational expenses. Additionally, AstraZeneca's experience illustrates how upholding budget regulations through their system has led to considerable cost reductions by pinpointing resource redundancies. As they mentioned, "AstraZeneca upheld budget regulations through their system."
In practical applications, organizations such as Pfizer have effectively shortened time-to-market by utilizing ctms software for enhanced budget oversight and . Pfizer stated, "Pfizer reduced time-to-market using ." These examples highlight the , making it a vital tool for improving financial oversight and ensuring the success of clinical trials.
Comprehensive Training and Support: Ensuring Successful CTMS Implementation
To fully leverage the capabilities of ctms software, organizations must invest in for their teams, particularly regarding . Effective should encompass all components of the , ranging from basic functionalities to advanced features. This includes the critical stages of CSV such as:
- User Requirement Specifications (URS)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
Furthermore, to address any challenges that may arise during implementation. This ensures that staff are well-versed in and best practices. By equipping teams with a thorough understanding of CSV procedures and to utilize ctms software, organizations can optimize the advantages of ctms software for clinical management systems. Ultimately, this leads to improved overall while upholding .
Data Security and Privacy: Protecting Sensitive Information in Clinical Trials with CTMS
is equipped with robust protocols designed to protect sensitive data collected during . Key attributes, such as AES-256 encryption for data at rest and TLS 1.2 for data in transit, along with access controls and comprehensive audit trails, ensure that only authorized personnel can access trial information.
Additionally, the system aids organizations in complying with protection regulations, including and HIPAA, by providing tools for managing . It underscores the critical need for to assess compliance with these regulations, which is vital for maintaining information security.
Moreover, the implementation of technical controls, such as and secure file transfer protocols (SFTP and HTTPS), further enhances the safeguarding of sensitive information. By prioritizing , the system not only secures participant details but also bolsters the overall integrity of the research process.
Privacy protection experts emphasize that the importance of confidentiality in research management cannot be overstated; effective information governance is essential for sustaining trust among participants and regulatory agencies alike. For instance, ctms software facilitates adherence to GDPR by incorporating features that manage patient consent and usage preferences, ensuring that organizations meet legal obligations while protecting sensitive .
Maintaining comprehensive logs of and user access is also crucial for demonstrating compliance during audits.
Conclusion
CTMS software is fundamentally transforming the clinical trial management landscape by providing a robust suite of tools that enhance efficiency, ensure regulatory compliance, and improve overall study outcomes. This innovative technology not only streamlines processes and centralizes data management but also fosters collaboration among stakeholders, ultimately paving the way for more successful clinical trials.
Key benefits of CTMS software are paramount, including:
- Improved data management
- Assurance of regulatory compliance
- Increased efficiency through automation
- Real-time reporting capabilities
- Enhanced collaboration
- Scalability to meet diverse needs
- Cost-effectiveness
- Comprehensive training and support
- Robust data security measures
Each of these elements is critical in simplifying clinical trial processes and enabling organizations to adapt to the evolving demands of medical research while upholding high standards of quality and compliance.
The importance of adopting CTMS software cannot be overstated. As the complexity of clinical trials escalates, leveraging such systems becomes essential for organizations striving to enhance operational efficiencies and achieve successful outcomes. By investing in CTMS solutions, organizations position themselves to navigate the intricate landscape of clinical research more effectively, ultimately contributing to advancements in healthcare and improved patient outcomes.
Frequently Asked Questions
What is AVS Life Sciences and what do they offer?
AVS Life Sciences is a company that provides a robust framework for research management, focusing on compliance challenges in the clinical trial industry through comprehensive regulatory and quality solutions, including the integration of clinical trial management system (CTMS) software.
How does AVS Life Sciences support clinical trial management?
AVS Life Sciences supports clinical trial management by leveraging CTMS software to ensure meticulous management of all aspects of study administration, from planning to execution, in accordance with GxP, ISO, and QSR standards.
What are the benefits of using CTMS software in clinical trials?
CTMS software centralizes clinical trial information, granting stakeholders immediate access to vital details, mitigating information silos, enhancing data integrity, and improving operational efficiency. It also facilitates compliance with GxP and FDA regulations.
How does CTMS software improve data management?
CTMS software improves data management by streamlining information processes, which allows team members to operate with the most up-to-date information, leading to enhanced accuracy and reliability of study information while aligning with regulatory compliance standards.
What impact does CTMS software have on regulatory compliance?
CTMS software automates compliance tracking and documentation processes, significantly reducing the administrative burden on clinical trial teams, improving data accuracy by 25%, and reducing audit durations by 30%, thus ensuring adherence to GMP and ISO standards.
Can you provide an example of how AVS Life Sciences has demonstrated its commitment to regulatory compliance?
AVS Life Sciences demonstrated its commitment to regulatory compliance through a case study where they upgraded a biotechnology GMP facility, emphasizing regulatory compliance and quality assurance, showcasing their proficiency in navigating complex compliance landscapes.
What should organizations consider for maximizing the benefits of CTMS software?
Organizations should prioritize proper training and seamless integration of CTMS software into their existing workflows to fully realize its benefits in clinical trial management.
