8 Benefits of Computer Software Assurance (CSA) for Compliance Officers

Overview
The primary advantages of Computer Software Assurance (CSA) for compliance officers encompass:
- Enhanced risk management
- Increased efficiency
- Improved data integrity
- Cost-effectiveness
- Flexibility
- Collaboration
- Continuous improvement
- Strategic advantage
CSA effectively addresses compliance challenges by:
- Streamlining validation processes
- Reducing documentation burdens
- Fostering a culture of accountability and innovation
This enables organizations to navigate regulatory challenges with confidence while upholding high quality and compliance standards. By leveraging CSA, compliance officers can not only meet regulatory requirements but also position their organizations for sustained success in an increasingly complex landscape.
Introduction
The pharmaceutical and life sciences sectors are increasingly navigating a complex landscape of regulatory compliance, where the stakes are high and the margin for error is slim. In this context, Computer Software Assurance (CSA) emerges as a pivotal solution, offering compliance officers a suite of benefits that streamline processes, enhance data integrity, and ultimately drive innovation. However, with the rapid evolution of regulations and the pressing need for efficiency, organizations must consider:
- How can they effectively leverage CSA not only to meet compliance standards but also to gain a competitive edge in the market?
AVS Life Sciences: Comprehensive CSA Solutions for Regulatory Compliance
AVS Life Sciences offers a strong array of computer software assurance (CSA) solutions that are specifically tailored for the pharmaceutical and life sciences sectors. As a leading provider of comprehensive compliance and quality solutions, AVS empowers clients to navigate the complexities of oversight landscapes with confidence. Their offerings in computer software assurance (CSA) encompass validation, quality management, and engineering support, all focused on enhancing adherence and operational efficiency. Leveraging extensive industry expertise in biopharmaceuticals, medical devices, and nutraceuticals, AVS Life Sciences enables organizations to implement strategies for computer software assurance (CSA) that align with Good Manufacturing Practices (GMP) and other compliance standards.
The benefits of implementing computer software assurance (CSA) are significant, with companies reporting validation time reductions of 30-50%. This efficiency allows teams to shift their focus from excessive documentation to . As the market for computer software assurance (CSA) is anticipated to grow at a CAGR of 13% from 2025 to 2032, the importance of risk-based approaches and critical thinking in validation processes is becoming increasingly paramount. Such a shift not only simplifies adherence efforts but also enhances overall software reliability and quality, addressing the pressing challenges faced by life sciences companies today.
AVS Life Sciences distinguishes itself as a trusted partner in this dynamic landscape, recognized for its capacity to deliver rapid value creation and meticulous project execution, all while emphasizing computer software assurance (CSA). Their solutions for computer software assurance (CSA) are vital in ensuring that clients uphold high standards of quality while adeptly managing compliance-driven projects.

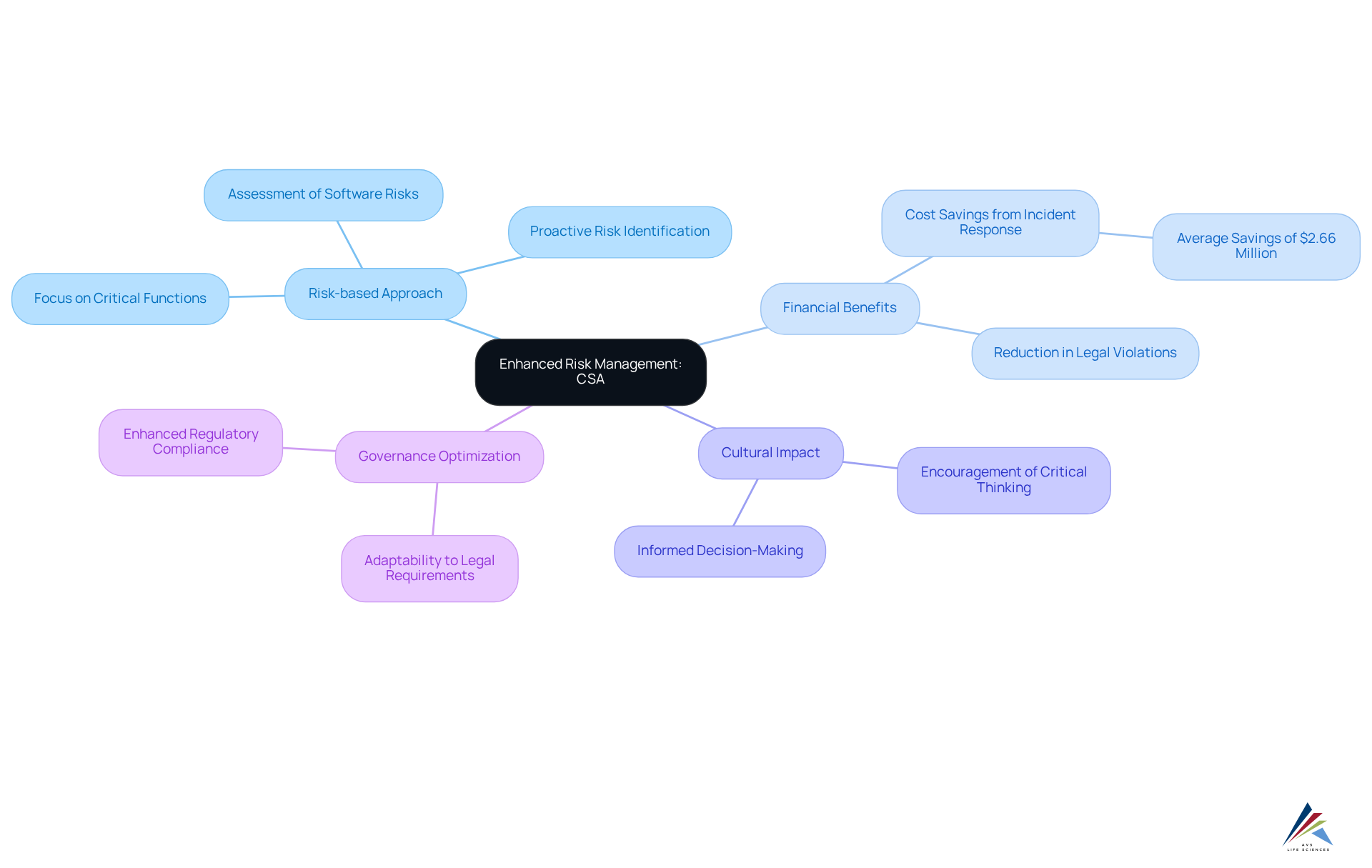
Enhanced Risk Management: Mitigating Compliance Risks with CSA
Computer software assurance (CSA) significantly enhances risk management by implementing a risk-based approach that prioritizes critical system functions. This methodology allows oversight personnel to focus on areas with the highest potential effect on data integrity and product quality. By proactively recognizing and tackling regulatory risks early in the software lifecycle, entities can significantly reduce the likelihood of legal violations and the penalties that come with them.
For instance, entities that frequently evaluate their incident response plans save an average of $2.66 million in breach expenses, underscoring the financial advantages of proactive risk management. Furthermore, the principles of computer software assurance (CSA) foster a culture of critical thinking and informed decision-making, enabling teams to concentrate on what truly matters—patient safety and integrity.
Consequently, entities can optimize their governance structures, ensuring they remain nimble and adaptable to evolving legal requirements. This strategic emphasis not only safeguards patients but also enhances the overall , positioning entities for long-term success in a complex oversight environment.
Increased Efficiency: Streamlining Validation Processes with CSA
Computer software assurance (CSA) significantly enhances validation processes by alleviating the documentation burden that has long characterized compliance in the life sciences sector. By prioritizing essential functions and employing a risk-based strategy, computer software assurance (CSA) enables entities to optimize their validation efforts, which reduces unnecessary testing and documentation. This pivotal shift not only accelerates validation timelines but also reallocates resources to more critical tasks. As a result, compliance officers can ensure their teams operate with greater efficiency, leading to expedited product development cycles and improved time-to-market. Notably, entities that have embraced computer software assurance (CSA) report up to an 80% reduction in validation time dedicated to documentation, which facilitates a more agile response to market demands. This efficiency enhancement is vital in an industry where the pace of innovation can dictate competitive advantage.
Furthermore, the collaboration between project managers and quality professionals is essential for maximizing the benefits of computer software assurance (CSA), which ensures that validation processes are both efficient and aligned with overarching project goals. To further bolster the validation framework, entities should integrate the stages of the Computer System Validation (CSV) process, which encompasses:
- Planning
- Defining User Requirement Specifications (URS)
- Design Specifications
- Building and Configuring a System
- Installation Qualification (IQ) testing
- Operational Qualification (OQ) testing
- Performance Qualification (PQ) testing
- Reporting
Additionally, employing a detailed process checklist for CSV can guarantee adherence and quality assurance, ultimately enhancing operational effectiveness in the pharmaceutical and biotechnology sectors.

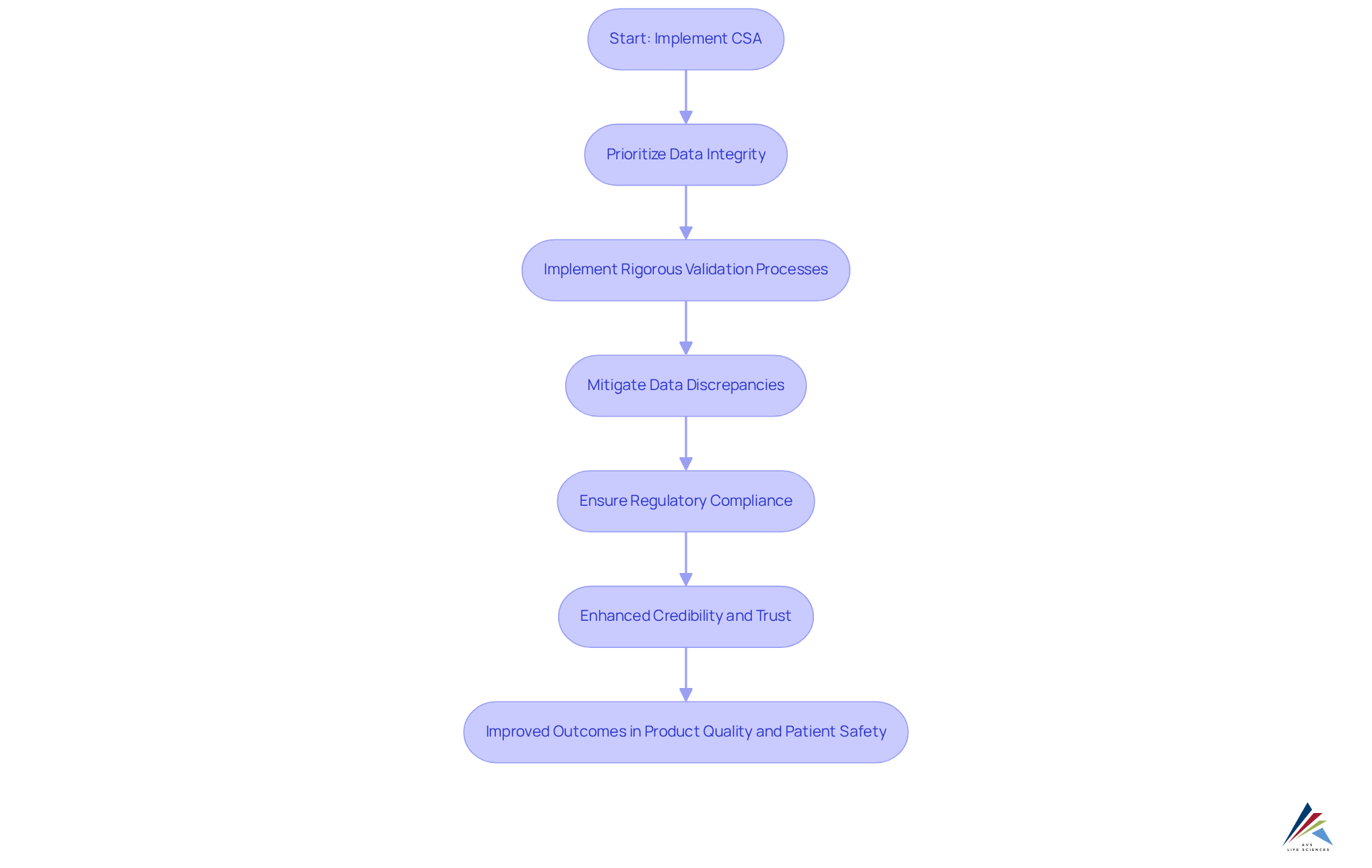
Improved Data Integrity: Ensuring Accurate and Reliable Data with CSA
In the life sciences industry, ensuring data integrity is not just essential; it is imperative. Computer software assurance (CSA) is crucial for achieving this goal. By prioritizing the maintenance of accurate and reliable data throughout the software lifecycle, computer software assurance (CSA) effectively addresses compliance challenges head-on.
Implementing rigorous validation processes that target critical data points allows firms to effectively mitigate data discrepancies, which have been reported to affect up to 30% of pharmaceutical companies. This proactive approach not only guarantees adherence to but also enhances the entity's credibility, cultivating trust among stakeholders and regulatory bodies.
As Tim King aptly noted, 'Data Privacy Week enables entities of all sizes to reflect on their critical data and evaluate methods to ensure its safety and security.' By embracing computer software assurance (CSA), companies can significantly strengthen their data integrity efforts, ultimately leading to improved outcomes in product quality and patient safety.
It is time for organizations to take action and engage with AVS Life Sciences to fortify their compliance solutions.
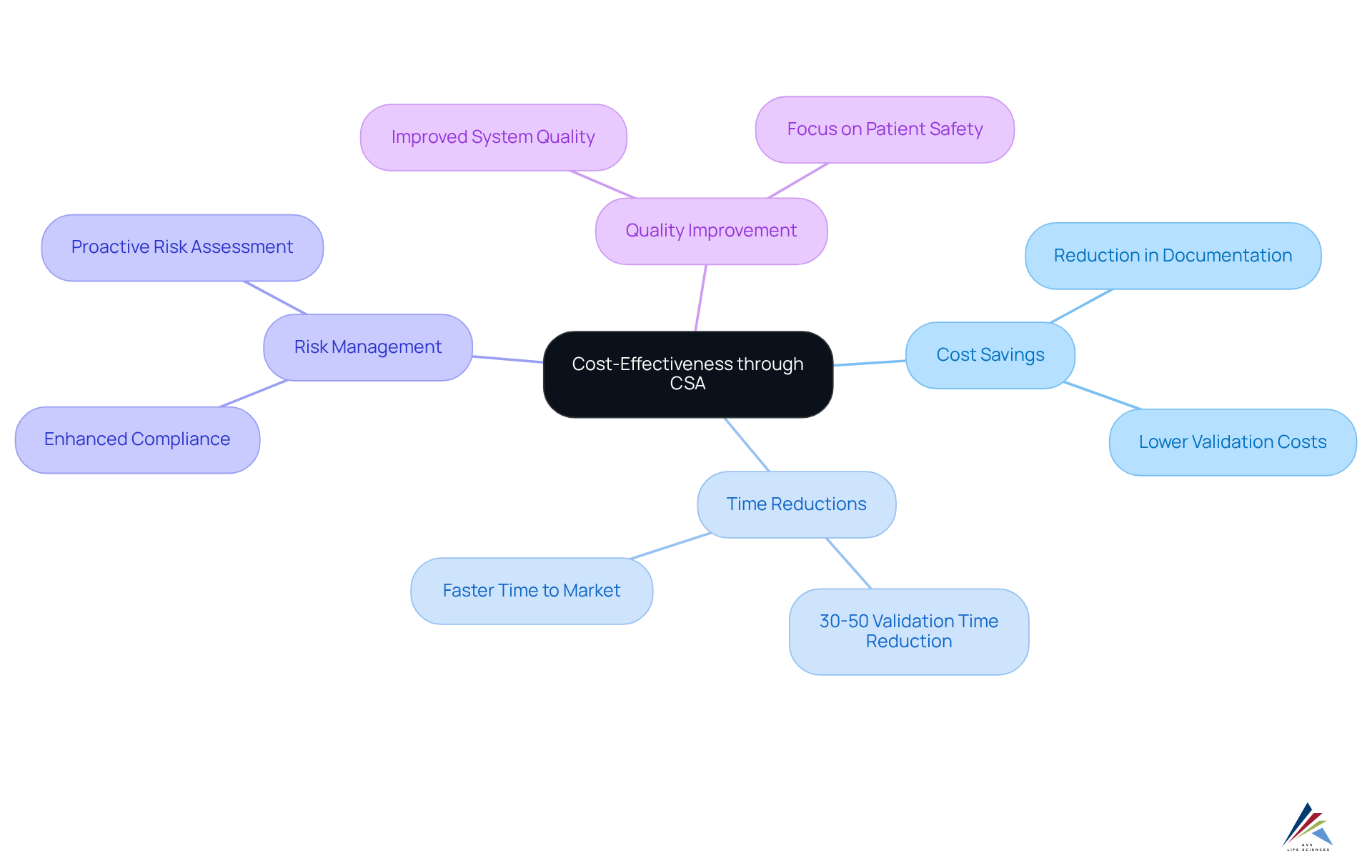
Cost-Effectiveness: Reducing Validation Costs through CSA Implementation
Implementing computer software assurance (CSA) offers significant cost-saving opportunities for companies by streamlining validation processes and greatly reducing the necessity for extensive documentation. This method not only reduces the resources allocated to regulatory activities but also proves particularly advantageous for organizations operating with constrained budgets or seeking to optimize operational costs.
For example, companies that have embraced CSA report validation time reductions ranging from 30% to 50%, enabling teams to redirect their focus towards innovation rather than documentation. Moreover, the computer software assurance (CSA) framework emphasizes risk-based decision-making, which enhances compliance with regulations while diminishing the likelihood of incurring costly penalties associated with regulatory noncompliance.
This proactive approach to risk management translates into additional financial benefits, positioning CSA as a strategic investment for professionals intent on boosting efficiency while ensuring regulatory adherence. By aligning validation processes with computer software assurance (CSA) principles, organizations can achieve superior system quality and expedited time to market, ultimately fostering a more agile and cost-effective operational model.
To fully leverage the advantages of CSA, compliance officers should contemplate integrating AVS Life Sciences' proven , which can further streamline compliance efforts and amplify overall operational effectiveness.
Flexibility: Adapting CSA to Diverse Regulatory Environments
Computer Software Assurance (CSA) offers a flexible framework that can be customized to address the diverse compliance needs of different jurisdictions. This adaptability is essential for entities operating on an international scale or within different compliance environments. By customizing computer software assurance (CSA) strategies to align with specific regulations, compliance officers can ensure their organizations remain compliant while also benefiting from the efficiencies that computer software assurance (CSA) offers.
For instance, AVS Life Sciences successfully assisted a leading biotechnology firm in upgrading their production area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This case exemplifies how a and regulatory compliance can lead to significant advancements. Such flexibility not only enhances adherence to regulations but also promotes innovation and agility in product development.
The positive outcomes observed during the facility upgrade included improved documentation practices and enhanced quality assurance measures, underscoring the value of a customized compliance strategy.

Collaboration: Enhancing Teamwork and Communication with CSA
Computer software assurance (CSA) significantly enhances collaboration among teams by promoting open communication and shared responsibilities. By involving cross-functional teams in the validation process, diverse perspectives are integrated, leading to more effective regulatory strategies. This collaborative approach not only improves the quality of regulatory efforts but also fosters a culture of accountability and continuous improvement within the organization.
A transformative case study involving AVS Life Sciences illustrates this point effectively. They assisted a leading in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. During this project, challenges emerged, such as anomalies in test results due to improperly installed barcode scanner cameras, which were initially overlooked. However, AVS's thorough investigation and documentation efforts yielded critical lessons learned, prompting the client’s QC laboratory team to reassess their business processes for gaps that permitted unreliable test results. This project was completed on time and within budget, underscoring AVS's commitment to quality management and regulatory standards.
The partnership allowed the client to focus on developing medicines while AVS ensured complete traceability throughout the transition process. Furthermore, data indicates that 70% of workers believe that enhanced collaboration positively impacts productivity and time efficiency, highlighting the significance of computer software assurance (CSA) in promoting teamwork and communication in procedural practices. By leveraging computer software assurance (CSA), organizations can cultivate an environment where adherence goes beyond mere regulatory obligation, evolving into a collective commitment to excellence.

Continuous Improvement: Leveraging CSA for Ongoing Compliance Enhancements
Computer software assurance (CSA) promotes a culture of continuous improvement within organizations, enabling regular assessments and enhancements of compliance processes. This proactive approach allows pharmaceutical companies to implement computer software assurance (CSA) and report , demonstrating its effectiveness in streamlining processes.
By leveraging computer software assurance (CSA), compliance officers can implement methodologies that facilitate ongoing evaluation and enhancement of adherence strategies, especially in alignment with GXP and FDA regulations. Furthermore, the concept of computer software assurance (CSA) promotes a proactive mindset among regulatory teams, fostering innovation and excellence.
According to the FDA, 'the main goal of computer software assurance (CSA) in the pharmaceutical context is to ensure that software-controlled systems consistently operate as intended while fulfilling compliance requirements.' This dedication to ongoing enhancement not only assists entities in staying ahead of regulatory changes but also improves overall effectiveness, ultimately resulting in better patient safety and product quality.
Additionally, the risk framework of computer software assurance (CSA) categorizes systems based on their risk levels, allowing organizations to focus their validation efforts where they matter most. The thorough computer system validation procedure, encompassing phases like Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), is vital for guaranteeing adherence and quality assurance in life sciences.
As highlighted by industry leaders, the shift towards computer software assurance (CSA) signifies a significant progression in the approach to adherence, emphasizing the importance of critical thinking and risk management in assurance activities.
AVS Life Sciences is prepared to assist officers in managing these complexities with expert solutions in GMP adherence, validation, and engineering for the pharmaceutical and biotechnology sectors.

Training and Support: Empowering Teams with CSA Knowledge
Effective implementation of computer software assurance (CSA) hinges on robust training and support for teams. Organizations must prioritize comprehensive training programs that equip employees with the essential knowledge and skills to adeptly navigate processes related to computer software assurance (CSA). By fostering a culture of ongoing learning and development, regulatory officers can guarantee their teams are well-equipped to implement computer software assurance (CSA) strategies effectively. This not only improves adherence results but also enables employees to take responsibility for their roles in maintaining regulatory standards.
As Helen Keller aptly stated, 'Alone we can do so little; together we can do so much.' This collective empowerment encourages a proactive attitude towards regulations, ultimately benefiting the entity as a whole.

Strategic Advantage: Gaining a Competitive Edge with CSA
Implementing computer software assurance (CSA) gives companies a significant competitive advantage in the life sciences sector. Introduced by the FDA in September 2022, computer software assurance (CSA) serves to streamline compliance processes and enhance data integrity, thereby enabling faster product market entry while upholding rigorous quality standards. This agility not only enhances but also positions companies advantageously against competitors still reliant on traditional, cumbersome validation methods.
For instance, AVS Life Sciences recently assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on schedule and within budget. Our support included conducting a gap analysis and overseeing final equipment installation, which allowed the client to focus on developing targeted antibodies for cancer, ultimately enhancing patient outcomes.
Companies that implement computer software assurance (CSA) have reported a significant reduction in documentation burdens—up to 80%—allowing them to concentrate on critical thinking and risk management. As noted by the FDA, this shift emphasizes patient safety and product quality over extensive documentation.
Compliance officers can strategically leverage computer software assurance (CSA) to enhance their organization's market position, thereby driving business success through improved innovation and responsiveness to regulatory changes. Important lessons learned during this project have prompted the QC laboratory team and Quality team to evaluate their processes, ensuring continuous improvement.
Organizations that embrace computer software assurance (CSA) are likely to experience marked improvements in their market positioning, ultimately leading to greater profitability and sustainability.

Conclusion
Implementing Computer Software Assurance (CSA) transcends mere regulatory necessity; it represents a strategic advantage that empowers compliance officers within the life sciences sector. By adopting CSA, organizations can significantly enhance operational efficiency, streamline validation processes, and ensure data integrity, all while mitigating compliance risks. This multifaceted approach not only simplifies adherence to regulatory standards but also cultivates a culture of innovation and continuous improvement.
The article delineates several key benefits of CSA, including:
- Improved risk management through a proactive focus on critical system functions
- Substantial reductions in validation time and costs
- Flexibility to adapt to diverse regulatory environments
Furthermore, the emphasis on collaboration and training guarantees that teams are well-equipped to navigate the complexities of compliance, ultimately leading to enhanced patient safety and product quality. These insights underscore the critical importance of integrating CSA solutions into compliance strategies for sustainable success.
In an ever-evolving regulatory landscape, the adoption of Computer Software Assurance is imperative for organizations striving to maintain a competitive edge. By leveraging AVS Life Sciences' expertise in CSA, compliance officers can not only meet but exceed regulatory expectations, fostering innovation and operational excellence. Embracing CSA is a proactive measure towards achieving sustainable growth and ensuring that organizations remain agile and responsive to regulatory changes, ultimately benefiting both the business and the patients they serve.
Frequently Asked Questions
What is AVS Life Sciences known for?
AVS Life Sciences is known for offering a comprehensive array of computer software assurance (CSA) solutions specifically tailored for the pharmaceutical and life sciences sectors, focusing on regulatory compliance and quality management.
How does AVS Life Sciences support clients in regulatory compliance?
AVS Life Sciences empowers clients by providing validation, quality management, and engineering support, helping them navigate the complexities of compliance with Good Manufacturing Practices (GMP) and other standards.
What are the benefits of implementing computer software assurance (CSA)?
Implementing CSA can lead to significant benefits, including validation time reductions of 30-50%, allowing teams to focus on innovation rather than excessive documentation.
What is the expected market growth for computer software assurance (CSA)?
The market for computer software assurance (CSA) is anticipated to grow at a compound annual growth rate (CAGR) of 13% from 2025 to 2032.
How does CSA enhance risk management?
CSA enhances risk management by implementing a risk-based approach that prioritizes critical system functions, allowing organizations to proactively identify and mitigate regulatory risks early in the software lifecycle.
What financial advantages can proactive risk management provide?
Entities that frequently evaluate their incident response plans can save an average of $2.66 million in breach expenses, highlighting the financial benefits of proactive risk management.
In what ways does CSA streamline validation processes?
CSA streamlines validation processes by alleviating the documentation burden, optimizing validation efforts, and reallocating resources to critical tasks, ultimately leading to expedited product development cycles.
What is the potential reduction in validation time reported by entities using CSA?
Entities that have adopted CSA report up to an 80% reduction in validation time dedicated to documentation.
What stages are involved in the Computer System Validation (CSV) process?
The stages of the CSV process include Planning, Defining User Requirement Specifications (URS), Design Specifications, Building and Configuring a System, Installation Qualification (IQ) testing, Operational Qualification (OQ) testing, Performance Qualification (PQ) testing, and Reporting.
How can entities ensure adherence and quality assurance in the validation framework?
Employing a detailed process checklist for CSV can help guarantee adherence and quality assurance, enhancing operational effectiveness in the pharmaceutical and biotechnology sectors.
