7 Ways How AVS Supports Brands with Compliant Packaging and Label Review

Overview
AVS Life Sciences empowers brands by ensuring compliant packaging and label review through expert regulatory consulting, comprehensive training programs, and thorough validation services designed to uphold industry standards. This article highlights the compliance challenges faced by the pharmaceutical sector and illustrates how AVS's strategic solutions effectively mitigate these risks. By enhancing operational efficiency, AVS positions itself as a trusted partner in navigating the complexities of regulatory landscapes. The detailed insights provided herein serve to inform and engage compliance officers, prompting them to consider AVS Life Sciences as their go-to resource for achieving compliance excellence.
Introduction
Navigating the intricate landscape of packaging compliance presents a formidable challenge for many brands, particularly within the highly regulated life sciences sector. AVS Life Sciences stands out as a pivotal ally, offering specialized consulting services that empower organizations to confidently meet stringent regulatory standards. As companies pursue operational excellence while ensuring compliance, a crucial question emerges: how can AVS effectively bridge the gap between regulatory demands and efficient packaging processes? This article delves into seven key ways AVS supports brands in achieving compliant packaging and label review, illuminating the transformative impact of their expertise.
AVS Life Sciences: Quality Compliance Consulting for Packaging and Labeling
AVS Life Sciences specializes in quality regulatory consulting tailored for containers and labeling, demonstrating how AVS supports brands with compliant packaging and label review to ensure that clients navigate the complexities of regulatory demands with precision. Their expert advisors collaborate closely with organizations to devise comprehensive strategies that guarantee all materials comply with industry standards, including:
- [Good Manufacturing Practices (GMP)](https://canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/gmp-guidelines-0001/document.html)
- ISO standards
- [Quality System Regulations (QSR)](https://fastercapital.com/topics/successful-labeling-compliance-approaches.html)
This process demonstrates how AVS supports brands with compliant packaging and label review by encompassing meticulous reviews of label content, design, and materials, which significantly mitigates the risk of non-compliance during audits and inspections. By emphasizing regulatory adherence, AVS not only safeguards client interests but also enhances operational efficiency, establishing itself as a trusted partner in the pharmaceutical sector. Engage with AVS Life Sciences to fortify your compliance strategies and secure your position in the market.
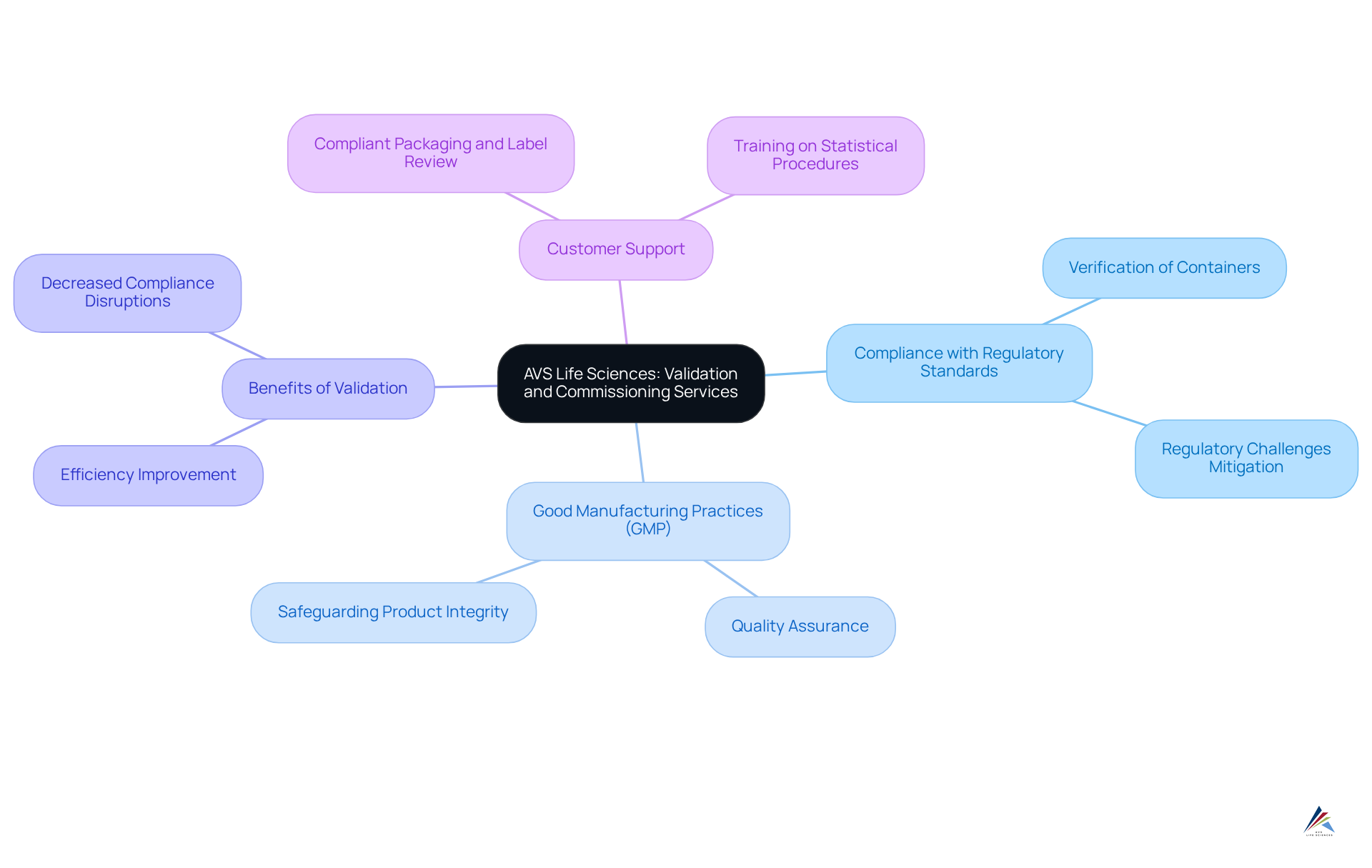
AVS Life Sciences: Validation and Commissioning Services for Compliant Packaging
AVS Life Sciences delivers robust validation and commissioning services essential for ensuring compliance with regulatory standards. This includes a thorough verification of containers, processes, and materials to guarantee adherence to Good Manufacturing Practices (GMP). By employing stringent validation procedures, AVS not only aids customers in mitigating costly regulatory challenges but also enhances the efficiency and reliability of their wrapping operations.
The recent advancements in validation processes for 2025 highlight the critical nature of these services, as organizations increasingly acknowledge that effective validation is vital for maintaining product integrity and regulatory compliance. Companies that adopt comprehensive validation protocols, for example, report a marked decrease in compliance-related disruptions, underscoring the efficacy of these strategies.
Experts in GMP emphasize that validating container processes transcends mere regulatory obligation; it is a fundamental aspect of quality assurance that safeguards both the product and the consumer. With AVS Life Sciences at the forefront, customers can navigate the complexities of with confidence, illustrating how AVS supports brands with compliant packaging and label review while ensuring their operations align with standards and remain effective.
AVS Life Sciences: Regulatory Submissions Guidance for Packaging Compliance
AVS Life Sciences provides specialized guidance on regulatory submissions, essential for brands aiming to navigate highly regulated markets. Their seasoned consultants meticulously assist clients in preparing and submitting the required documentation to regulatory bodies, ensuring strict compliance with established standards. This support is crucial in streamlining the approval process, significantly minimizing the risk of delays that can stem from inadequate submissions.
As we approach 2025, the regulatory submission landscape for containers is undergoing significant changes, with the FDA processing over 300,000 submissions annually. The timeline for regulatory approvals related to containers can be extensive, especially for applications that necessitate resubmissions, which often face median delays of 435 days. To address these challenges, AVS Life Sciences advocates for best practices such as:
- Early collaboration with biostatisticians
- Comprehensive documentation preparation
Both are vital for successful submissions.
Regulatory affairs specialists underscore the importance of detailed documentation for packages, emphasizing that clear and precise submissions enhance the likelihood of first-time approvals. By leveraging AVS's expertise, clients can adeptly navigate the complexities of regulations and understand how AVS supports brands with compliant packaging and label review to ensure their containers meet all legal requirements while expediting their time to market.

AVS Life Sciences: Comprehensive Training Programs for Packaging Compliance
AVS Life Sciences presents comprehensive training programs meticulously designed to equip customer teams with essential knowledge on packaging regulations, including Good Manufacturing Practices (GMP) and ISO standards. These programs ensure that all staff members are adept in regulatory expectations, significantly reducing the risk of adherence issues stemming from knowledge gaps. Notably, 89% of workers express a desire for training that is available anytime and anywhere, while 91% seek personalized training relevant to their roles.
AVS's initiatives are in perfect alignment with current trends that underscore the critical importance of continuous learning in the workplace. By investing in these robust training solutions, customers not only enhance their internal capabilities but also foster a culture of adherence that is vital for navigating the complexities of the life sciences sector.
Research indicates that companies with comprehensive employee training programs experience 218% higher income per employee compared to those lacking formalized training, underscoring the tangible benefits of prioritizing GMP and ISO training.
As the industry evolves, AVS Life Sciences remains committed to delivering cutting-edge training, including the upcoming 'Good Manufacturing Practices (GMP) 101' course scheduled for September 18-19, 2025. This course is designed to equip participants with the , ensuring they remain at the forefront of adherence and operational excellence.

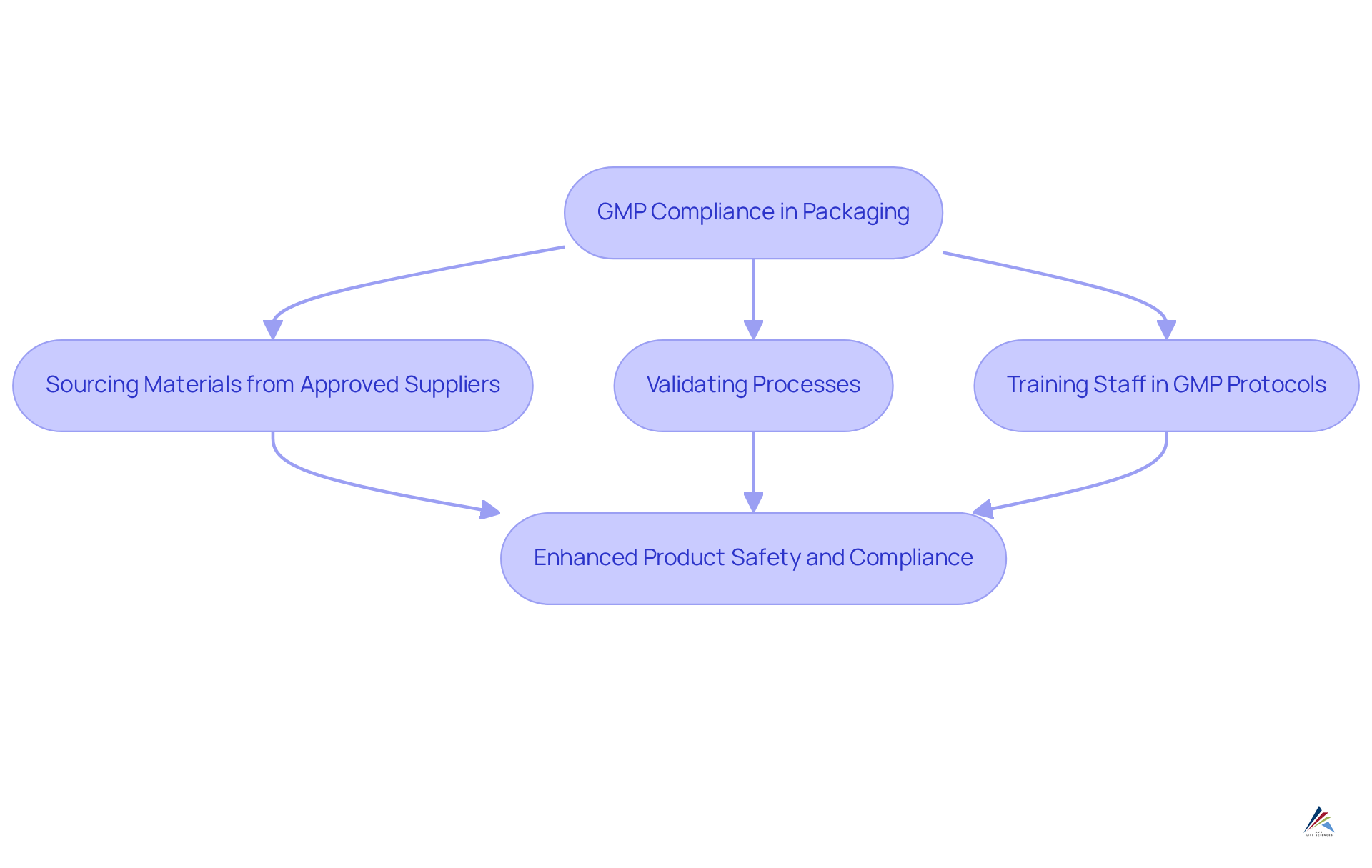
Good Manufacturing Practices (GMP): Ensuring Compliance in Packaging
AVS Life Sciences emphasizes the critical role of [Good Manufacturing Practices (GMP)](https://avslifesciences.com/industry-news/cders-quality-management-maturity-program) in compliance, particularly highlighted by our successful transformation of a biotechnology customer's facility from a Biosafety Level 1 to a Level 2 GMP facility for lentivirus production. Our advisors work in close collaboration with customers to establish and maintain throughout the packing process. This encompasses:
- Sourcing all wrapping materials from approved suppliers
- Validating processes
- Training staff in GMP protocols
By adhering to these stringent GMP standards, customers can ensure the safety and quality of their products, thereby enhancing their compliance with FDA regulations and improving patient outcomes.
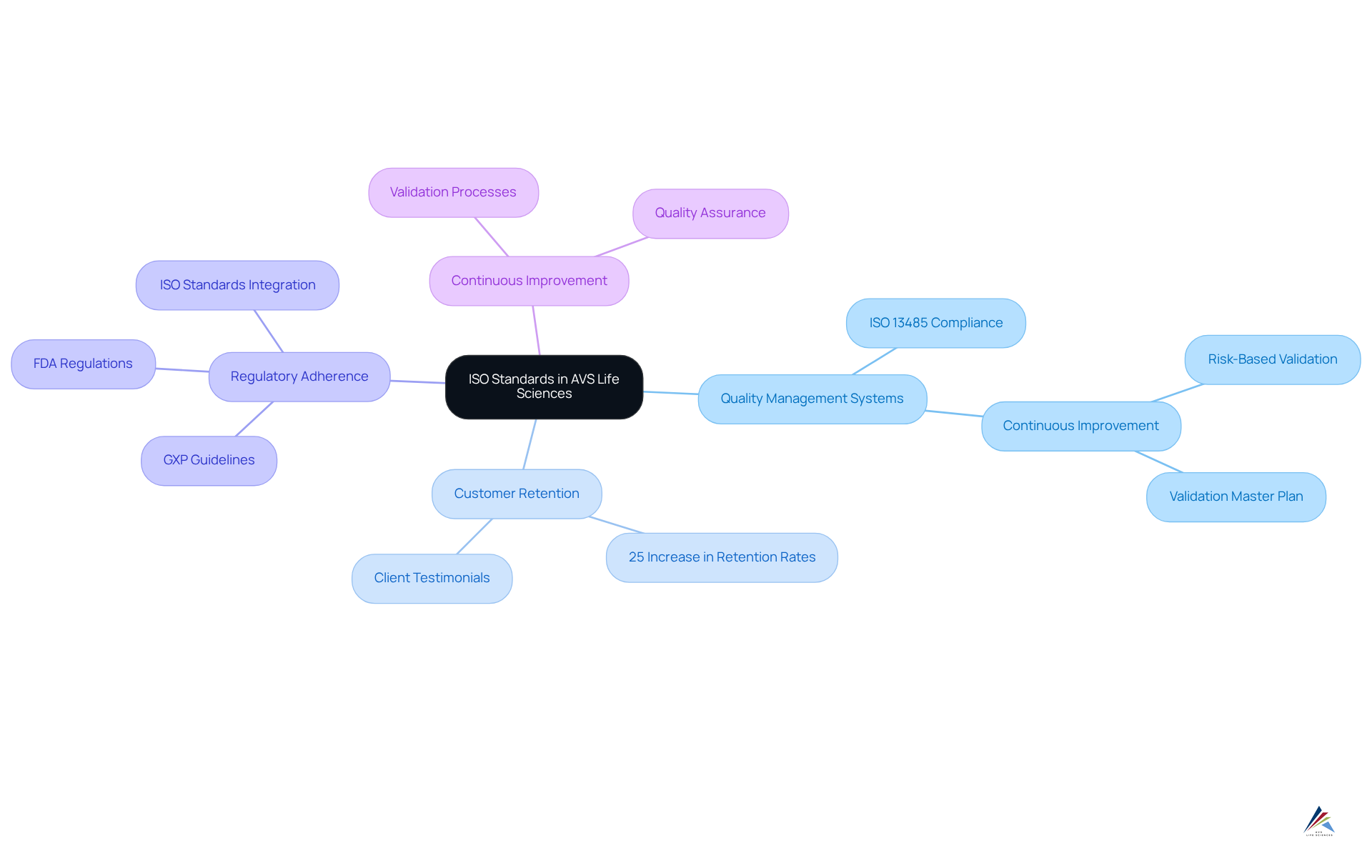
ISO Standards: AVS Life Sciences' Approach to Compliant Packaging
AVS Life Sciences effectively incorporates ISO standards into its product regulations strategy, ensuring that clients' operations conform to international quality benchmarks. This alignment with ISO requirements fosters the implementation of robust that promote continuous improvement and vigilant compliance monitoring. Such a proactive strategy not only enhances the quality of containers but also significantly mitigates the risk of non-compliance.
Organizations adopting ISO standards often report a 25% increase in customer retention rates, highlighting the tangible benefits of quality assurance linked to ISO implementation. Furthermore, the latest ISO standards underscore the necessity for rigorous validation processes, which are critical for maintaining product safety and efficacy. Quality management specialists assert that integrating ISO principles into wrapping operations is essential for achieving excellence and sustaining a competitive advantage in the pharmaceutical industry.
Additionally, AVS Life Sciences adheres to GXP guidelines and FDA regulations, ensuring that all aspects of wrapping and labeling meet stringent adherence standards. As one specialist noted, 'Validation is a core function that directly aids in meeting ISO and FDA standards, not merely a documentation necessity.'
AVS Life Sciences' commitment to these standards exemplifies how AVS supports brands with compliant packaging and label review, showcasing its dedication to empowering customers through exceptional packaging solutions.
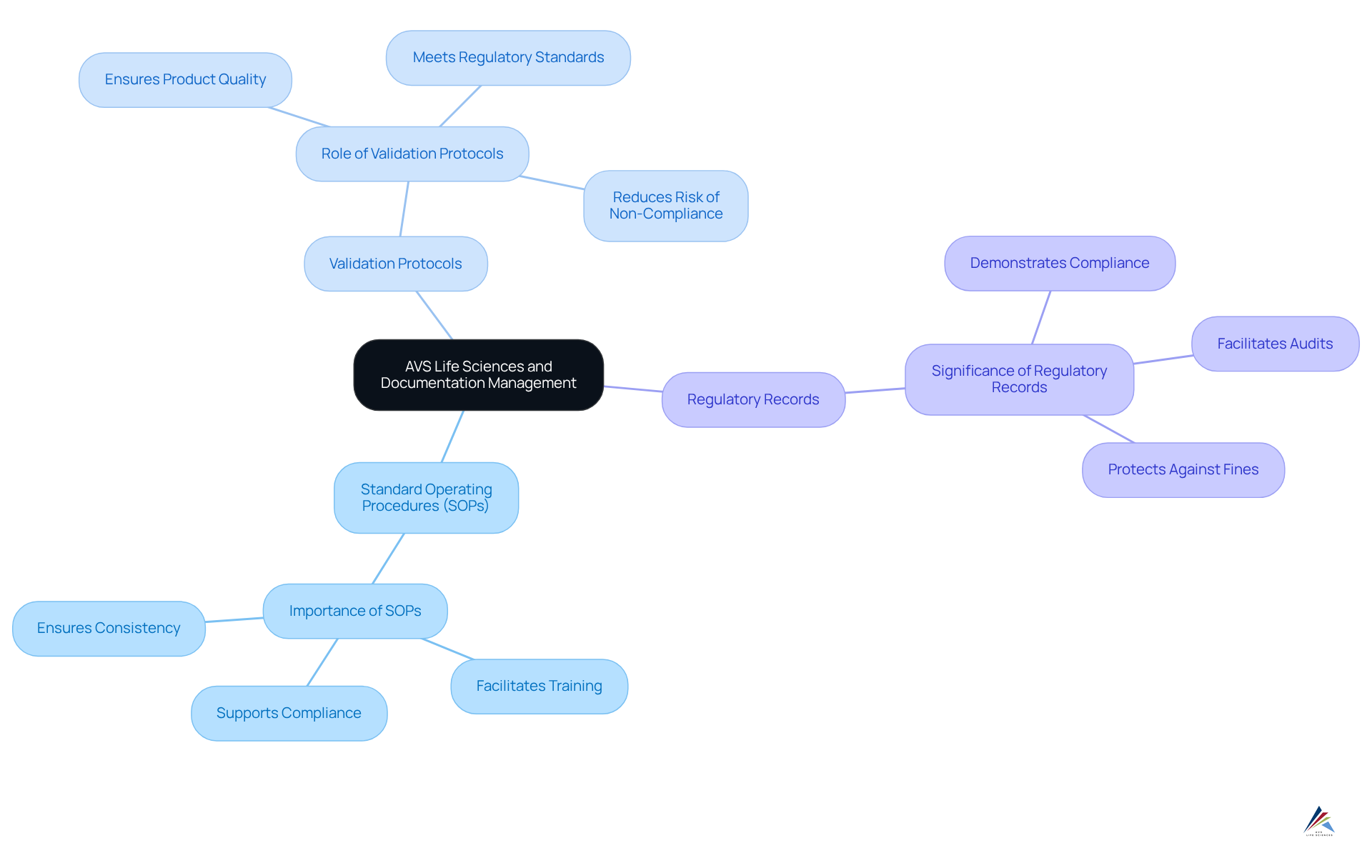
Documentation Management: AVS Life Sciences' Role in Packaging Compliance
AVS Life Sciences plays a crucial role in effective documentation management for packaging adherence. Their skilled advisors assist clients in developing and maintaining essential documentation, including:
- Standard Operating Procedures (SOPs)
- Validation protocols
- Regulatory records
Precise and current documentation is vital; it not only demonstrates adherence during audits and inspections but also significantly reduces the likelihood of non-adherence findings. Organizations implementing robust documentation practices can expect a marked decrease in audit-related issues, with effective documentation management being a key factor in achieving successful outcomes.
Regulatory specialists emphasize that the integrity of documentation is essential in navigating the complexities of regulatory requirements, making it imperative for organizations to prioritize comprehensive documentation practices. By leveraging AVS Life Sciences' expertise, clients can ensure their documentation meets industry standards, ultimately fostering a culture of adherence and operational excellence.
Balancing Compliance and Efficiency: AVS Life Sciences' Strategic Solutions
AVS Life Sciences recognizes the critical need to balance adherence with operational efficiency in production processes. Their strategic solutions are designed to while ensuring rigorous compliance with regulatory requirements. By leveraging cutting-edge technology and industry-leading methodologies, AVS empowers clients to optimize their workflow processes, effectively reducing costs and accelerating time-to-market without compromising regulatory standards.
Industry leaders emphasize that integrating technology into container processes not only enhances efficiency but also fortifies adherence, making it an indispensable aspect of modern operations. As organizations navigate the complexities of regulatory landscapes, the way how AVS supports brands with compliant packaging and label review positions them as a reliable partner in achieving operational excellence.

Client Testimonials: Success Stories of AVS Life Sciences in Packaging Compliance
Client testimonials highlight how AVS supports brands with compliant packaging and label review, demonstrating its transformative impact on enhancing adherence to compliance standards across various brands. Numerous customers have reported substantial improvements in their regulatory frameworks, illustrating how AVS supports brands with compliant packaging and label review, resulting in a significant reduction in audit findings and heightened operational efficiency following their partnership.
For instance, organizations that leveraged AVS's consulting services have seen success rates soar, with 81% of clients affirming how AVS supports brands with compliant packaging and label review, which notably streamlined their packaging processes.
These success narratives not only demonstrate the effectiveness of AVS's but also reflect the company's steadfast commitment to client satisfaction and excellence within the life sciences sector. As a result, AVS Life Sciences has established itself as a dependable ally, showcasing how AVS supports brands with compliant packaging and label review, and achieving an impressive 80% repeat business rate that further validates its role in advancing regulatory compliance across the pharmaceutical industry.

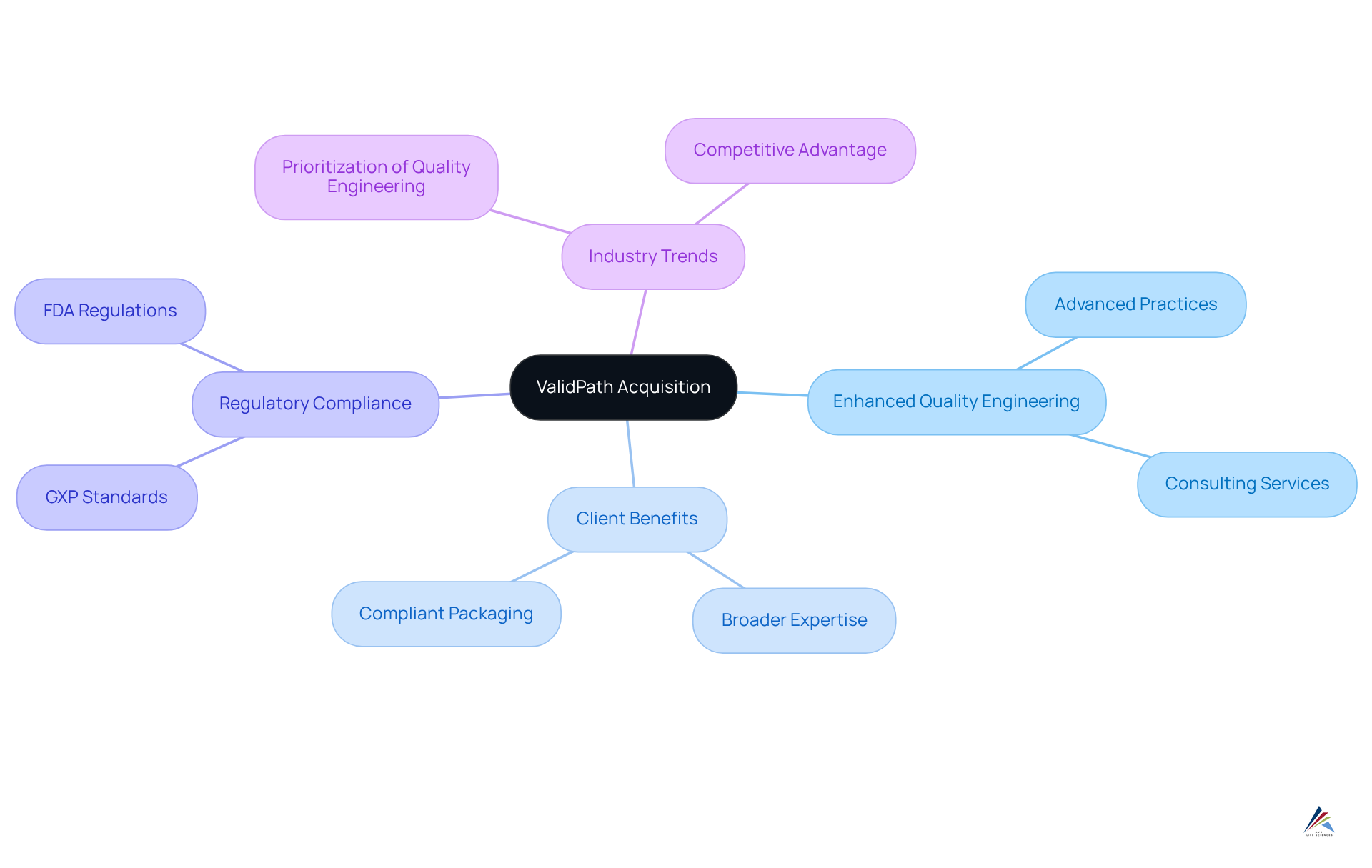
ValidPath Acquisition: Enhancing AVS Life Sciences' Quality Engineering for Packaging Compliance
The acquisition of ValidPath has significantly bolstered AVS Life Sciences' quality engineering capabilities, particularly in product regulations. This strategic enhancement positions AVS to provide more robust solutions, seamlessly integrating into their consulting services. Clients benefit from a broader spectrum of expertise, demonstrating how AVS supports brands with compliant packaging and label review, ensuring their packaging adheres to regulatory standards, including GXP and FDA regulations, while achieving the highest quality benchmarks.
Current trends indicate that organizations are increasingly prioritizing quality engineering to adeptly navigate complex regulatory environments. Industry analysts emphasize that such advancements in quality engineering are essential for sustaining competitive advantage and ensuring product integrity in the marketplace.
Moreover, AVS Life Sciences' remarkable 80% repeat business rate highlights the trust and satisfaction clients place in their services, reinforcing the significance of these advancements in quality management and regulatory compliance.
Conclusion
AVS Life Sciences emerges as an essential partner for brands navigating the complex terrain of compliant packaging and label review. By offering tailored consulting services that prioritize regulatory adherence, AVS empowers organizations to meet stringent industry standards, ensuring their packaging processes are efficient and compliant. This unwavering commitment to quality compliance not only protects client interests but also enhances operational capabilities, positioning AVS as a trusted ally within the pharmaceutical sector.
The article underscores several key components of AVS's services, such as:
- Comprehensive training programs
- Robust validation and commissioning services
- Expert guidance on regulatory submissions
Each of these elements is pivotal in cultivating a culture of compliance, ultimately minimizing the risk of non-adherence during audits and inspections. The focus on Good Manufacturing Practices (GMP) and ISO standards further highlights the significance of quality assurance in packaging, illustrating how AVS aids brands in achieving operational excellence.
In a rapidly changing regulatory landscape, prioritizing compliance transcends mere obligation; it represents a strategic advantage. Organizations are urged to capitalize on AVS Life Sciences' expertise to refine their packaging compliance strategies, ensuring competitiveness while safeguarding product integrity. By embracing these insights and solutions, companies can realize substantial enhancements in operational efficiency and customer satisfaction, ultimately driving success in the pharmaceutical industry.
Frequently Asked Questions
What services does AVS Life Sciences provide for packaging and labeling compliance?
AVS Life Sciences specializes in quality regulatory consulting for containers and labeling, offering compliant packaging and label review, ensuring adherence to industry standards such as Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
How does AVS Life Sciences ensure compliance during audits and inspections?
AVS conducts meticulous reviews of label content, design, and materials, which significantly mitigates the risk of non-compliance during audits and inspections, thereby safeguarding client interests and enhancing operational efficiency.
What are the benefits of AVS Life Sciences' validation and commissioning services?
AVS Life Sciences provides robust validation and commissioning services that verify containers, processes, and materials to ensure compliance with GMP, helping clients mitigate regulatory challenges and improve the efficiency and reliability of their wrapping operations.
Why is validation important for regulatory compliance?
Validation is critical for maintaining product integrity and regulatory compliance. Companies that implement comprehensive validation protocols report fewer compliance-related disruptions, highlighting its importance in quality assurance.
What guidance does AVS Life Sciences offer for regulatory submissions?
AVS Life Sciences provides specialized guidance on preparing and submitting documentation to regulatory bodies, which is essential for brands navigating regulated markets. This assistance helps streamline the approval process and minimize risks of delays.
What best practices does AVS recommend for successful regulatory submissions?
AVS advocates for early collaboration with biostatisticians and comprehensive documentation preparation, both of which are vital for successful regulatory submissions and enhancing the likelihood of first-time approvals.
What challenges are present in the regulatory submission landscape as of 2025?
The regulatory submission landscape is changing, with the FDA processing over 300,000 submissions annually. Applications requiring resubmissions often face median delays of 435 days, making detailed and precise documentation crucial for timely approvals.
