7 Ways How AVS Handles Facility Registration and FDA Updates for Clients

Overview
AVS Life Sciences adeptly addresses facility registration and FDA updates for clients through a systematic approach that encompasses expert guidance, tailored training programs, and advanced technology solutions. This article highlights how AVS's comprehensive strategies, including automated compliance monitoring and customized documentation practices, empower organizations to navigate regulatory complexities.
By enhancing adherence to FDA standards, these strategies ultimately improve operational efficiency and compliance outcomes. Organizations face significant compliance challenges, yet AVS Life Sciences provides robust solutions designed to streamline processes and ensure regulatory alignment.
Engage with AVS Life Sciences to elevate your compliance efforts and achieve excellence in operational performance.
Introduction
AVS Life Sciences stands at the forefront of regulatory compliance, expertly navigating the complexities of facility registration and FDA updates. In an era where the industry faces increasing scrutiny and evolving regulations, organizations are compelled not only to meet compliance standards but also to excel in operational efficiency. This article explores the innovative strategies employed by AVS, showcasing how their tailored solutions and cutting-edge technology empower clients to surmount regulatory challenges.
What key practices can transform compliance management into a streamlined process?
How can organizations leverage these insights to enhance their own regulatory frameworks?
The answers lie within the expertise of AVS Life Sciences.
AVS Life Sciences: Streamlining Facility Registration Processes
AVS Life Sciences demonstrates how AVS handles facility registration and FDA updates for clients by addressing the compliance challenges faced by facilities in the registration process with a systematic approach. By leveraging extensive knowledge of regulatory frameworks, including the Food Safety Modernization Act (FSMA) and the Bioterrorism Act, our team of specialists—comprising over 300 seasoned associates worldwide—assists customers in preparing and submitting essential documentation. This guarantees adherence to FDA requirements, particularly in how AVS handles facility registration and FDA updates for clients, with the impending enforcement for product listing starting on July 1, 2024.
Through pre-registration consultations, AVS experts guide individuals in understanding how AVS handles facility registration and FDA updates for clients, helping them avoid common pitfalls. Our customized strategies not only expedite the registration process but also enhance the overall regulatory posture of the facilities involved. By applying best practices, such as Standard Operating Procedures (SOPs) and emphasizing data integrity in Computer System Validation (CSV), while adhering to GxP and FDA guidelines, AVS improves efficiency in maneuvering through the oversight environment. This ultimately supports customers in achieving their compliance objectives.
Understanding FDA Regulations for Facility Registration
The FDA mandates that all facilities engaged in the manufacturing, processing, packing, or holding of food, drugs, and medical devices register with the agency. This registration is not merely a one-time obligation; it requires periodic renewal, and facilities must comply with specific guidelines outlined in the Federal Food, Drug, and Cosmetic Act. Notably, compliance rates for FDA facility registrations have shown remarkable improvement, with the FDA conducting 18,169 inspections in 2023—a 17.6% increase from the previous year.
AVS Life Sciences provides customers with comprehensive insights into how AVS handles facility registration and FDA updates for clients, highlighting the importance of understanding their responsibilities to maintain operational integrity and avoid compliance penalties. A transformative case study exemplifies AVS's successful enhancement of a biotechnology GMP facility. Through our expertise in quality assurance and adherence to standards, the customer progressed from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, significantly boosting their manufacturing capabilities while strictly following guidelines. As one compliance officer aptly stated, 'Understanding FDA guidelines is not just about compliance; it's about ensuring the safety and efficacy of our products.'
This perspective highlights the vital role that AVS plays in how AVS handles facility registration and FDA updates for clients, helping individuals navigate the complexities of compliance obligations effectively and illustrating the substantial ramifications of non-adherence.
Leveraging Technology for Efficient FDA Updates
AVS Life Sciences utilizes cutting-edge technology to enhance how AVS handles facility registration and FDA updates for clients, effectively addressing the pressing compliance challenges faced by the industry. By implementing automated systems that monitor compliance changes, clients benefit from real-time alerts about relevant updates. This proactive strategy significantly reduces the risk of non-compliance, ensuring that facilities consistently adhere to the latest regulations. In fact, AI-driven real-time monitoring can decrease adherence issues by 40%, demonstrating the effectiveness of AVS's approach.
Furthermore, AVS provides comprehensive training on these technologies, emphasizing the importance of mastering essential competencies prior to FDA inspections, as recommended by UL Solutions. Industry leaders stress that automation plays a vital role in enhancing compliance, with automated systems streamlining workflows and improving accuracy in documentation. For instance, over 60% of top pharma companies have adopted AI-based automation for at least one stage of drug manufacturing, showcasing the industry's shift towards technology.
Additionally, AI-driven data analysis can reduce submission errors by as much as 35%, further illustrating the impact of automation. AVS Life Sciences exemplifies this method, showcasing how technology can transform compliance management into a more efficient and trustworthy process. By engaging with AVS, organizations can learn how AVS handles facility registration and FDA updates for clients, enabling them to navigate the complexities of compliance and elevate their operational standards to meet regulatory expectations.
Training Programs for Compliance with FDA Standards
AVS Life Sciences presents comprehensive training programs meticulously crafted to enlighten clients about how AVS handles facility registration and FDA updates for clients, along with FDA standards and regulatory requirements. These programs cover essential topics, including how AVS handles facility registration and FDA updates for clients, Good Manufacturing Practices (GMP), and documentation protocols.
By equipping employees with essential knowledge and skills, AVS cultivates a culture of adherence within organizations, significantly mitigating the risk of violations and enhancing operational efficiency. Current trends underscore that effective GMP training must be continuous and engaging, integrating real-world applications and recent industry developments to sustain employee interest and knowledge retention.
Insights from regulatory trainers highlight the necessity of structured on-the-job training (SOJT) and regular assessments to ensure that employees not only comprehend but can also implement FDA standards proficiently. This thorough approach to training fulfills legal standards while preparing organizations for sustained success in navigating the complexities of how AVS handles facility registration and FDA updates for clients.
A transformative case study exemplifies this: AVS assisted a leading biotechnology company in upgrading its GMP facility, confronting challenges such as initial test result anomalies due to improperly installed barcode scanners. By addressing these issues and capturing vital lessons learned, AVS ensured that the staff was well-trained in quality assurance protocols. This partnership not only facilitated adherence but also allowed the client to focus on developing life-saving medications, underscoring the direct impact of effective training on operational success.
Effective Documentation Management for Regulatory Compliance
Efficient documentation management is paramount for navigating regulatory landscapes, and how AVS handles facility registration and FDA updates for clients is what keeps AVS Life Sciences at the forefront of this critical area. The company employs comprehensive document control systems that guarantee all compliance documents are meticulously maintained, readily accessible, and consistently updated. This encompasses:
- The development of standardized templates for essential documents
- Regular audits of documentation practices
- Extensive training for staff on proper documentation protocols
Compliance experts assert that efficient documentation not only streamlines the audit process but also significantly enhances overall operational effectiveness, ultimately yielding improved outcomes in inspections. AVS ensures its documentation practices meet the highest quality management criteria by adhering to FDA regulations and GXP standards, which illustrates how AVS handles facility registration and FDA updates for clients. For instance, implementing compliance analytics can boost reporting efficiency by 82%, exemplifying the tangible benefits of a robust documentation management system. By prioritizing these practices, AVS demonstrates how AVS handles facility registration and FDA updates for clients, empowering them to mitigate risks associated with non-compliance and enhancing their preparedness for successful audits.
Navigating Challenges in Regulatory Compliance
Organizations frequently encounter a myriad of challenges in regulatory adherence, particularly within the life sciences sector, where regulations evolve rapidly and resource constraints are common. A significant 70% of corporate risk and regulatory experts have noted a transition toward a more strategic approach to compliance, underscoring the imperative for organizations to adapt effectively.
AVS Life Sciences equips clients to navigate these complexities by providing tailored solutions, specifically focusing on how AVS handles facility registration and FDA updates for clients to meet their specific needs. This process includes:
- Conducting thorough gap assessments to pinpoint deficiencies in compliance
- Offering strategic insights on regulatory changes
- Delivering continuous support to ensure adherence
As one consultant aptly stated, 'Overcoming legal obstacles demands not only comprehending the guidelines but also foreseeing changes and preparing accordingly.'
AVS's commitment to proactive compliance management illustrates how AVS handles facility registration and FDA updates for clients, empowering them to thrive amidst regulatory challenges while ensuring alignment with their core business objectives.
Preparing for Audits and Inspections: Best Practices
Thorough preparation for audits and inspections is essential in the life sciences field, where adherence to regulatory standards is vital. AVS Life Sciences advocates for several best practices to bolster audit readiness.
-
Conducting mock audits stands out as a key strategy; this approach allows organizations to identify potential issues before the actual audit occurs. Such a proactive strategy not only emphasizes areas requiring enhancement but also fosters employee confidence in addressing regulatory challenges.
-
Maintaining organized documentation is crucial, as it facilitates a smoother audit process.
-
Ensuring that all staff are well-trained and aware of their specific roles during an audit can significantly enhance overall readiness.
-
Furthermore, open communication with auditors is another critical component; fostering transparency can lead to a more collaborative and constructive audit experience.
Statistics reveal that organizations employing mock audits report a marked improvement in compliance readiness. Notably, 89% of risk and compliance professionals acknowledge that such practices expedite time-to-compliance across multiple frameworks. By implementing these best practices, organizations can effectively enhance their audit readiness and minimize the risk of non-compliance findings, ultimately safeguarding their reputation and operational integrity.
Enhancing Communication for Successful Facility Registration
Effective communication is paramount for clients to understand how AVS handles facility registration and FDA updates. AVS Life Sciences emphasizes how AVS handles facility registration and FDA updates for clients by ensuring open channels of communication between customers and governing entities, keeping all parties informed and coordinated throughout the registration process. This commitment encompasses:
- Regular updates on application statuses
- Prompt responses to inquiries
- Meticulous documentation of all communications
By fostering a culture of transparency and responsiveness, AVS empowers clients to navigate the complexities of how AVS handles facility registration and FDA updates for clients, resulting in greater efficiency and effectiveness.
Professionals in affairs stress that clear communication not only improves adherence but also fosters trust, which is vital for successful interactions with oversight agencies. For instance, AVS Life Sciences has successfully facilitated numerous client-regulatory body communications, illustrating how AVS handles facility registration and FDA updates for clients, which demonstrates that proactive engagement can lead to smoother approvals and a more streamlined registration process.
A transformative case study illustrates this: AVS assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. Throughout this project, AVS upheld open communication, which was vital for tackling challenges and ensuring adherence to standards.
AVS utilized specific methodologies such as gap analysis and customized communication strategies to ensure all compliance requirements were met effectively. Through these efforts, AVS Life Sciences illustrates how effective communication can greatly influence success in meeting regulations.
Tailored Solutions for Unique Client Needs in Facility Registration
AVS Life Sciences understands that how AVS handles facility registration and FDA updates for clients is not a one-size-fits-all process. Each customer presents unique challenges and requirements, prompting the company to offer customized solutions that address specific organizational needs. This includes:
- Tailored training programs designed to enhance regulatory adherence
- Specialized documentation templates that streamline the registration process
- Dedicated support throughout every stage of registration
With expertise in GXP, FDA regulations, and the development of Standard Operating Procedures (SOPs), AVS ensures that customers are well-equipped to meet regulatory standards. Moreover, AVS emphasizes effective documentation methods and addresses data integrity deviations to further bolster adherence. For instance, AVS has developed training modules that highlight the intricacies of regulatory standards, significantly improving success rates among customers.
Industry experts assert, 'Tailored regulatory strategies are essential for navigating the complexities of facility registration effectively.' By prioritizing customized services and leveraging their extensive quality management practices, AVS empowers customers to understand how AVS handles facility registration and FDA updates for clients, allowing them to navigate the complexities with confidence and ensuring they meet regulatory standards efficiently.
Continuous Improvement in Regulatory Compliance Practices
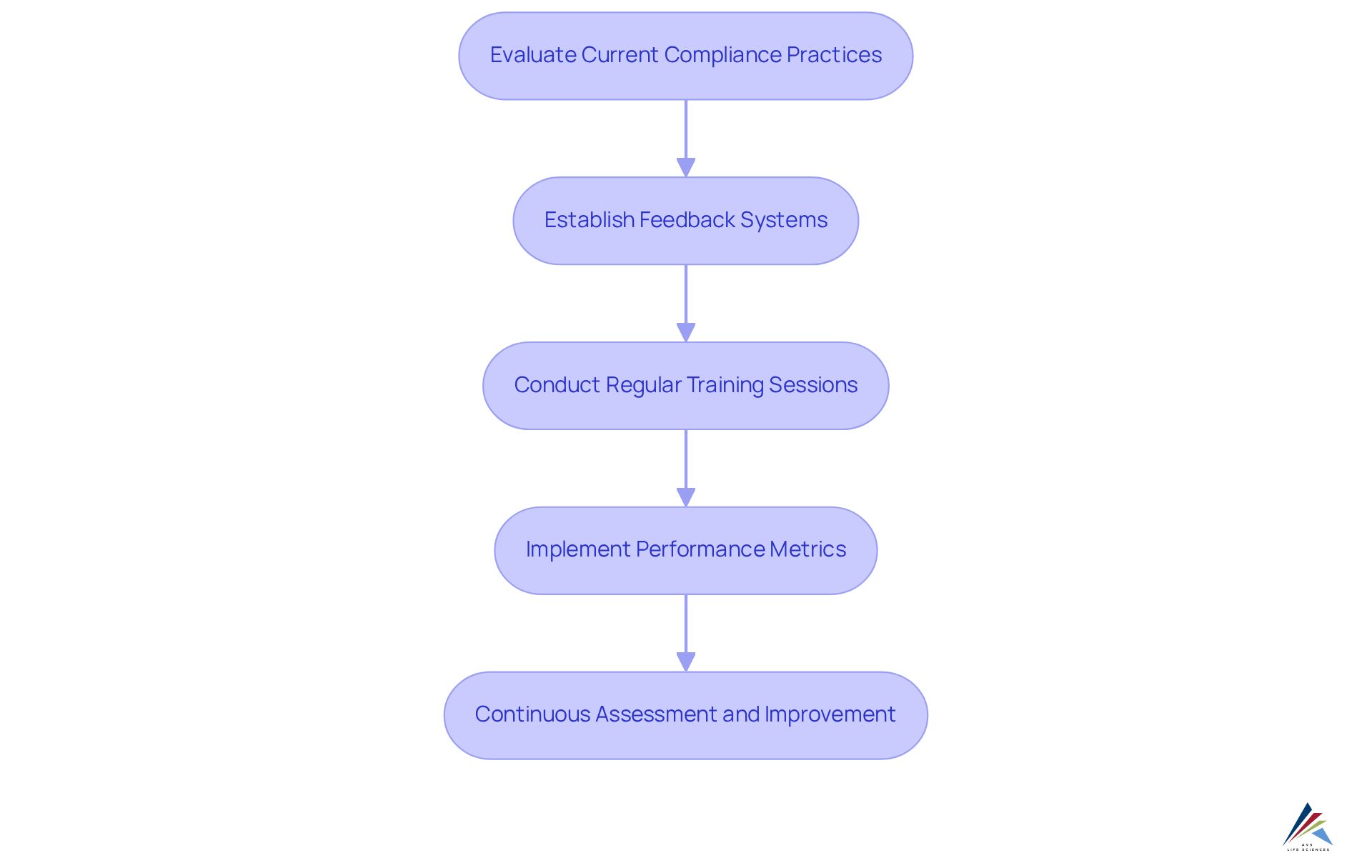
At AVS Life Sciences, continuous improvement transcends mere principle; it stands as a fundamental operational strategy. The company actively empowers clients to routinely evaluate and enhance their adherence practices, ensuring alignment with evolving regulations and industry standards. This proactive approach includes the establishment of robust feedback systems, essential for assessing adherence effectiveness. Regular training sessions play a pivotal role, equipping staff with the necessary knowledge to navigate regulatory challenges effectively. Performance metrics are employed to measure the success of these initiatives, enabling organizations to pinpoint areas for enhancement.
By fostering a culture of perpetual improvement, AVS enables organizations not only to meet current regulatory standards but also to anticipate and prepare for future adherence challenges. This commitment to adaptability is vital in a landscape where 60% of executives report increased investment in regulatory compliance, underscoring its growing importance. Organizations that implement effective feedback systems, as evidenced by numerous case studies, have experienced significant improvements in adherence outcomes, highlighting the tangible benefits of AVS's methodology. As industry leaders emphasize the necessity of adapting to regulatory changes, AVS Life Sciences positions itself as a dedicated partner by showcasing how AVS handles facility registration and FDA updates for clients, assisting them in navigating the complexities of regulatory management. To fully leverage the advantages of AVS's services, pharmaceutical compliance officers must engage in regular assessments and training, ensuring their organizations remain at the forefront of compliance excellence.
Conclusion
AVS Life Sciences exemplifies a comprehensive approach to managing facility registration and FDA updates, tailored to meet the unique needs of each client. By integrating regulatory expertise with advanced technology, AVS not only simplifies the registration process but also ensures that clients remain compliant with evolving FDA standards. This commitment to personalized service and strategic guidance positions AVS as a vital partner in navigating the complexities of regulatory compliance.
Throughout the article, key strategies such as:
- Pre-registration consultations
- Effective documentation management
- Continuous training programs
have been highlighted. AVS's use of technology, including automated compliance monitoring and AI-driven data analysis, significantly enhances operational efficiency and reduces the risk of non-compliance. Moreover, the emphasis on open communication fosters a collaborative environment that is essential for successful interactions with regulatory bodies.
In an industry where regulatory landscapes are constantly shifting, the importance of proactive compliance management cannot be overstated. Organizations are encouraged to leverage AVS Life Sciences' expertise to not only meet current standards but also to anticipate future challenges. By prioritizing continuous improvement and tailored solutions, clients can enhance their operational integrity and ensure that they are well-prepared for upcoming FDA requirements. Engaging with AVS is not just a step toward compliance; it is a strategic investment in the future success and sustainability of any facility within the life sciences sector.
Frequently Asked Questions
What is the role of AVS Life Sciences in facility registration and FDA updates?
AVS Life Sciences assists clients in navigating the facility registration process and ensuring compliance with FDA requirements by providing systematic approaches and leveraging extensive knowledge of regulatory frameworks.
What regulatory frameworks does AVS Life Sciences focus on?
AVS Life Sciences focuses on regulatory frameworks such as the Food Safety Modernization Act (FSMA) and the Bioterrorism Act to help clients with facility registration and compliance.
How does AVS Life Sciences help clients avoid common pitfalls in the registration process?
AVS Life Sciences offers pre-registration consultations where experts guide clients in understanding the registration process, helping them avoid common pitfalls and expedite their registration.
What strategies does AVS Life Sciences employ to enhance regulatory compliance?
AVS Life Sciences employs customized strategies, best practices like Standard Operating Procedures (SOPs), and emphasizes data integrity in Computer System Validation (CSV) to enhance regulatory compliance.
What are the FDA's requirements for facility registration?
The FDA requires all facilities involved in the manufacturing, processing, packing, or holding of food, drugs, and medical devices to register with the agency, which includes periodic renewals and adherence to specific guidelines.
How has the compliance rate for FDA facility registrations changed recently?
Compliance rates for FDA facility registrations have improved, with the FDA conducting 18,169 inspections in 2023, marking a 17.6% increase from the previous year.
Can you provide an example of AVS Life Sciences' impact on a client's facility?
AVS Life Sciences successfully enhanced a biotechnology GMP facility's capabilities, progressing it from a Biosafety Level 1 to a Level 2 GMP facility while ensuring compliance with quality assurance standards.
How does AVS Life Sciences use technology in the registration process?
AVS Life Sciences utilizes cutting-edge technology, including automated systems that monitor compliance changes and provide real-time alerts, significantly reducing the risk of non-compliance.
What are the benefits of AI-driven real-time monitoring in compliance management?
AI-driven real-time monitoring can decrease adherence issues by 40% and reduce submission errors by as much as 35%, demonstrating its effectiveness in enhancing compliance management.
What training does AVS Life Sciences provide regarding compliance technologies?
AVS Life Sciences offers comprehensive training on compliance technologies, emphasizing the importance of mastering essential competencies prior to FDA inspections.
