7 Strategies for Managing Nitrosamine Impurities Effectively

Overview
The article addresses the significant challenges associated with managing nitrosamine impurities in pharmaceuticals, underscoring the necessity for rigorous compliance and quality control measures. It elaborates on effective strategies, such as the adoption of advanced analytical techniques and comprehensive risk assessment strategies. These approaches are critical for ensuring product safety and meeting regulatory standards within the pharmaceutical industry. By implementing these solutions, organizations can enhance their compliance posture and safeguard public health.
Introduction
The pharmaceutical industry is confronted with a critical challenge: the widespread presence of nitrosamine impurities, which are recognized as probable human carcinogens and present serious risks to public health. As manufacturers navigate stringent regulatory standards and the looming threat of expensive recalls, the implementation of effective management strategies becomes imperative.
How can companies not only meet the demands of evolving regulations but also guarantee the safety and quality of their products amidst such formidable contaminants? This article delves into seven pivotal strategies that can empower pharmaceutical firms to adeptly maneuver through the complexities of nitrosamine impurity management, all while prioritizing consumer health.
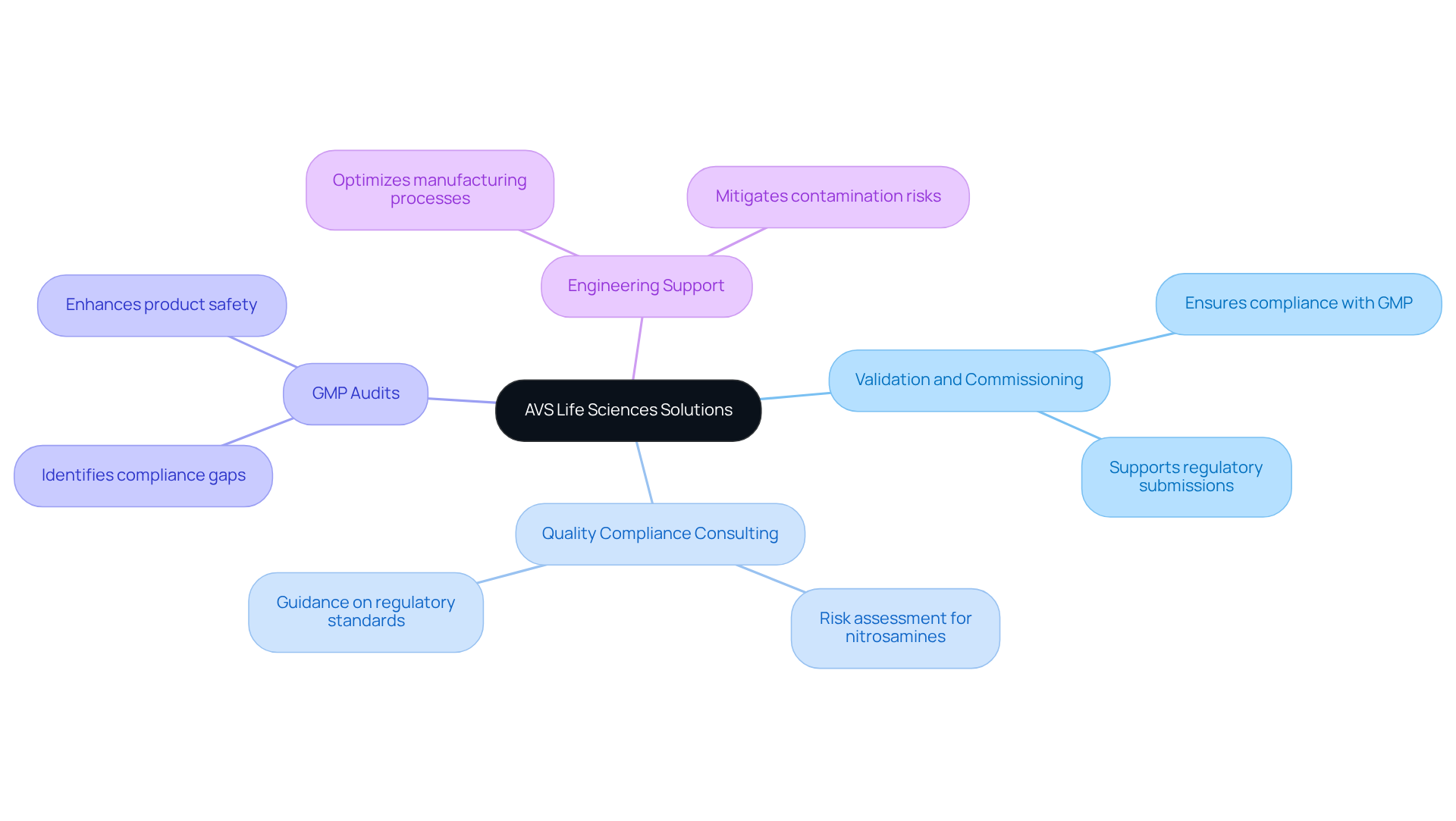
AVS Life Sciences: Comprehensive Regulatory Solutions for Nitrosamine Impurities
presents a comprehensive suite of compliance solutions meticulously designed to address impurities in pharmaceuticals. Their offerings include:
- Validation and commissioning
- Quality compliance consulting
- GMP audits
- Engineering support
All aimed at ensuring adherence to Good Manufacturing Practices (GMP) and other stringent regulatory standards. With a dedicated team of over 300 skilled experts, AVS Life Sciences equips clients to adeptly navigate the complexities of managing harmful substances. This commitment not only ensures compliance but also protects product safety and quality, which is a critical concern due to the presence of nitrosamine impurities that are classified as probable human carcinogens.
Notably, 29.6% of active pharmaceutical ingredients (APIs) identified in the Global Substance Registration System (GSRS) database are potential precursors, underscoring the pervasive nature of this issue. Furthermore, AVS Life Sciences enjoys an impressive 80% repeat business rate, a testament to client satisfaction and trust in their services. By capitalizing on their expertise in GMP audits and comprehensive quality management solutions, AVS Life Sciences empowers clients to meet the highest safety standards while mitigating the risks associated with nitrosamine impurities.
Their proactive approach to managing these contaminants is vital, especially considering the potential consequences of non-compliance, which can include costly product recalls and legal ramifications. As Dr. Pravin Karmuse articulates, pharmaceutical manufacturers need to effectively address nitrosamine impurities challenges and solutions.
Understanding Nitrosamine Impurities: Origins and Risks in Pharmaceuticals
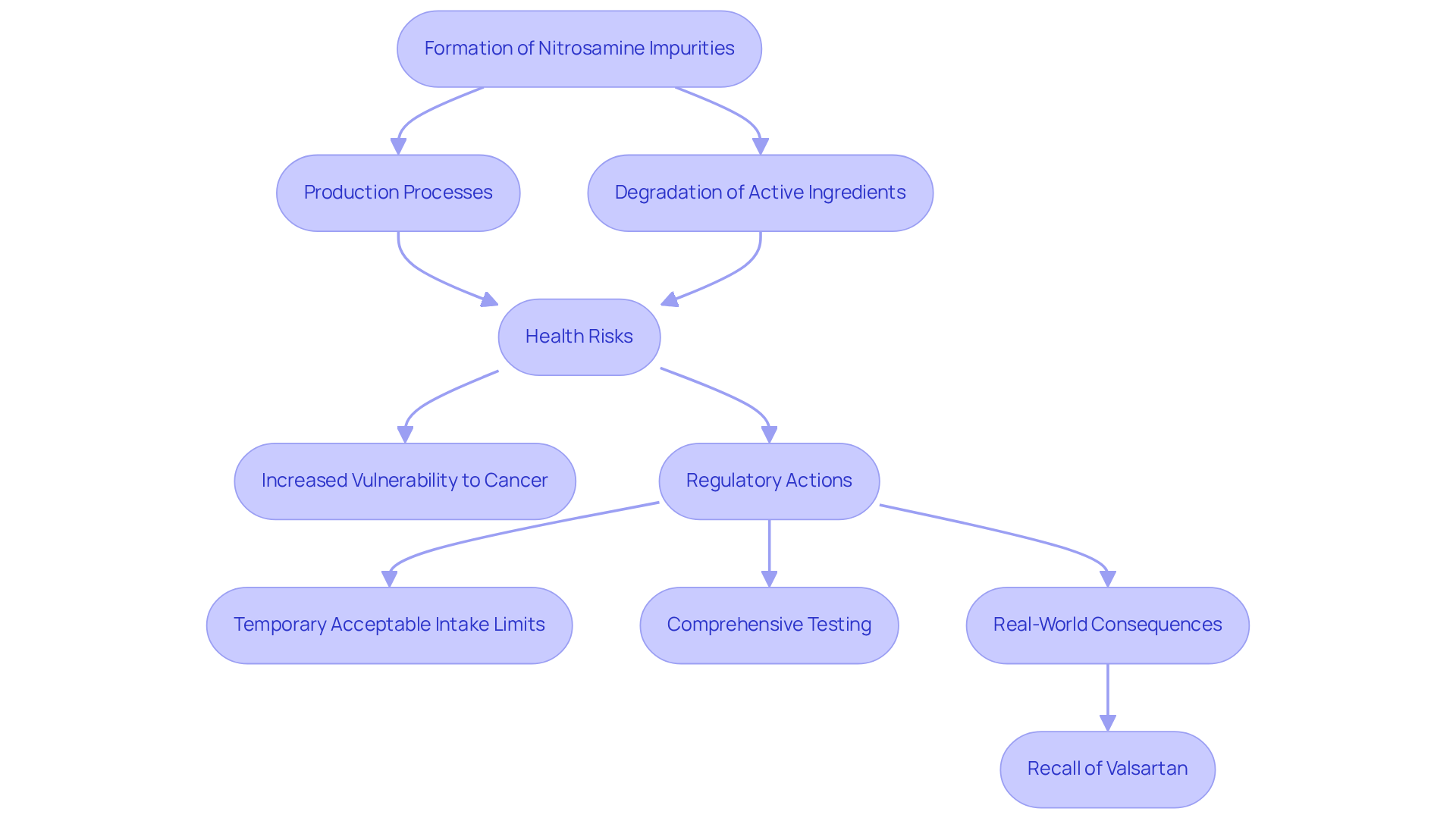
Nitrosamine impurities are chemical compounds that can form during the production of pharmaceuticals, primarily through reactions involving nitrites and amines. The presence of nitrosamine impurities classifies them as probable human carcinogens, raising significant safety concerns. The presence of nitrosamine impurities in drug products is linked to , including an increased vulnerability to cancer.
For instance, contamination can occur during production processes or through the degradation of active ingredients, highlighting the necessity for stringent manufacturing practices. Health authorities emphasize that even minimal exposure to nitrosamine impurities can lead to cancer, underscoring the critical need for effective management strategies.
In response, regulatory authorities have established temporary acceptable intake limits, reflecting the urgency of addressing these issues within the pharmaceutical sector. Real-world examples, such as the recall of valsartan due to contamination, illustrate the potential consequences of inadequate oversight and the imperative for comprehensive testing and compliance actions to safeguard public health.
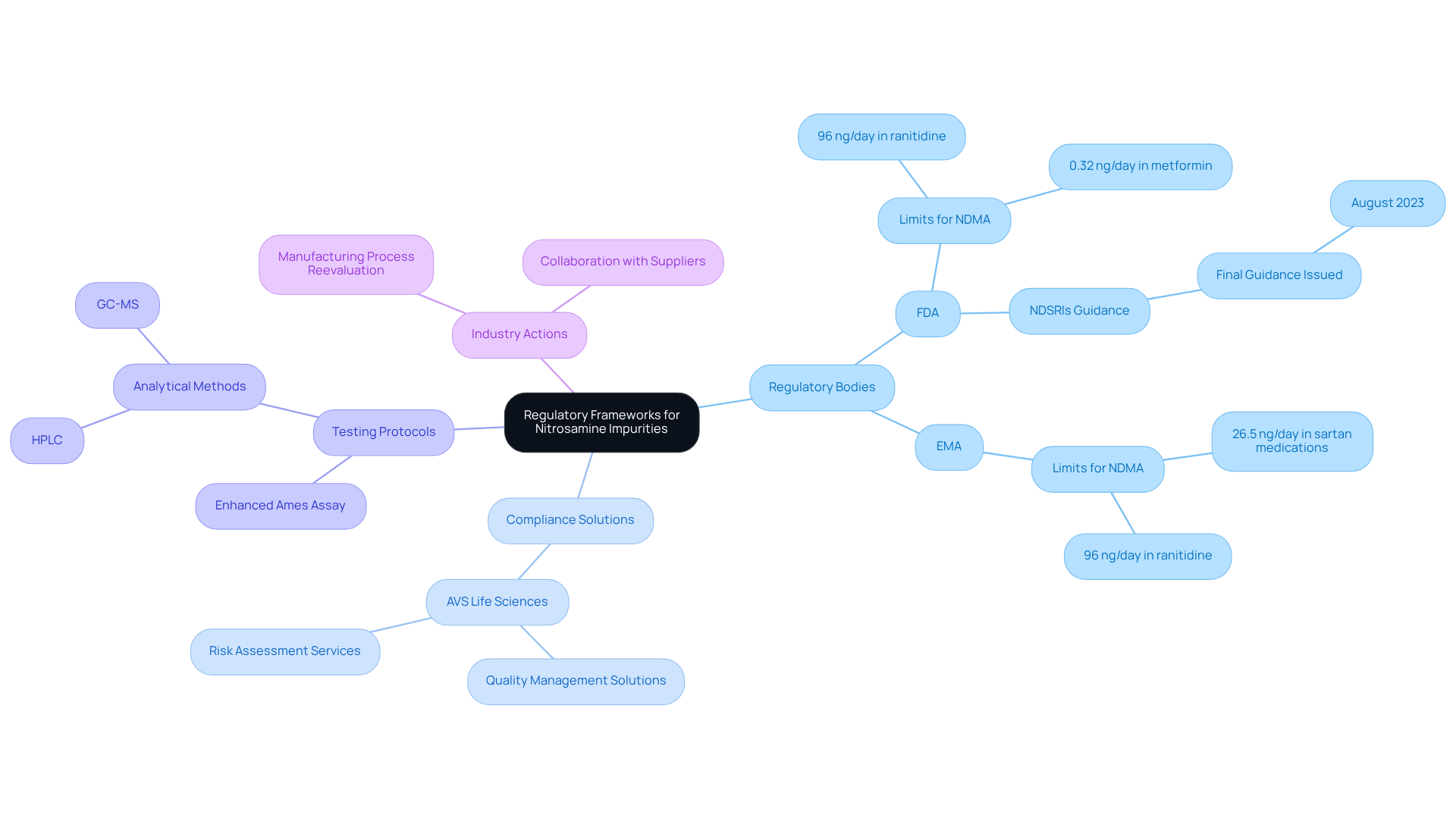
Regulatory Frameworks: Navigating Compliance for Nitrosamine Impurities
The oversight environment for harmful impurities is evolving rapidly, driven by organizations such as the FDA and EMA, which have established stringent protocols aimed at mitigating associated risks.
AVS Life Sciences offers comprehensive quality management and compliance solutions tailored specifically for the life sciences sector, ensuring that manufacturers conduct thorough risk assessments pertinent to their products and processes, in accordance with GXP and FDA standards. This diligence is critical for .
Notably, the FDA's recent updates underscore the imperative of adopting robust testing protocols and upholding transparent reporting practices concerning any identified nitrosamine impurities in pharmaceutical products. Adhering to these regulations transcends mere legal obligation; it is vital for protecting public health and cultivating consumer trust within the pharmaceutical industry.
Statistics indicate that a significant number of pharmaceutical companies are currently reevaluating their manufacturing processes to align with these guidelines, reflecting a broader commitment to quality and safety in drug development.
Insights from regulatory experts at AVS Life Sciences highlight that ongoing collaboration with suppliers and investment in advanced analytical technologies, alongside adherence to standard operating procedures (SOPs), are crucial for the effective management of related compounds, ultimately enhancing the industry's ability to navigate this complex regulatory landscape.
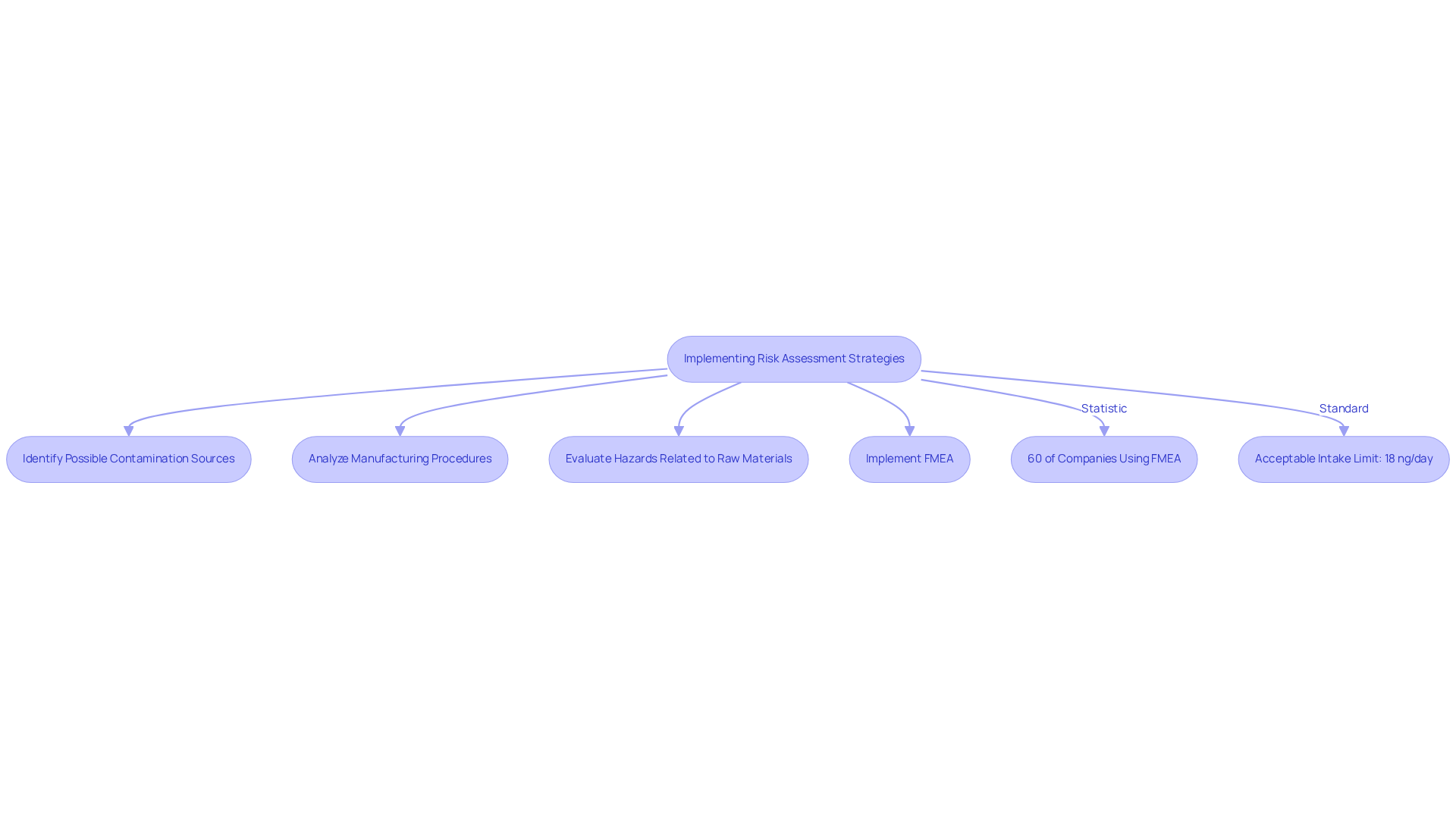
Implementing Risk Assessment Strategies for Nitrosamine Management
Establishing a thorough evaluation strategy is crucial for managing nitrosamine impurities effectively. This involves recognizing possible , analyzing manufacturing procedures, and evaluating the hazards related to raw materials. Organizations must adopt a proactive stance, employing tools such as Failure Mode and Effects Analysis (FMEA) to systematically assess uncertainties and develop mitigation strategies.
Recent research published in the Journal of Pharmaceutical Sciences indicates that approximately 60% of pharmaceutical companies are now utilizing FMEA for assessing hazards associated with specific contaminants, demonstrating a growing trend towards proactive management of potential issues.
Furthermore, the Acceptable Intake Limit for the combined drug substance-related impurities (NDSRIs) is set at 18 ng/day, underscoring the standards that companies must adhere to in their assessments. Regular evaluations and updates to the assessment process are essential to adapt to new discoveries and regulatory changes, including the recent recall due to NDSRI detection in August 2023.
Continuous education and updates on risk management concerning harmful substances are vital to ensure that companies remain compliant and safeguard product integrity.
Analytical Techniques: Detecting Nitrosamine Impurities in Pharmaceuticals
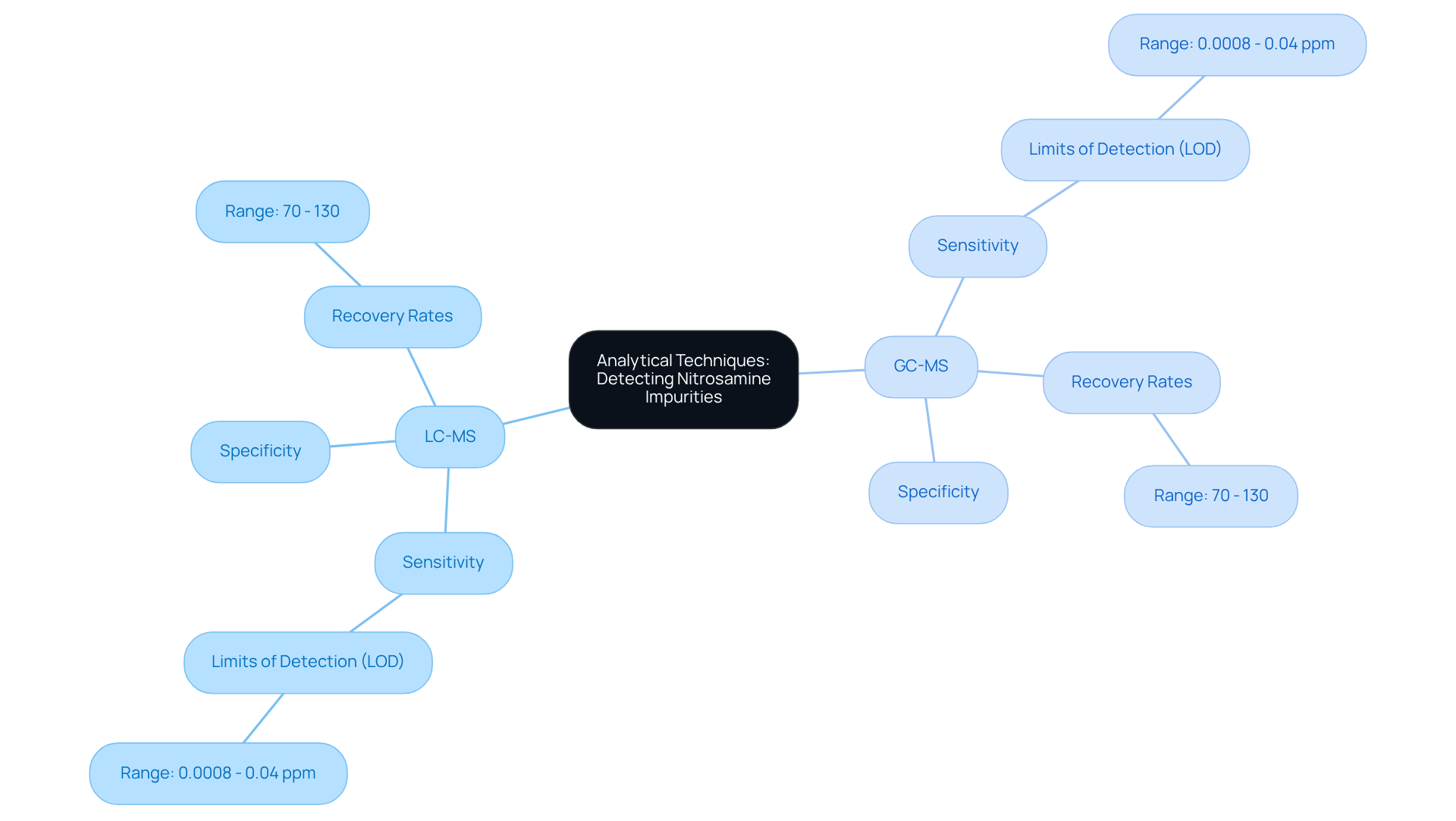
Identifying nitroso-related contaminants necessitates the application of sophisticated analytical methods, with Liquid Chromatography-Mass Spectrometry (LC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS) standing out as the most notable techniques. These methods are celebrated for their , allowing for the identification of trace levels of nitrosamines, often detected at concentrations as low as 0.01 ppm. Recent advancements have significantly enhanced their capabilities, enabling the simultaneous examination of various contaminants—an essential requirement given the stringent limits imposed on these substances.
For example, a highly sensitive GC-MS/MS method has been validated to quantify nine nitrosamine impurities, demonstrating excellent linearity across relevant concentration ranges. The limits of detection (LOD) for these methods typically range from 0.0008 to 0.04 ppm, thus ensuring compliance with regulatory standards. Furthermore, the recovery rates for these analytical methods consistently fall within the acceptable range of 70% to 130%, affirming their reliability in pharmaceutical testing.
Experts underscore the critical need for regular testing and validation of these methods to uphold product safety and regulatory compliance. Investing in cutting-edge analytical tools, coupled with comprehensive training for personnel, emerges as a vital strategy for effectively monitoring chemical levels. As the pharmaceutical sector grapples with challenges related to harmful substances, the ongoing advancement and refinement of LC-MS and GC-MS methods will be pivotal in ensuring drug safety and efficacy.
Quality Control Measures: Mitigating Nitrosamine Impurities in Drug Production
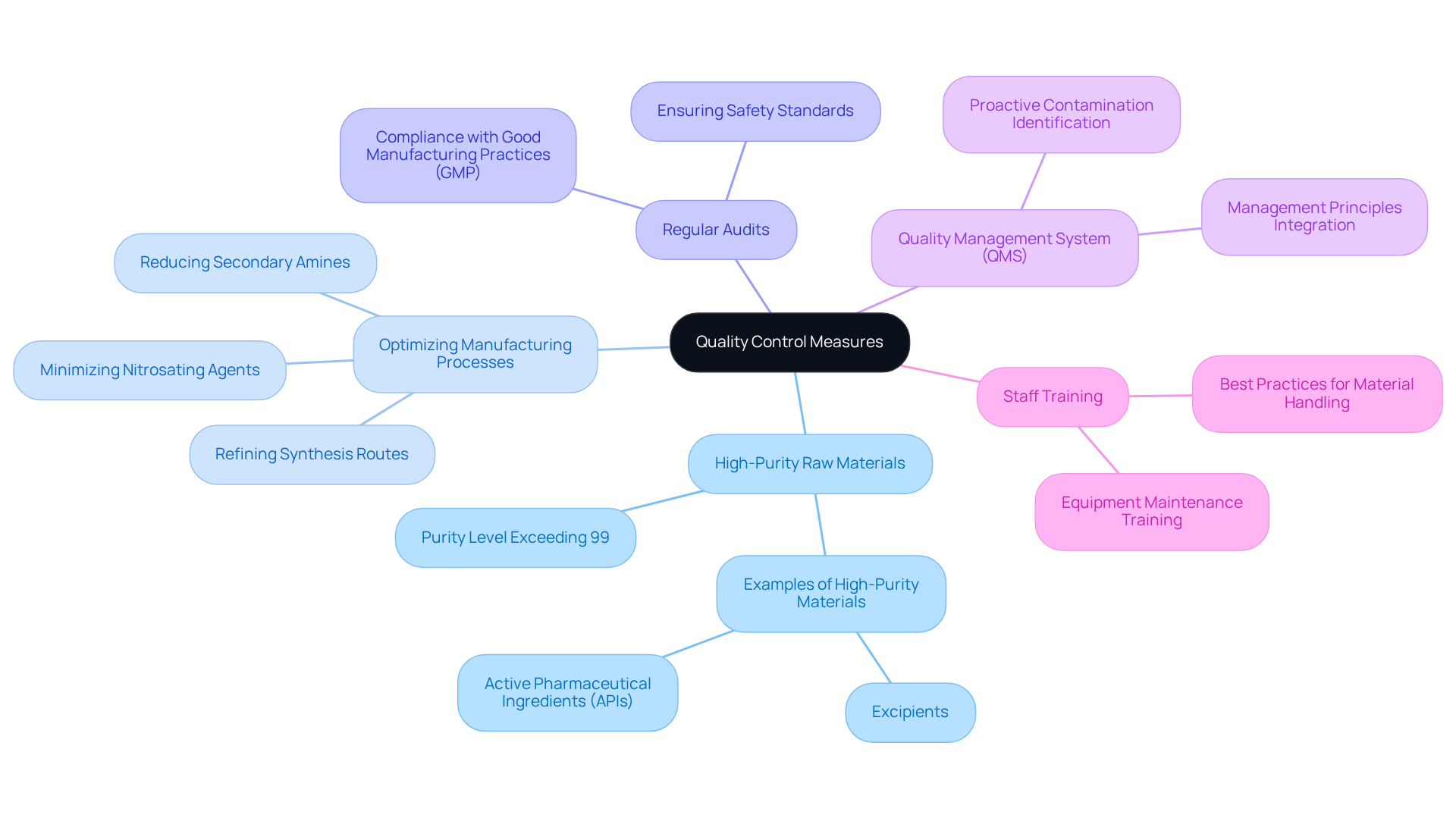
To effectively reduce impurities in drug production, companies must adopt rigorous quality control measures. Engaging with AVS Life Sciences provides tailored consulting solutions that enhance quality management and regulatory compliance. A primary strategy involves selecting high-purity raw materials, significantly diminishing the risk of harmful compound formation. For instance, utilizing raw materials with a purity level exceeding 99% can lead to a substantial reduction in harmful compounds, as impurities often stem from lower-quality inputs. Notable examples of high-purity raw materials include specific grades of excipients and active pharmaceutical ingredients (APIs) certified to meet stringent purity standards.
Optimizing manufacturing processes is equally essential; this encompasses refining synthesis routes and minimizing the use of nitrosating agents and secondary amines, which are known contributors to nitrosamine impurities. Regular audits of production facilities are vital for maintaining compliance with Good Manufacturing Practices (GMP) and ensuring that all processes align with established safety standards. Establishing a robust quality management system (QMS) that incorporates management principles allows organizations to proactively identify and address potential contamination sources before they affect product quality. The FDA underscores the in preventing contamination issues.
Moreover, comprehensive training programs for staff on best practices for handling materials and maintaining equipment are crucial. Such training not only enhances operational efficiency but also cultivates a culture of quality and safety within the organization. Statistics indicate that implementing strict quality control measures can reduce harmful substances by as much as 50%, underscoring the significance of these practices in safeguarding patient health and ensuring adherence to regulatory standards. By prioritizing high-purity raw materials and strong quality management, pharmaceutical firms can substantially mitigate the risks associated with harmful contaminants, with the professional support of AVS Life Sciences.
Training Programs: Keeping Compliance Officers Informed on Nitrosamine Regulations
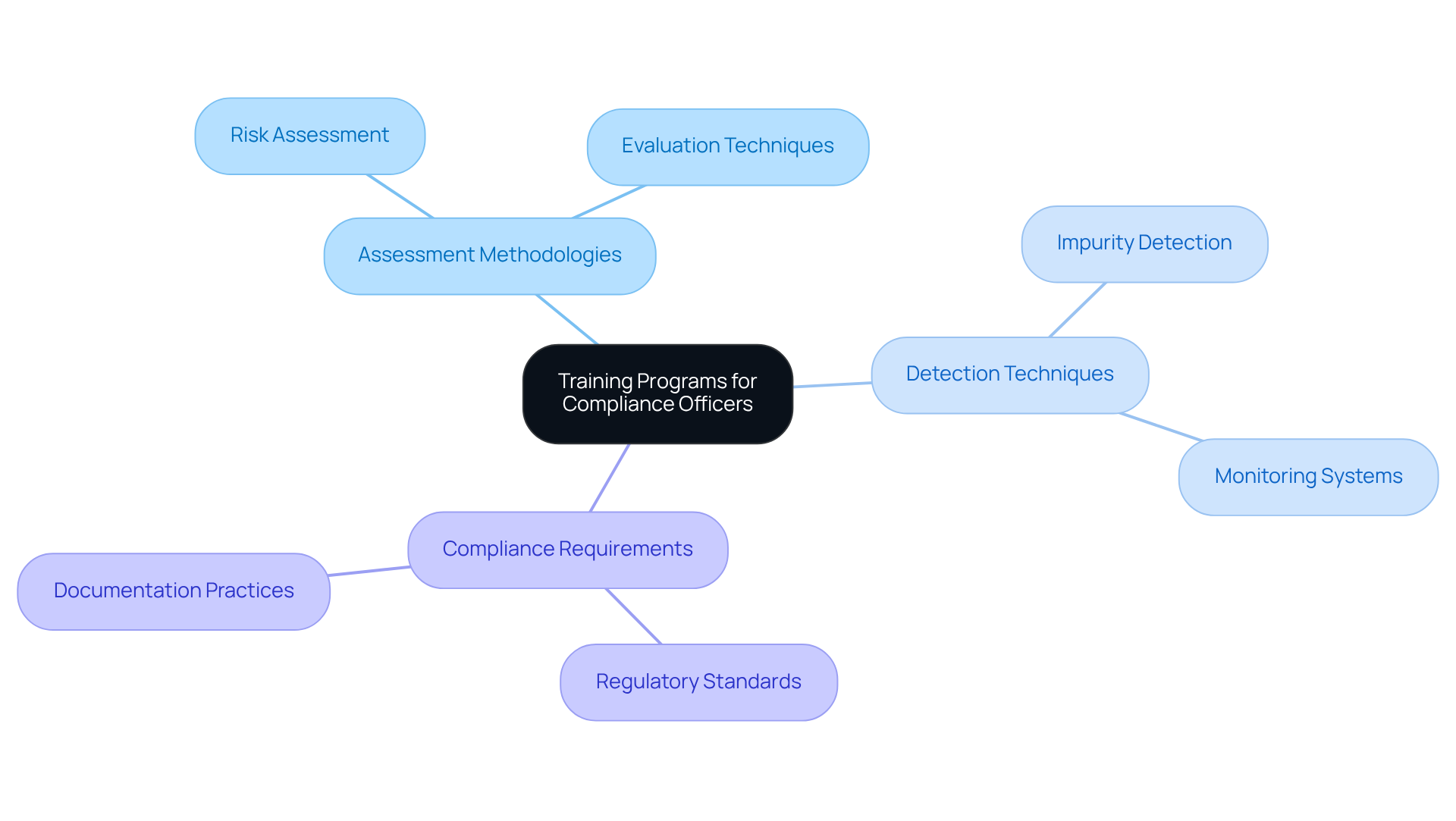
Training programs for compliance officers are not just beneficial; they are essential for ensuring that these professionals remain updated on the ever-evolving regulations concerning impurities. These programs must encompass critical subjects such as:
Regular workshops and seminars are pivotal in keeping compliance teams informed about the latest developments in the field, fostering a culture of continuous improvement and vigilance within the organization. For example, recent workshops have focused on assessing risks associated with specific compounds, equipping participants with practical skills to effectively identify and mitigate potential dangers.
Industry leaders emphasize that comprehensive training not only enhances knowledge but also significantly influences adherence to detection and management protocols, ultimately safeguarding public health and maintaining regulatory standards. By prioritizing these educational initiatives, organizations can ensure their compliance officers are thoroughly prepared to navigate the complexities of nitrosamine regulations.
Public Health Implications: The Impact of Nitrosamine Impurities
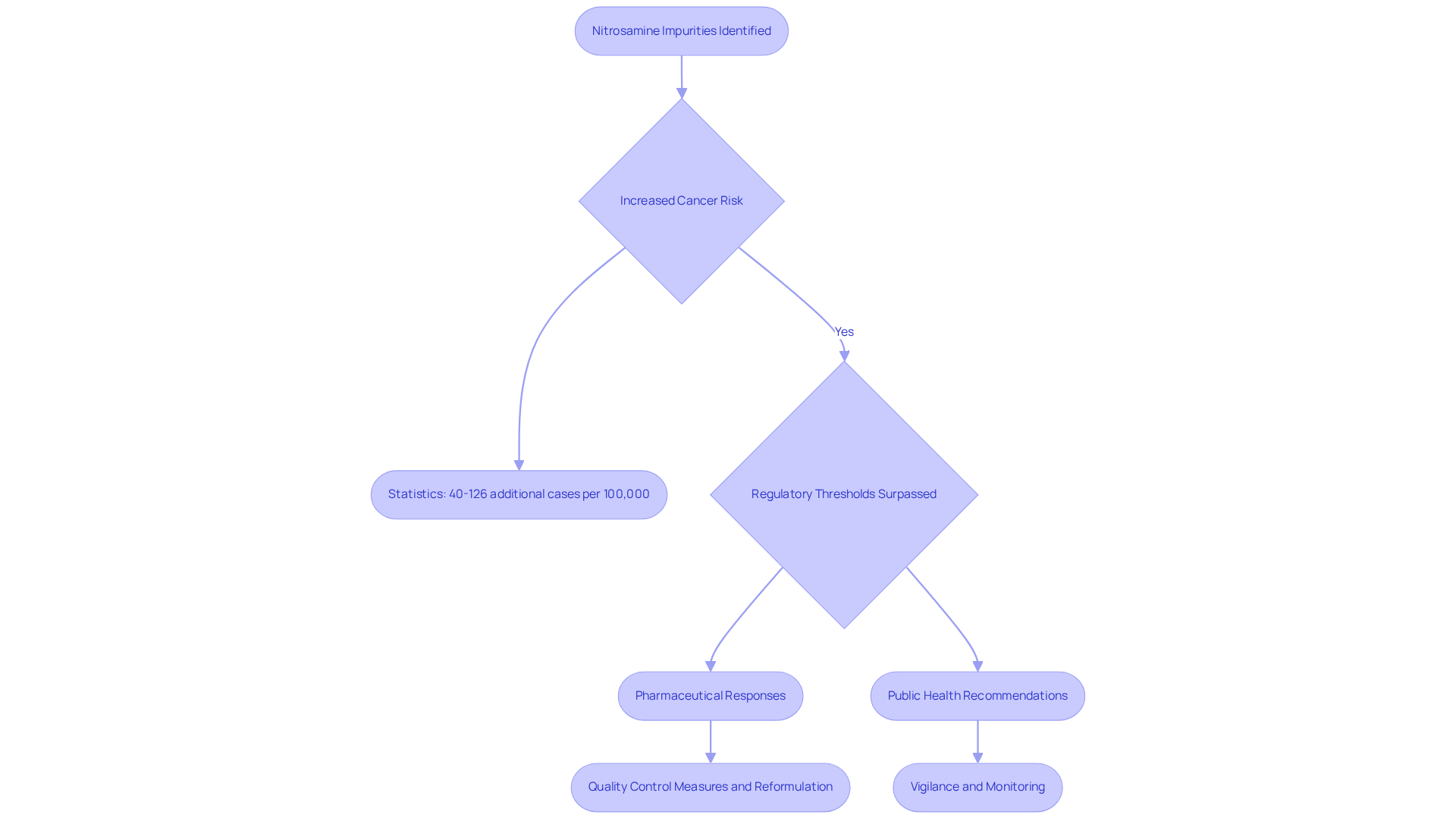
Nitrosamine impurities in pharmaceuticals pose critical public health challenges, primarily due to their association with heightened cancer risk. Long-term exposure to these compounds can significantly elevate the chances of developing various cancers, underscoring the necessity for rigorous testing and monitoring protocols to protect consumer health.
Recent statistics reveal that the , particularly N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), surpasses acceptable thresholds established by regulatory bodies. Estimates indicate an additional 40 to 126 cancer cases per 100,000 individuals exposed to NDMA through contaminated medications such as valsartan.
In response, pharmaceutical companies are increasingly prioritizing the elimination of nitrosamine impurities, implementing stringent quality control measures and reformulation strategies to ensure product safety. Health organizations emphasize the importance of addressing contamination by these hazardous substances, noting that the presence of these carcinogenic compounds in widely used medications can undermine public trust and health outcomes.
As the industry confronts these challenges, continuous vigilance and proactive measures are imperative to safeguard consumers and maintain the integrity of pharmaceutical products.
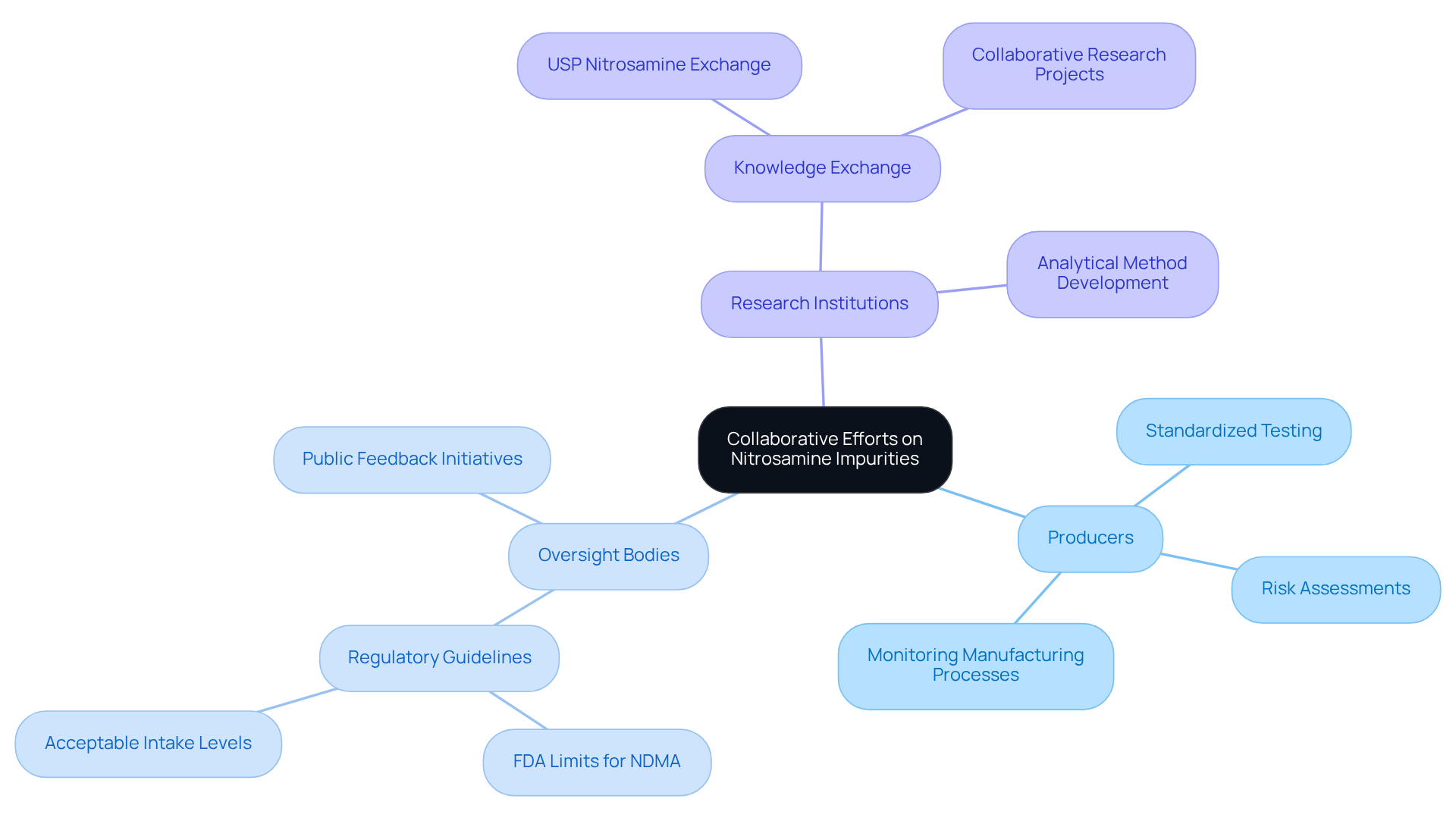
Collaborative Efforts: Industry Stakeholders Tackling Nitrosamine Impurities
Addressing nitrosamine impurities requires a coordinated effort among various stakeholders in the pharmaceutical sector, such as producers, oversight bodies, and research institutions. These partnerships are vital for the exchange of knowledge, resources, and best practices, ultimately leading to the development of and improved evaluation frameworks.
Notably, collaborations between producers and oversight organizations have proven effective in establishing stringent guidelines and evaluation frameworks that include low acceptable daily intake limits for nitrosamine impurities, such as the FDA's limit of 96 ng/day for N-nitrosodimethylamine (NDMA), aimed at safeguarding public health.
Industry specialists emphasize that fostering a culture of collaboration enhances the sector's overall response to challenges, ensuring that mitigation strategies are not only effective but also compliant with regulatory expectations.
The establishment of initiatives like the USP Nitrosamine Exchange exemplifies how collaborative efforts can facilitate the sharing of analytical methods and regulatory updates, thereby enhancing compliance and risk management across the sector.
Furthermore, with approximately 40.4% of examined active pharmaceutical ingredients (APIs) identified as potential precursors, the urgency for such collaborative initiatives is underscored. Additionally, the FDA's recent solicitation for public feedback on contaminants highlights the ongoing dialogue and necessity for stakeholder engagement in addressing these critical issues.
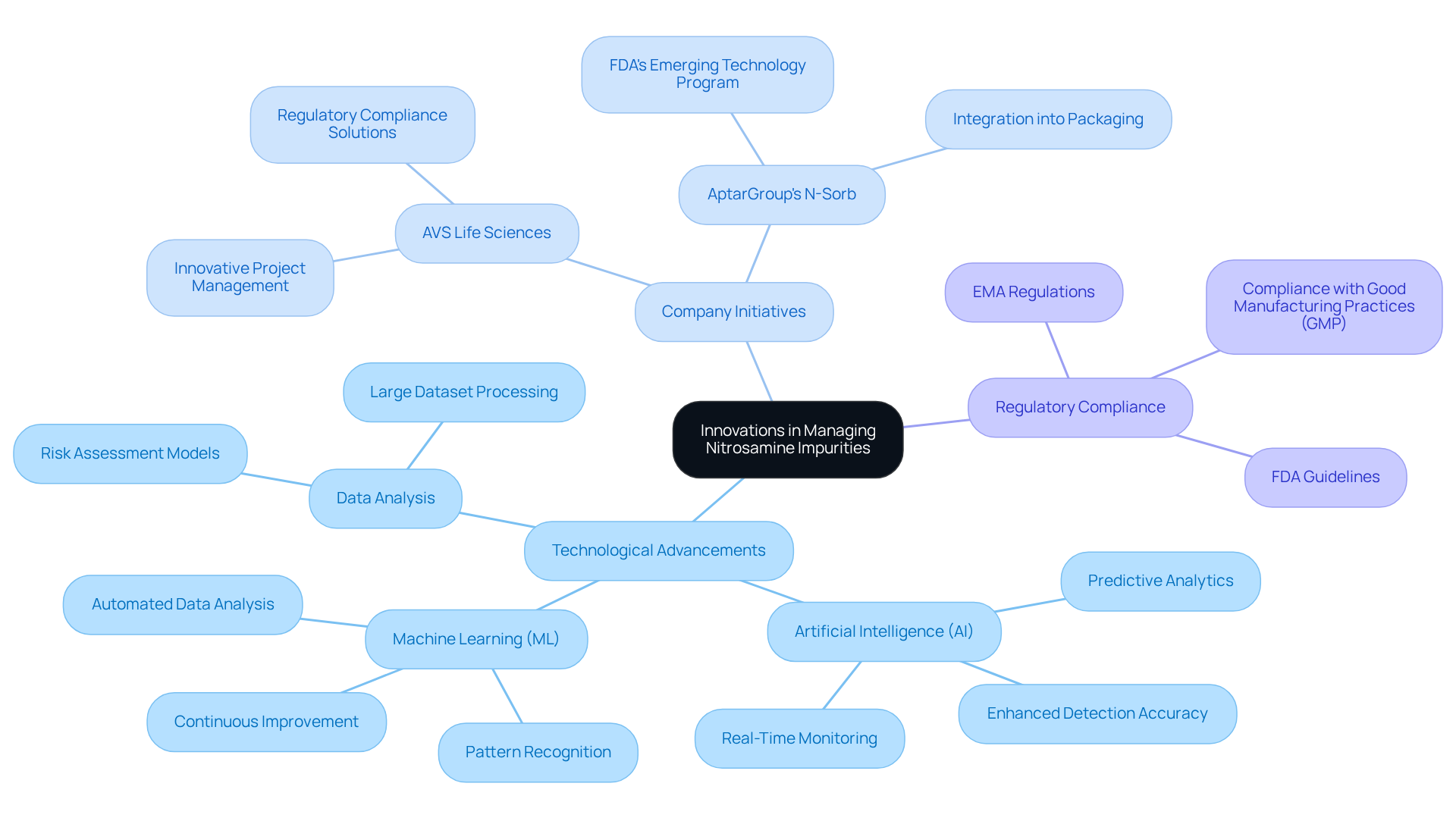
Future Directions: Innovations in Managing Nitrosamine Impurities
The future of managing nitrosamine impurities in pharmaceuticals is increasingly connected to innovation and technological advancements, especially through the initiatives of leading providers such as AVS Life Sciences. New analytical methods, particularly artificial intelligence (AI) and machine learning (ML), are transforming detection and evaluation processes. These technologies enable the analysis of extensive datasets, allowing for the identification of patterns and potential contamination risks that traditional methods may overlook. AI-driven systems can significantly enhance the precision of detecting harmful compounds, identifying even the tiniest concentrations in intricate samples, thus greatly lowering false negatives and ensuring adherence to strict compliance standards.
Moreover, AVS Life Sciences exemplifies the industry's commitment to mitigating nitrosamine impurities through its innovative project management and regulatory compliance solutions. The creation of innovative substances, such as AptarGroup's N-Sorb solution, which has been included in the FDA's Emerging Technology Program, demonstrates how partnerships with specialists can yield successful approaches for handling contaminants. This technology can be integrated into various packaging formats, effectively scavenging impurities and .
By adopting these advancements, pharmaceutical firms can enhance their capability to manage associated risks and improve overall product safety. The integration of AI and ML into nitrosamine impurities testing processes, as supported by AVS Life Sciences, is expected to lead to faster results and reduced turnaround times, facilitating quicker market entry for safe pharmaceutical products. As the industry continues to evolve, the proactive application of these technologies will be crucial in addressing the challenges posed by nitrosamine impurities and ensuring consumer safety.
Conclusion
Managing nitrosamine impurities in pharmaceuticals is a critical endeavor that necessitates a multifaceted approach to ensure compliance, product safety, and public health. Effective strategies, as highlighted throughout the article, encompass rigorous quality control measures, advanced analytical techniques, and ongoing training for compliance officers, all aimed at mitigating the risks associated with these harmful contaminants.
Key insights reveal the importance of proactive risk assessment strategies, such as employing Failure Mode and Effects Analysis (FMEA) and leveraging cutting-edge technologies like Liquid Chromatography-Mass Spectrometry (LC-MS) for precise detection. Moreover, collaboration between industry stakeholders—including pharmaceutical manufacturers and regulatory bodies—is essential for establishing standardized testing methods and maintaining transparent communication regarding nitrosamine levels.
The significance of addressing nitrosamine impurities cannot be overstated. As the pharmaceutical industry navigates the complexities of compliance and safety, a commitment to innovation and collaboration will be paramount. Stakeholders are encouraged to prioritize these strategies and invest in training and technology to safeguard public health and enhance consumer trust in pharmaceutical products. By doing so, the industry can effectively tackle the challenges posed by nitrosamine impurities and ensure a safer future for all.
Frequently Asked Questions
What are nitrosamine impurities and why are they a concern in pharmaceuticals?
Nitrosamine impurities are chemical compounds that can form during the production of pharmaceuticals, primarily through reactions involving nitrites and amines. They are classified as probable human carcinogens, raising significant safety concerns due to their association with serious health hazards, including an increased vulnerability to cancer.
What services does AVS Life Sciences provide to address nitrosamine impurities?
AVS Life Sciences offers a comprehensive suite of compliance solutions that includes validation and commissioning, quality compliance consulting, GMP audits, and engineering support, all aimed at ensuring adherence to Good Manufacturing Practices (GMP) and other regulatory standards.
How prevalent are nitrosamine impurities in pharmaceuticals?
Notably, 29.6% of active pharmaceutical ingredients (APIs) identified in the Global Substance Registration System (GSRS) database are potential precursors to nitrosamine impurities, underscoring the widespread nature of this issue.
What are the potential consequences of non-compliance with regulations regarding nitrosamine impurities?
Non-compliance can lead to costly product recalls and legal ramifications, highlighting the importance of effective management strategies for nitrosamine impurities.
What role do regulatory authorities play in managing nitrosamine impurities?
Regulatory authorities, such as the FDA and EMA, have established stringent protocols and temporary acceptable intake limits to mitigate risks associated with nitrosamine impurities, emphasizing the need for robust testing protocols and transparent reporting practices.
How does AVS Life Sciences assist pharmaceutical manufacturers in navigating compliance?
AVS Life Sciences provides tailored quality management and compliance solutions, ensuring that manufacturers conduct thorough risk assessments in accordance with GXP and FDA standards, which are critical for identifying and managing potential contamination.
What is the importance of collaboration and technology in managing nitrosamine impurities?
Ongoing collaboration with suppliers and investment in advanced analytical technologies, alongside adherence to standard operating procedures (SOPs), are crucial for effectively managing nitrosamine impurities and enhancing the industry's ability to navigate the complex regulatory landscape.
