7 Strategies for Effective ai-enabled validation in Compliance

Overview
The article presents seven strategies for effective AI-enabled validation in compliance, clearly addressing the challenges faced in regulatory adherence within the biopharmaceutical sector. These strategies not only enhance regulatory compliance but also significantly improve operational efficiency. By detailing how AI technologies automate processes, reduce errors, and provide real-time insights, the article illustrates the transformative potential of AI in compliance management. This shift towards a proactive and efficient practice is essential for organizations aiming to stay ahead in an increasingly complex regulatory landscape.
Introduction
In an era where regulatory compliance is increasingly complex, organizations are seeking innovative solutions to maintain their competitive edge. AI-enabled validation presents a transformative approach, streamlining processes and enhancing accuracy while ensuring adherence to industry standards. However, as companies navigate this evolving landscape, a significant challenge persists: how can they effectively implement these advanced technologies to not only meet compliance requirements but also drive operational excellence?
This article delves into seven key strategies for leveraging AI-enabled validation in compliance, revealing the potential for substantial improvements in efficiency and error reduction.
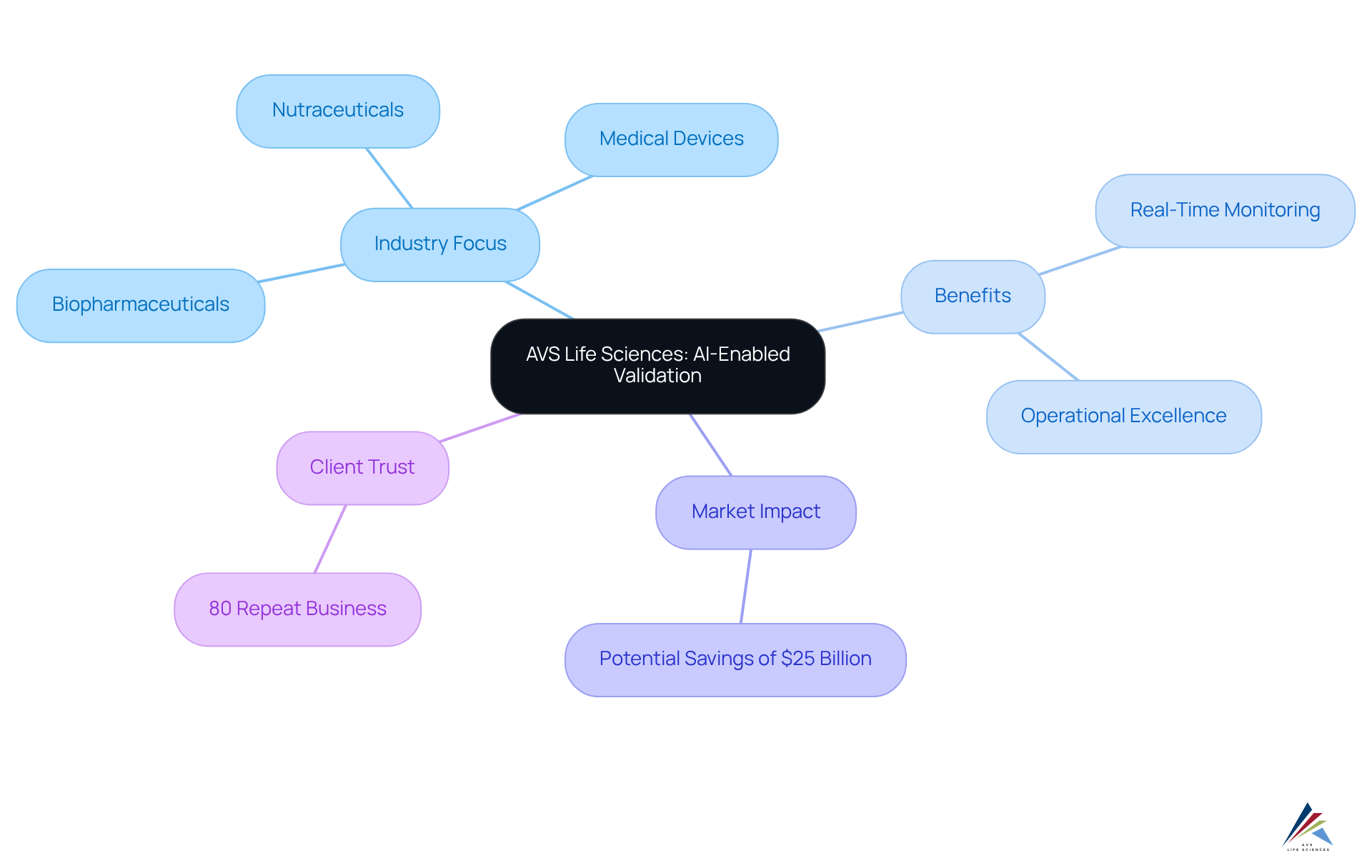
AVS Life Sciences: Pioneering AI-Enabled Validation for Compliance Excellence
AVS Life Sciences stands at the forefront of ai-enabled validation, harnessing advanced technologies to revolutionize compliance processes. The company, with extensive industry expertise and a dedicated focus on biopharmaceuticals, medical devices, and nutraceuticals, empowers clients to not only meet regulatory standards but also achieve operational excellence. By integrating AI-enabled validation into validation frameworks, AVS Life Sciences facilitates real-time monitoring and adaptive validation strategies—essential components in today’s rapidly evolving compliance landscape. This innovative approach is integral to their comprehensive oversight and quality solutions, ensuring quality and adherence throughout the drug development lifecycle.
With projections indicating that AI could save up to $25 billion in clinical development costs, AVS Life Sciences exemplifies the within the industry. The firm's commitment to quality is underscored by an impressive 80% repeat business rate, reflecting high client satisfaction and trust. As industry leaders underscore the significance of AI in compliance, AVS Life Sciences emerges as a dependable partner, adept at navigating the complexities of regulatory requirements while fostering continuous improvement. Engage with AVS Life Sciences to explore how their expertise can elevate your compliance strategies.
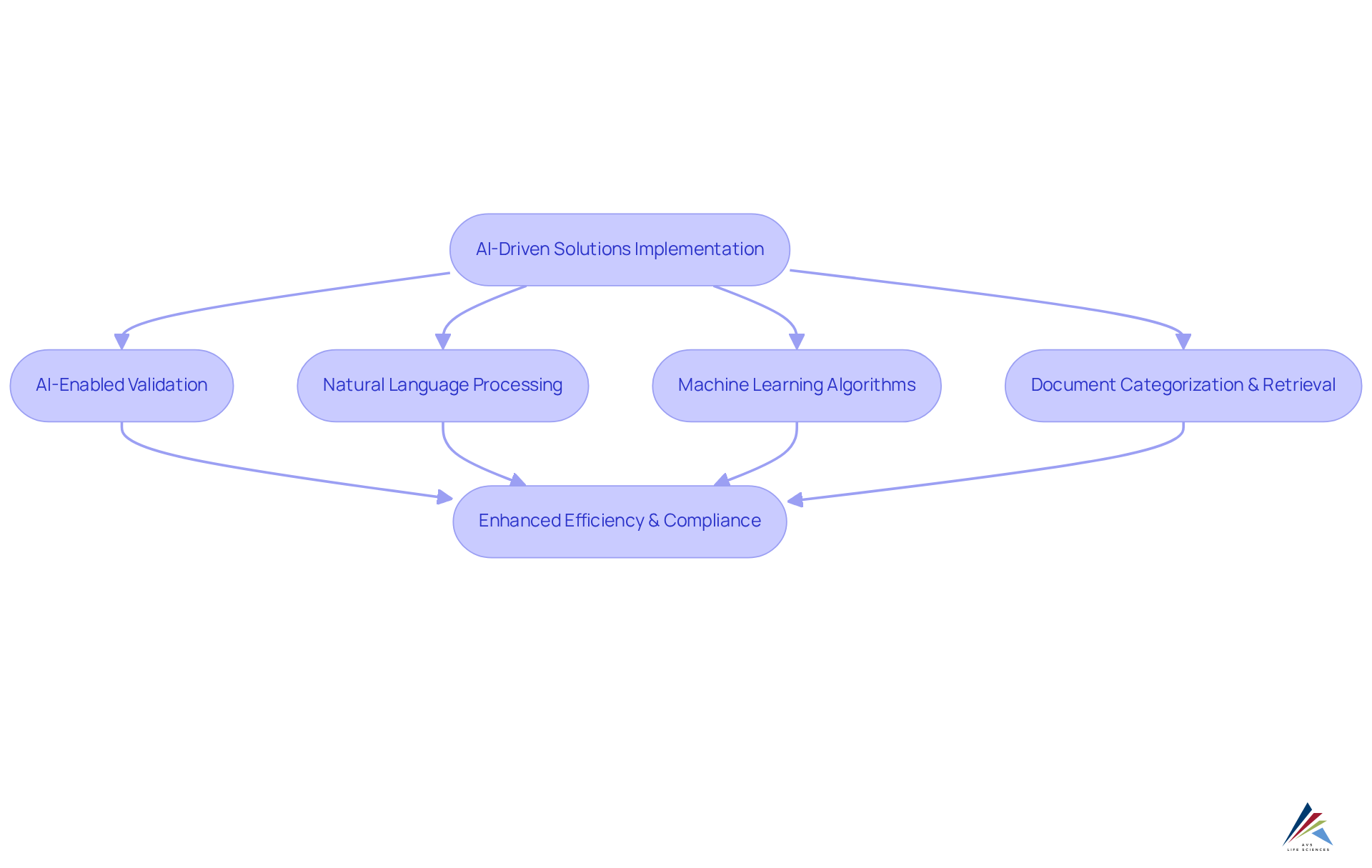
Streamline Documentation Management with AI-Driven Solutions
AI-driven solutions, through ai-enabled validation, are revolutionizing the documentation management process by automating the arrangement and retrieval of records, ensuring accuracy, currency, and accessibility. Organizations can implement ai-enabled validation alongside natural language processing and machine learning algorithms to efficiently categorize and retrieve documents, significantly reducing the time spent on manual searches. This automation not only enhances operational efficiency but also mitigates the risk of non-compliance due to missing or outdated documentation—an aspect that is critical in the biopharmaceutical sector, where ai-enabled validation is paramount for adherence to GXP and FDA regulations.
For instance, AVS Life Sciences has showcased the effectiveness of such solutions in a recent case study, assisting a leading biotechnology company in upgrading their GMP facility. This upgrade not only improved documentation practices but also ensured adherence to stringent . Notably, automation can minimize manual errors by as much as 90%. Organizations that have integrated ai-enabled validation into their documentation processes report enhanced accuracy and a significant reduction in administrative burdens, allowing teams to concentrate on strategic initiatives rather than routine tasks.
Moreover, with 83% of IT leaders recognizing workflow automation as essential for digital transformation and 60% of organizations achieving ROI within 12 months of implementation, the adoption of AI in documentation management is increasingly vital for firms striving to uphold compliance and operational excellence.
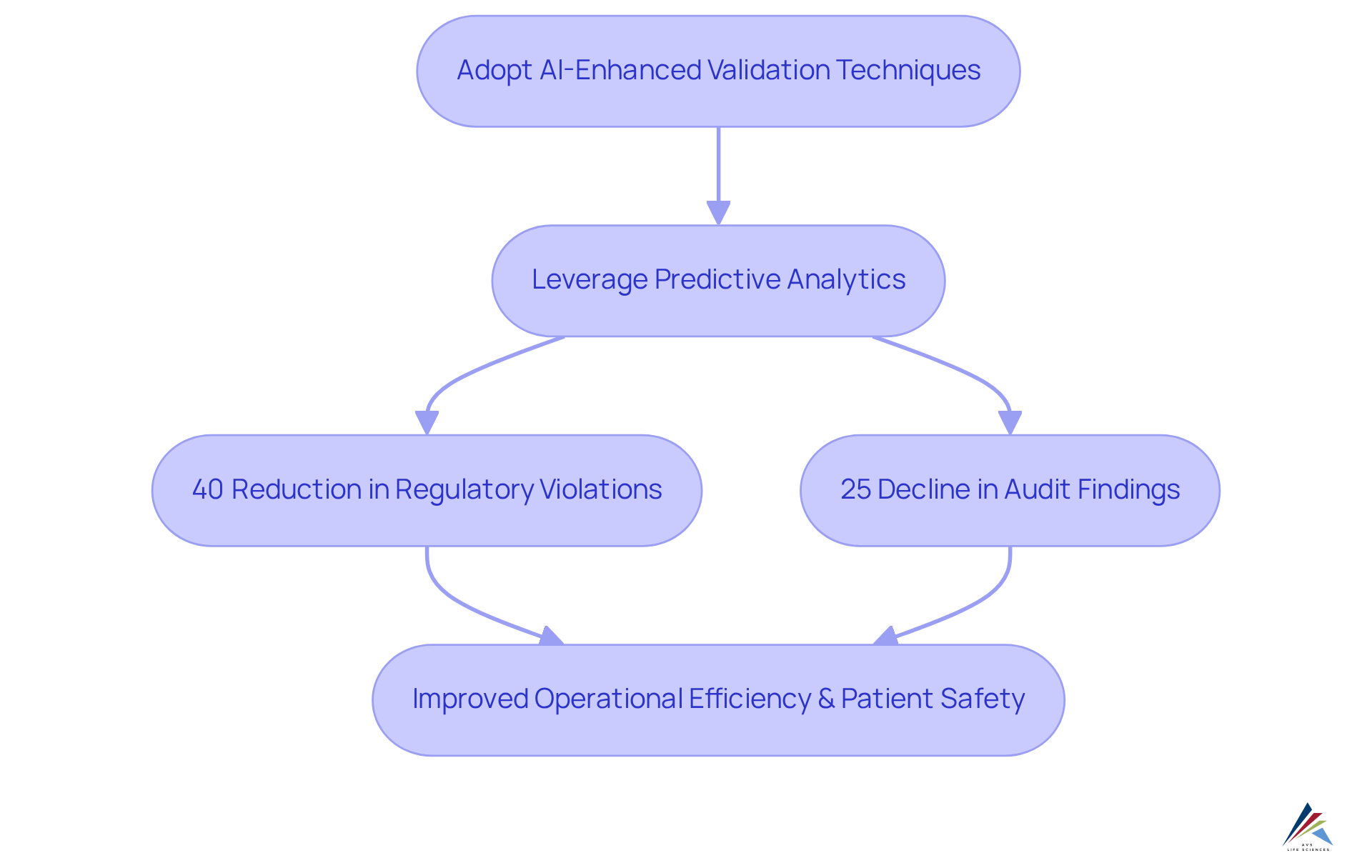
Adapt to Evolving Regulations with AI-Enhanced Validation Techniques
AI-enabled validation techniques empower organizations to swiftly adapt to evolving regulations. By leveraging predictive analytics, companies can foresee regulatory changes and modify their validation processes proactively. This progressive strategy not only ensures adherence but also fosters a culture of .
For instance, pharmaceutical firms employing predictive analytics have indicated a 40% reduction in regulatory violations and a 25% decline in audit findings. These results demonstrate the concrete advantages of these technologies.
AI systems can handle extensive compliance data, uncovering trends and potential impacts on existing ai-enabled validation processes. This capability allows organizations to sustain agility and adherence in a changing governance environment, ultimately improving operational efficiency and patient safety.
As highlighted by industry specialists, adopting predictive analytics is crucial for effectively managing the intricacies of regulatory requirements.
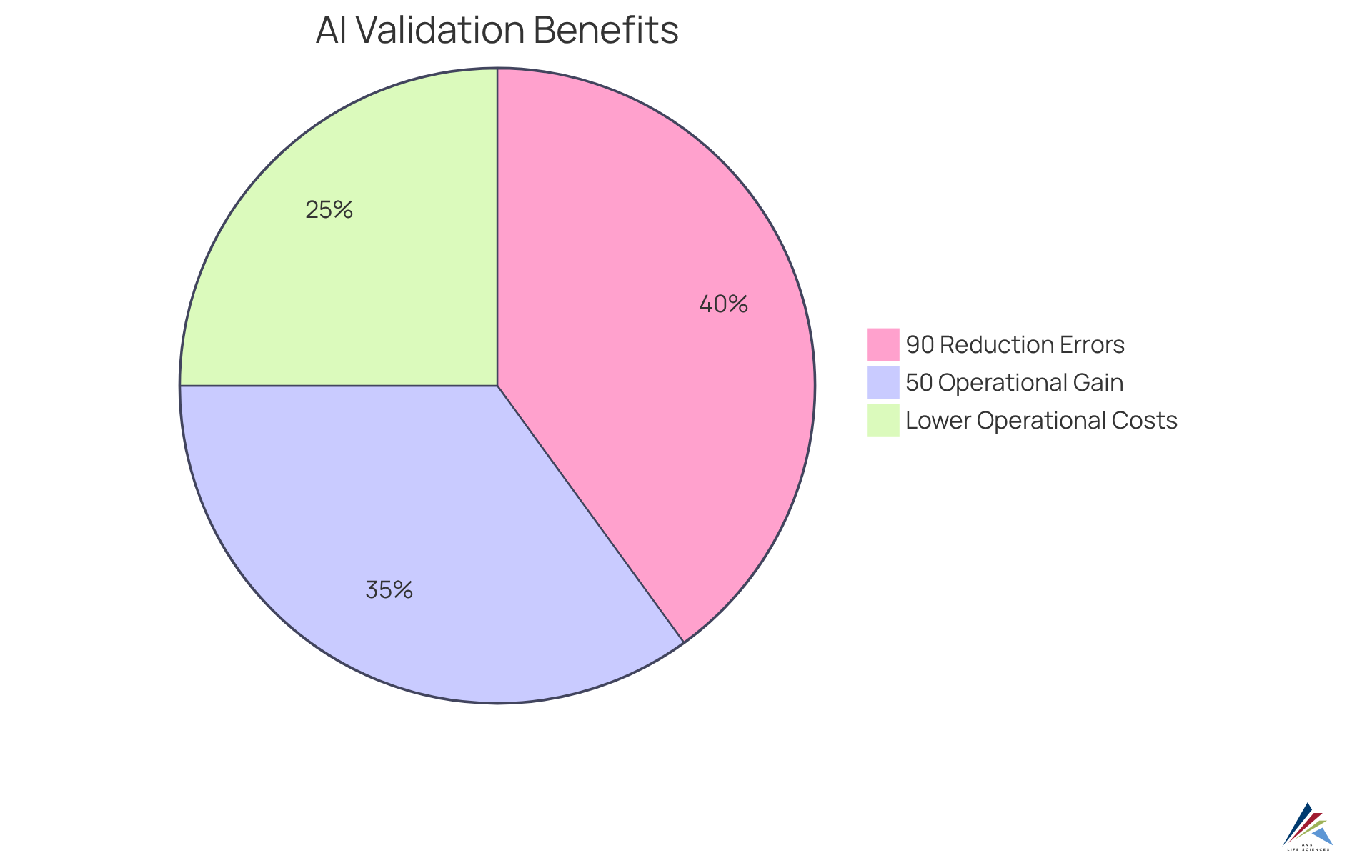
Enhance Operational Efficiency through Intelligent Validation Automation
AI-enabled validation is crucial for enhancing operational efficiency by minimizing manual intervention and streamlining workflows. By conducting routine checks and balances, automated verification procedures enable oversight teams to concentrate on more complex tasks that necessitate human supervision. This transformation not only accelerates the verification process but also significantly , ensuring that regulatory standards are consistently upheld.
Organizations that integrate intelligent automation can anticipate faster turnaround times and lower operational costs while upholding high-quality standards. For instance, companies utilizing automated systems report a remarkable 90% reduction in reporting errors and improved accuracy in documentation. Moreover, the implementation of AI-enabled validation in validation processes can yield operational efficiency gains of up to 50%, empowering teams to redirect their focus toward strategic initiatives rather than mundane tasks.
As the regulatory landscape continues to evolve, adopting intelligent automation is imperative for organizations seeking to bolster their operational capabilities and ensure robust management.
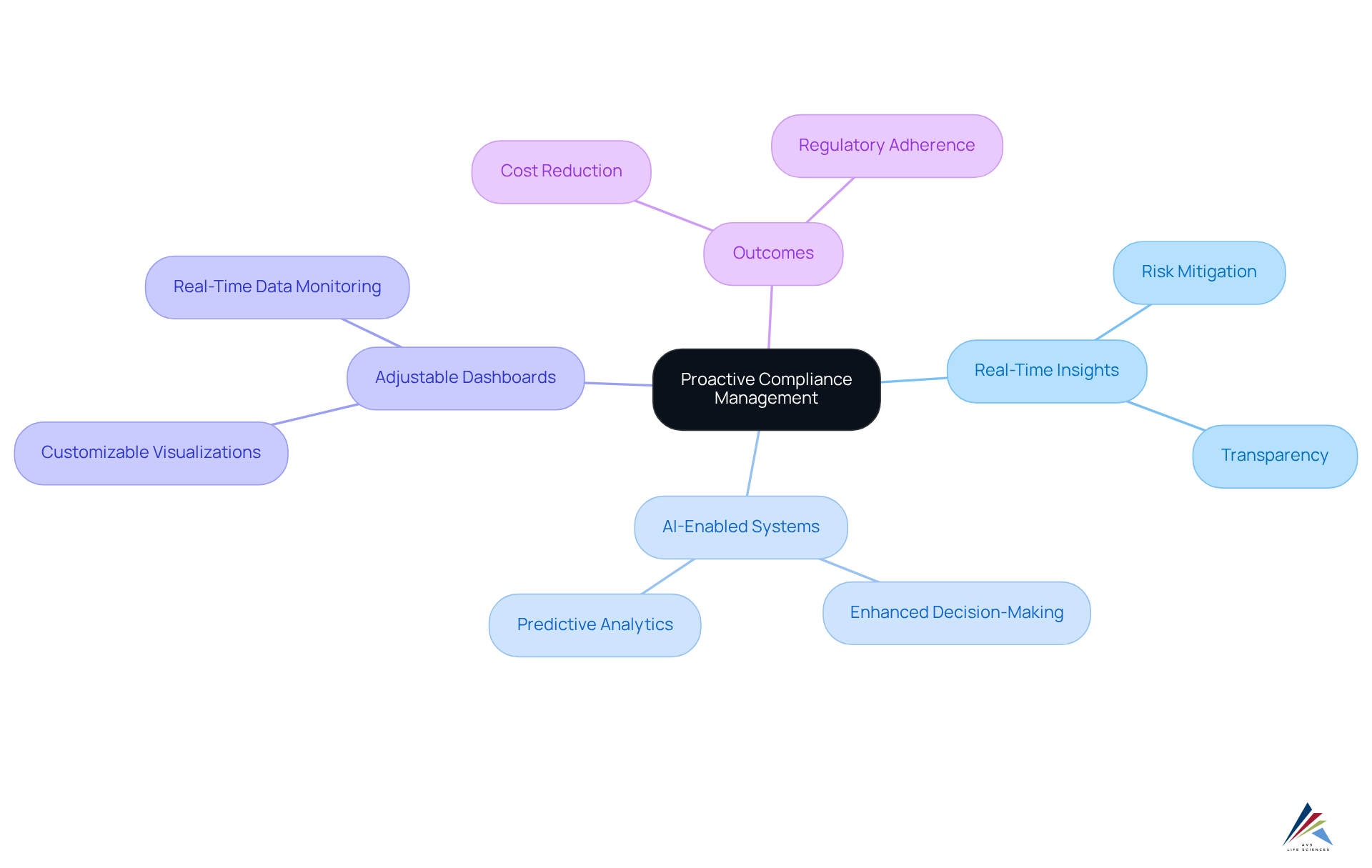
Leverage Real-Time Insights for Proactive Compliance Management
Utilizing real-time insights is crucial for efficient proactive regulatory management in the biopharmaceutical and life sciences fields. AI-enabled validation systems continuously track adherence metrics, offering notifications to teams about potential problems before they escalate. By employing adjustable dashboards and sophisticated analytics tools, organizations can visualize regulatory data in real-time, facilitating informed decision-making. This not only mitigates risks but also fosters a culture of accountability and transparency within the organization.
AVS Life Sciences exemplifies this approach through its comprehensive quality management and adherence solutions, which encompass GXP, FDA regulations, and standard operating procedures (SOPs). Empowered by real-time insights, regulatory teams can respond swiftly and effectively, ensuring strict adherence to standards.
For instance, organizations employing AI-enabled validation for regulatory oversight have reported substantial enhancements in their ability to manage metrics, with many achieving a 40% reduction in regulatory violations. Furthermore, a recent survey indicated that entities adopting data-driven regulatory programs experienced a 30% decrease in costs related to compliance.
As industry leaders emphasize, implementing a proactive adherence strategy is no longer optional; it is an essential element of maintaining trust and operational integrity in today's oversight environment. Compliance officers should consider integrating real-time analytics solutions while also assessing the associated costs and challenges of managing large volumes of data.
AVS Life Sciences' successful upgrade of a biotechnology GMP facility underscores the significance of quality assurance and regulatory compliance, demonstrating how effective management practices can lead to improved outcomes in the life sciences sector.
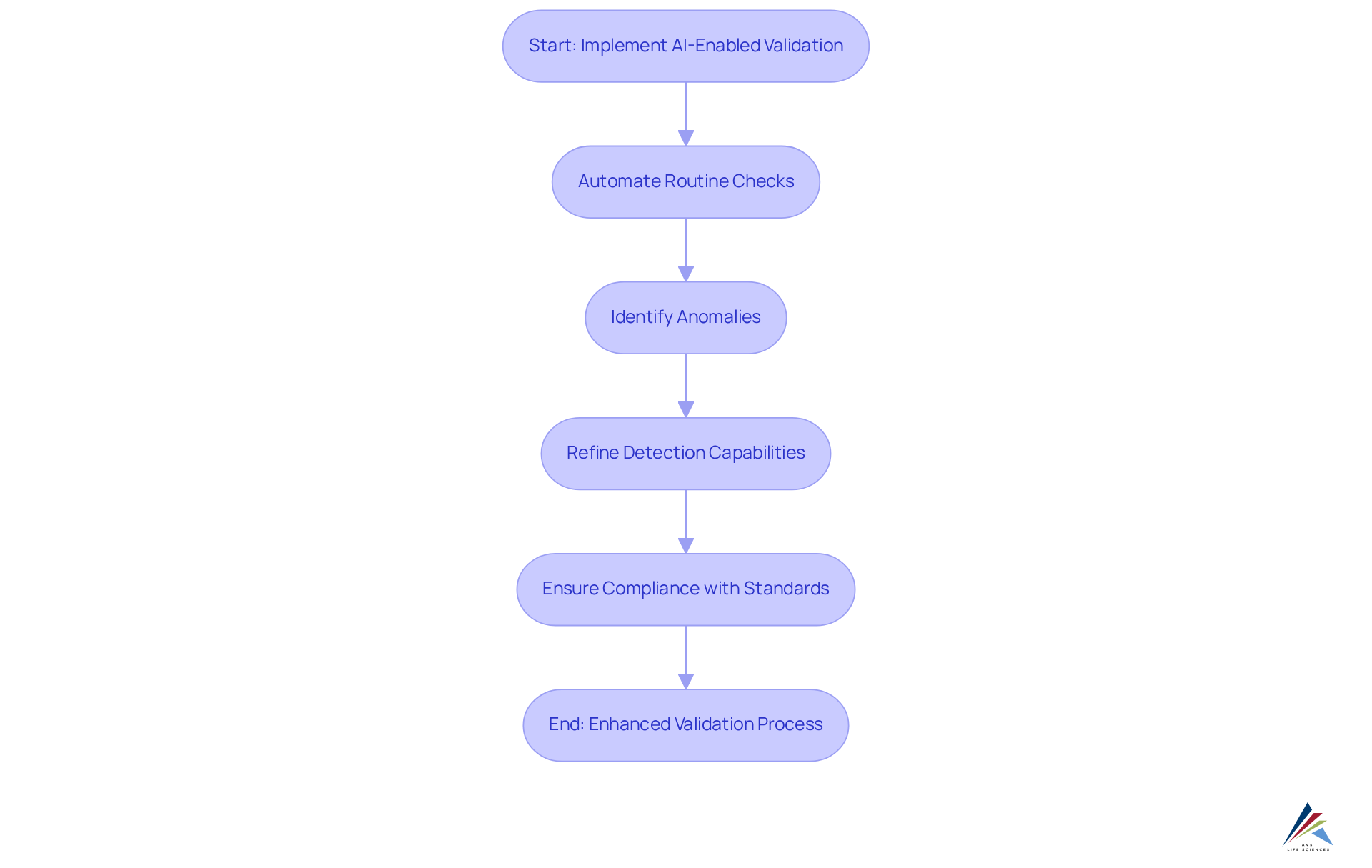
Minimize Errors with AI-Enabled Validation Systems
AI-enabled validation systems are essential for mitigating errors throughout the verification process. By automating routine checks and employing advanced algorithms, these systems effectively identify anomalies and inconsistencies often missed by manual methods. Organizations leveraging AI-driven verification have reported an impressive 60-75% reduction in error rates compared to traditional approaches. This capability not only enhances the accuracy of assessment outcomes but also ensures compliance with regulatory standards, such as GXP and FDA guidelines, is consistently maintained.
Furthermore, AI systems continuously learn from past errors, refining their detection capabilities and significantly decreasing the likelihood of future mistakes. This iterative improvement leads to a more reliable verification process, ultimately bolstering overall compliance efforts and fostering trust in data integrity. To fully harness the advantages of AI-enabled validation, organizations must follow a that incorporates comprehensive documentation practices at every stage. This approach not only fortifies compliance but also enhances quality management practices within the pharmaceutical and biotechnology industries.
Utilize Predictive Analytics to Foresee Compliance Challenges
Utilizing empowers entities to anticipate potential regulatory challenges and proactively mitigate associated risks. By analyzing historical data and identifying emerging patterns, predictive analytics can highlight areas of concern that may result in non-compliance. This foresight enables oversight teams to develop targeted strategies and allocate resources effectively, ensuring that potential issues are addressed before they escalate.
Organizations that have integrated predictive analytics into their regulatory frameworks report enhanced visibility into their compliance status, with 71% of users noting improved monitoring capabilities. Moreover, predictive analytics cultivates a culture of continuous improvement and risk awareness, prompting teams to stay vigilant and responsive to the evolving regulatory landscape.
As regulatory experts emphasize, a proactive approach to risk reduction is essential for navigating the complexities of compliance in the pharmaceutical industry, ultimately transforming adherence from a reactive obligation into a strategic advantage.

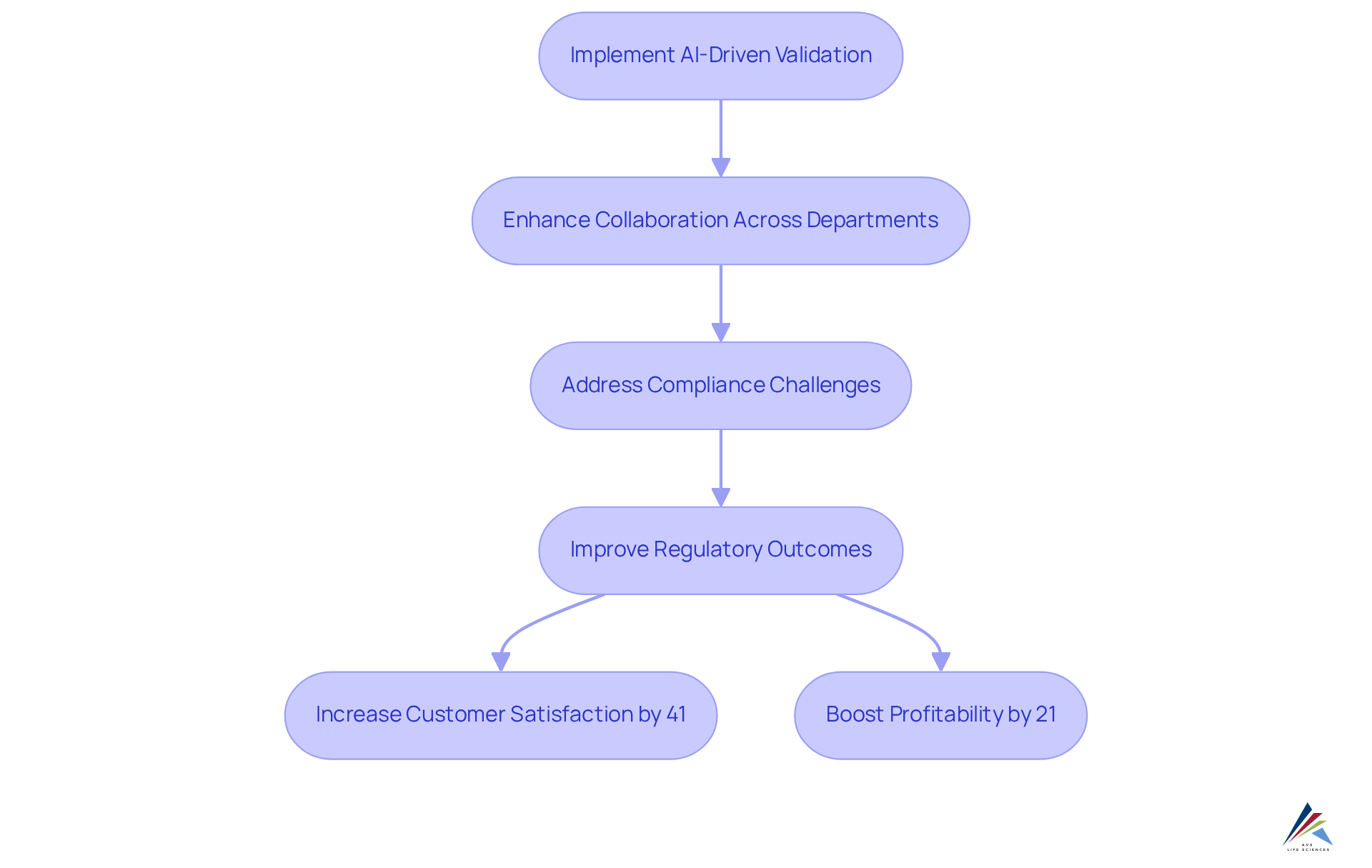
Foster Collaboration Across Departments with AI-Driven Validation
AI-enabled validation significantly enhances collaboration across departments by creating a centralized platform for communication and data sharing. This innovative approach uses ai-enabled validation to address compliance challenges head-on, enabling organizations to break down barriers and foster transparency. By utilizing ai-enabled validation tools, all stakeholders can align with regulatory goals, which not only enhances efficiency but also improves the quality of regulatory outcomes.
For instance, real-time communication supported by ai-enabled validation empowers teams to exchange insights and collaboratively address regulatory challenges. This nurtures a unified culture that propels . AVS Life Sciences exemplifies this approach through their extensive biopharmaceutical consulting services, focusing on quality management and regulatory adherence, including GXP and FDA regulations. Their successful upgrade of a biotechnology GMP facility illustrates how effective collaboration and adherence to quality assurance practices can lead to substantial improvements in operational outcomes.
Studies indicate that effective collaboration can lead to a 41% increase in customer satisfaction and a 21% boost in profitability. Furthermore, with 64% of employees wasting at least three hours a week due to collaboration inefficiencies, the demand for AI-driven solutions becomes even more compelling. As a result, organizations can expect improved performance and innovation, making the case for investing in these transformative technologies.
Invest in Training for Effective AI Implementation in Compliance
Investing in training is paramount for the successful integration of AI into regulatory processes. Organizations must implement comprehensive training programs that equip employees with vital skills, such as interpreting AI-generated insights, managing data responsibly, and adhering to regulatory standards. A culture of ongoing education not only enhances the benefits of AI but also strengthens regulatory initiatives. Training empowers employees, fostering confidence in their ability to to achieve regulatory goals. Notably, 50% of employees express trust in AI for unbiased feedback, indicating a growing confidence in AI tools. Furthermore, organizations that prioritize training report a 40% increase in employee engagement, which is crucial for maintaining standards in the rapidly evolving life sciences industry. Effective training examples include:
- Hands-on workshops
- Real-time simulations
These methods allow employees to practice using AI-enabled validation in compliance scenarios, ensuring they are well-prepared to navigate complex legal environments.

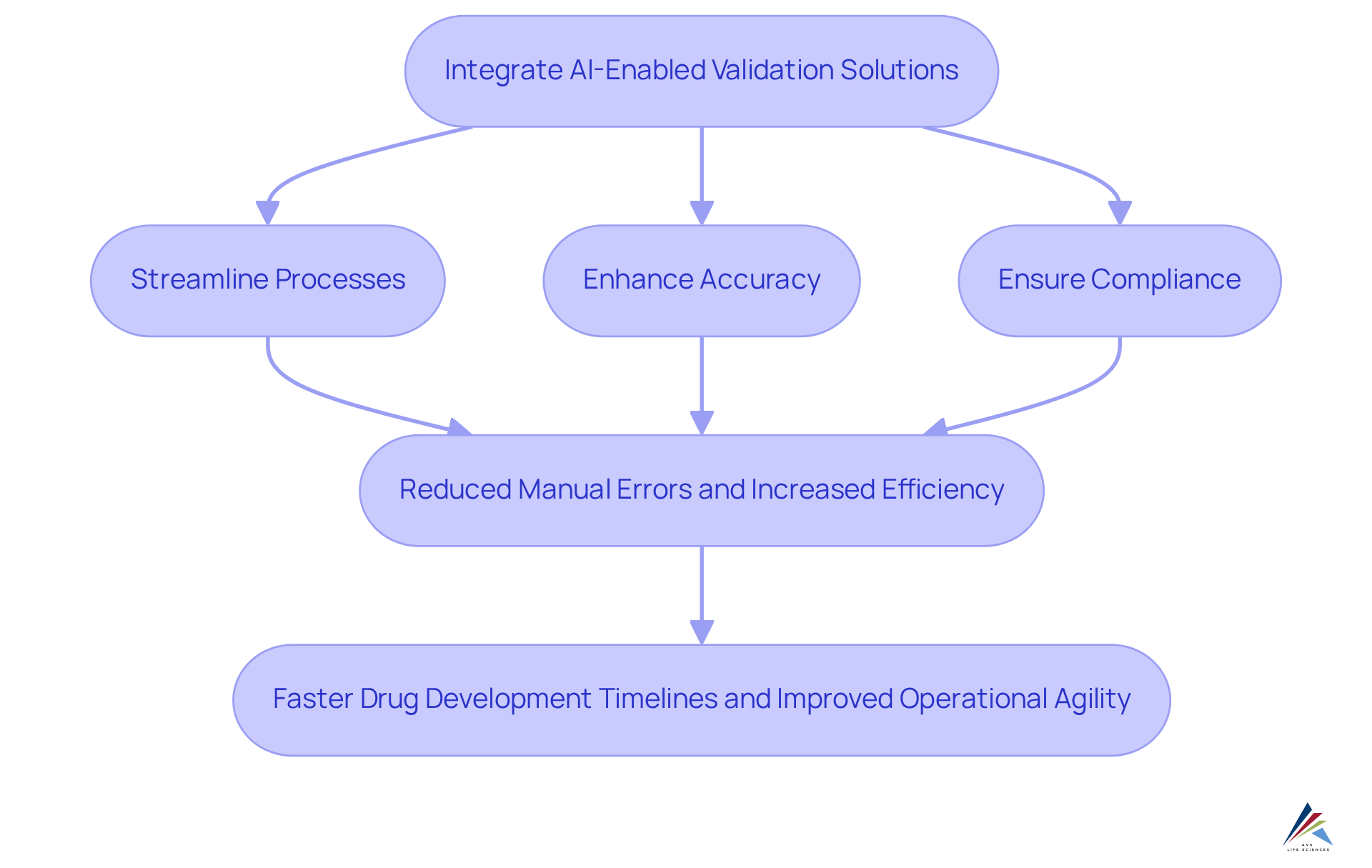
Transform Pharmaceutical Compliance with AI-Enabled Validation Solutions
AI-enabled validation solutions are revolutionizing pharmaceutical practices by streamlining processes, enhancing accuracy, and ensuring adherence to industry standards. By harnessing AI technologies, organizations can automate routine regulatory tasks, which significantly reduces manual errors and boosts overall efficiency.
For example, AI can compress drug development timelines from five years to as little as 12-18 months, underscoring its capacity to expedite compliance-driven initiatives. As the industry evolves, companies like Pfizer and Janssen are already integrating AI into their regulatory frameworks, demonstrating improved operational efficiency and agility in responding to regulatory changes.
This transformation not only but also equips organizations to swiftly adapt to market demands. Embracing AI-enabled validation is crucial for sustaining a competitive advantage and securing long-term success in the fast-paced pharmaceutical landscape.
Conclusion
AI-enabled validation is fundamentally reshaping compliance within the pharmaceutical industry, presenting organizations with innovative avenues to bolster accuracy, efficiency, and adherence to regulatory standards. By integrating advanced technologies, companies can automate routine tasks, reduce errors, and swiftly adapt to evolving regulations, ultimately positioning themselves for success in a competitive market.
This article explores several key strategies for implementing AI-enabled validation:
- Streamlining documentation management
- Leveraging predictive analytics
- Fostering interdepartmental collaboration
- Investing in comprehensive training programs
Each strategy underscores the transformative potential of AI, not only in enhancing compliance but also in driving operational excellence and improving overall organizational performance.
Embracing AI-enabled validation is not merely a trend; it is a strategic necessity for organizations seeking to thrive in the rapidly evolving pharmaceutical landscape. By adopting these advanced solutions, companies can mitigate compliance risks while unlocking significant efficiencies that lead to superior quality outcomes and enhanced patient safety. The call to action is unequivocal: organizations must invest in these technologies and cultivate a culture of continuous improvement to maintain an edge in compliance and operational excellence.
Frequently Asked Questions
What is AVS Life Sciences known for?
AVS Life Sciences is known for pioneering AI-enabled validation, focusing on revolutionizing compliance processes in the biopharmaceutical, medical device, and nutraceutical industries.
How does AI-enabled validation benefit compliance processes?
AI-enabled validation facilitates real-time monitoring and adaptive validation strategies, which are essential for maintaining quality and adherence throughout the drug development lifecycle.
What are the projected financial benefits of AI in clinical development?
Projections indicate that AI could save up to $25 billion in clinical development costs.
What is the client satisfaction rate for AVS Life Sciences?
AVS Life Sciences has an impressive 80% repeat business rate, reflecting high client satisfaction and trust.
How does AI improve documentation management?
AI-driven solutions automate the arrangement and retrieval of records, ensuring accuracy, currency, and accessibility, which enhances operational efficiency and reduces the risk of non-compliance.
What technologies are used alongside AI for documentation management?
AI-enabled validation is used alongside natural language processing and machine learning algorithms to efficiently categorize and retrieve documents.
What impact does automation have on manual errors in documentation?
Automation can minimize manual errors by as much as 90%, significantly improving accuracy in documentation practices.
What percentage of IT leaders recognize workflow automation as essential for digital transformation?
83% of IT leaders recognize workflow automation as essential for digital transformation.
How do AI-enabled validation techniques help organizations adapt to regulatory changes?
These techniques leverage predictive analytics to foresee regulatory changes and modify validation processes proactively, ensuring adherence and fostering a culture of ongoing enhancement.
What are the reported benefits of using predictive analytics in pharmaceutical firms?
Pharmaceutical firms using predictive analytics have reported a 40% reduction in regulatory violations and a 25% decline in audit findings.
Why is adopting predictive analytics important for organizations?
Adopting predictive analytics is crucial for effectively managing the complexities of regulatory requirements and improving operational efficiency and patient safety.
