7 Key Insights on PFAS in Cosmetics Legislation You Need

Overview
The article examines the significant implications of PFAS (per- and polyfluoroalkyl substances) legislation within the cosmetics industry, emphasizing the essential insights and changes manufacturers must address. Upcoming regulations, particularly the Toxic-Free Cosmetics Act, will mandate that manufacturers eliminate intentionally added PFAS from their products by 2025. This pivotal requirement aims to enhance consumer safety and propel the industry towards safer formulations. The urgency of these changes is underscored by increasing public health advocacy and proactive state-level legislative actions, compelling manufacturers to adapt swiftly to ensure compliance and foster consumer trust.
Introduction
The increasing scrutiny of chemical ingredients in cosmetics has ignited a seismic shift in regulatory landscapes, particularly regarding per- and polyfluoroalkyl substances (PFAS). As states like Washington enact the Toxic-Free Cosmetics Act, manufacturers find themselves at a critical juncture where compliance transcends mere legal obligation; it becomes a pathway to consumer trust and brand integrity. With over 350 PFAS-related bills introduced across the U.S., a pressing question arises: how can manufacturers adeptly navigate this intricate web of regulations while ensuring product safety and market relevance? This article explores seven key insights that illuminate the challenges and opportunities posed by the evolving PFAS legislation within the cosmetics industry.
AVS Life Sciences: Expert Regulatory Solutions for PFAS Compliance
AVS Life Sciences stands at the forefront of providing customized regulatory solutions that address the unique challenges posed by emerging legislation in the cosmetics sector. With a robust emphasis on , the company empowers clients to adeptly navigate the rapidly changing landscape of chemical regulations. This is particularly crucial as states like Washington enact laws such as the Toxic-Free Cosmetics Act, which includes pfas in cosmetics legislation by prohibiting harmful substances, including specific fluorinated compounds, in personal care products.
Specializing in the development of phase-appropriate quality and regulatory strategies, AVS Life Sciences enables manufacturers to fulfill stringent compliance requirements and mitigate the risk of penalties as new regulations are implemented. Their comprehensive approach guarantees that clients not only comply with existing laws but also anticipate future regulatory shifts, positioning them for success in a competitive market. By leveraging their extensive expertise, AVS Life Sciences assists clients in upholding high standards of safety and quality, ultimately fostering consumer trust and enhancing brand reputation in an industry that is under increasing scrutiny.
Toxic-Free Cosmetics Act: Key Provisions Impacting PFAS Regulations
The Toxic-Free Beauty Products Act, enacted on May 15, 2023, introduces crucial measures that reform chemical regulations in the beauty industry. Beginning on January 1, 2025, the Act clearly forbids the production, sale, and distribution of beauty products containing intentionally added PFAS in cosmetics legislation. This landmark legislation is designed to safeguard consumers from harmful chemicals while encouraging the development of safer alternatives in cosmetic formulations.
As Representative Sharlett Mena emphasized, consumers should not need to be toxicologists to purchase safe personal care items. Consequently, manufacturers must perform thorough ingredient assessments and may need to adjust formulations to meet these new standards. This shift not only enhances consumer safety but also aligns with , particularly for vulnerable populations such as women of color, who are often targeted by products containing harmful chemicals.
Ecology's findings revealed that many beauty products contain these toxic ingredients, underscoring the necessity of this legislation. Overall, the Toxic-Free Cosmetics Act signifies a major advancement in pfas in cosmetics legislation, encouraging [safer beauty products](https://toxicfreefuture.org/washington-state/campaign-washington-ban-toxic-cosmetics/additional-quotes) and safeguarding public health.
Recent Updates on PFAS Regulations in Cosmetics: What You Should Know
Recent updates on chemical regulations in beauty products reveal a significant shift towards stricter enforcement and broader bans. As of 2025, over 350 PFAS-related bills have been introduced across various states, with several already enacting laws related to PFAS in cosmetics legislation that prohibit the sale of cosmetics containing these substances. This trend underscores the urgency for to avoid penalties and ensure continued market access.
Minnesota, for example, has enacted an extensive regulatory initiative concerning per- and polyfluoroalkyl substances, requiring manufacturers to disclose intentionally incorporated substances in products sold within the state. The Minnesota Pollution Control Agency (MPCA) has extended the reporting deadline to July 1, 2026, allowing manufacturers additional time to prepare for compliance. As states continue to implement and enhance their chemical regulations, companies must navigate a complex regulatory environment characterized by varying definitions, reporting obligations, and effective dates.
Engaging with stakeholders and utilizing technology solutions for tracking obligations are essential strategies for companies aiming to align with these evolving regulations. AVS Life Sciences offers valuable consulting services, assisting manufacturers in developing state-by-state compliance matrices and updating internal protocols to effectively navigate the growing patchwork of regulations.
The upcoming 12 to 24 months will be crucial as both state and federal authorities strive to enhance oversight of PFAS in cosmetics legislation concerning certain chemicals. It is essential for manufacturers to remain informed and proactive in this rapidly changing landscape.
Manufacturers' Responsibilities Under PFAS Legislation: Compliance Challenges
Manufacturers now face significant obligations under related legislation, including the necessity for thorough ingredient evaluations and adherence to PFAS in cosmetics legislation to ensure their products are free from intentionally added substances. Compliance challenges arise from navigating the varying definitions of PFAS across states, maintaining accurate documentation, and implementing effective quality control measures.
To support pharmaceutical regulatory officers, it is crucial to establish a robust adherence framework that includes:
- Regular training on state-specific regulations
- The use of user manuals for best practices
- A proactive strategy for ingredient sourcing
Noncompliance can result in severe penalties, including recalls and damage to brand reputation. Firms that prioritize compliance not only safeguard their offerings but also enhance consumer confidence, which is essential in today's market.
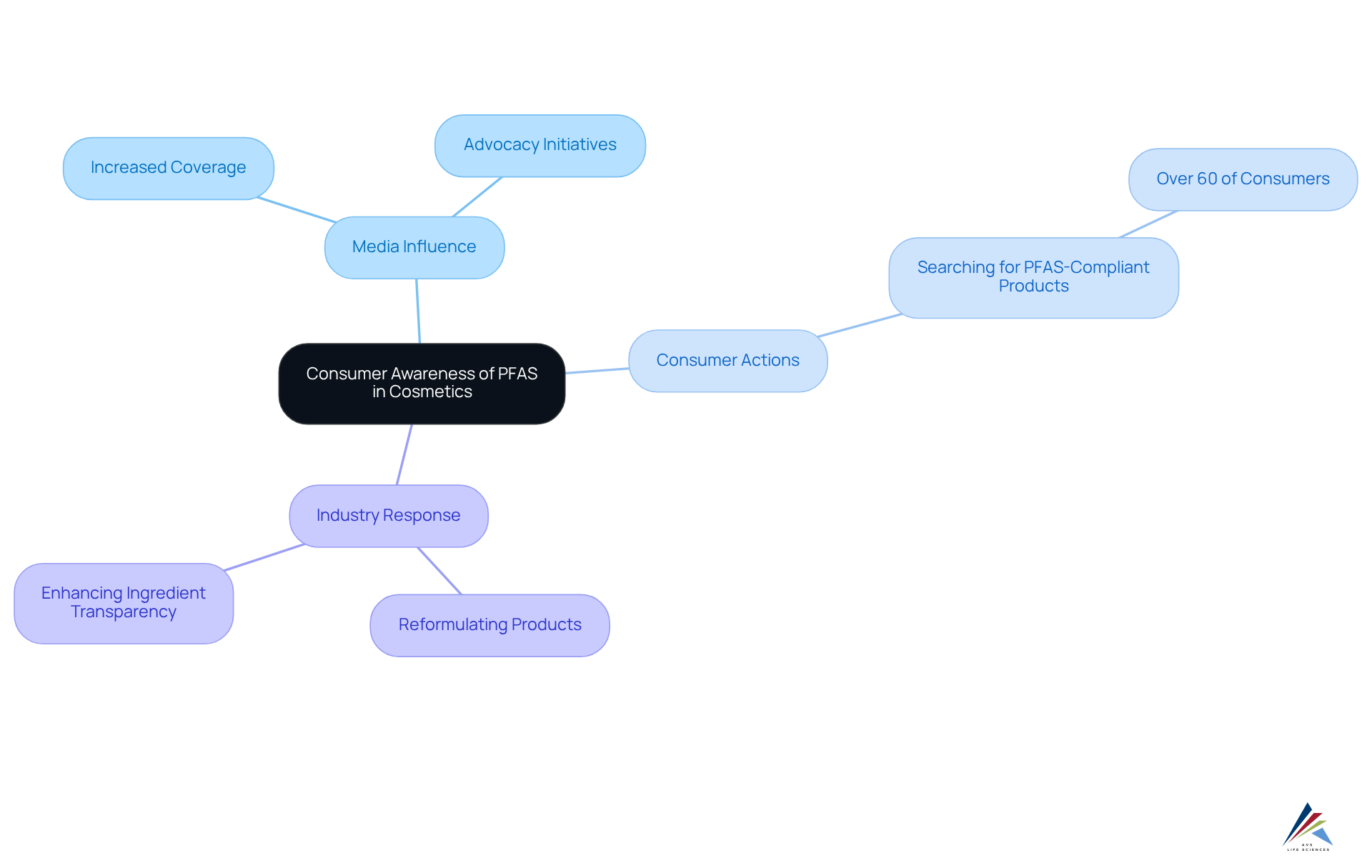
Consumer Awareness: Understanding PFAS Risks in Cosmetics
Consumer awareness regarding the risks associated with PFAS in cosmetics legislation is rapidly increasing, largely fueled by heightened media coverage and advocacy initiatives. Recent surveys suggest that a significant percentage of consumers—over 60%—are actively searching for items in accordance with PFAS in cosmetics legislation. This reflects a growing awareness of the associated with these substances, such as cancer and hormonal disruptions.
Consequently, this shift in consumer behavior is compelling manufacturers to enhance [transparency in their ingredient lists](https://treehugger.com/toxic-unlabeled-pfas-chemicals-found-cosmetics-5188974) and invest in [safer alternatives](http://avslifesciences.com/blog-post/10-essential-clinical-trial-management-software-solutions) to comply with PFAS in cosmetics legislation and align with market demand. Notably, various brands have started reformulating their products to remove harmful chemicals, responding to consumer preferences for safer, more sustainable options.
The media's role in influencing public perception is crucial, as it has significantly increased awareness and impacted consumer decisions, ultimately guiding the beauty industry toward enhanced accountability and safety.
Advocacy Groups: Driving Change in PFAS Cosmetics Legislation
Advocacy organizations are crucial in driving legislative change regarding harmful substances, including pfas in cosmetics legislation, by raising awareness about the risks associated with these chemicals and lobbying for more stringent regulations. Notable entities like the Environmental Working Group (EWG) and the Campaign for Safe Cosmetics have been instrumental in advocating for pfas in cosmetics legislation and restrictions on toxic chemicals in personal care products. Their persistent efforts have led to heightened scrutiny of beauty product ingredients, prompting lawmakers to take decisive action regarding , resulting in a surge of new legislation aimed at safeguarding consumers.
Recent statistics reveal that seven states have effectively enacted prohibitions on harmful chemicals in beauty products, which underscores the significance of pfas in cosmetics legislation and a growing public consensus in support of such initiatives. This momentum is bolstered by expert opinions that underscore the vital role of advocacy in shaping public health policy. Susan Goldhaber emphasizes that with various state prohibitions commencing as soon as January 1, 2025, the deliberate application of any and all per- and polyfluoroalkyl substances in beauty products is swiftly nearing its conclusion, which underscores the urgency of pfas in cosmetics legislation and compels producers to modify their offerings.
States such as California, Colorado, and Maine have proactively established prohibitions on harmful substances in beauty products, including considerations for pfas in cosmetics legislation, prior to the FDA's safety evaluation, exemplifying a forward-thinking regulatory strategy. The EWG's initiatives have been pivotal in galvanizing public support for these bans, showcasing successful lobbying efforts that have yielded tangible legislative outcomes. As these organizations persist in advocating for consumer safety, their influence is poised to significantly shape the future landscape of pfas in cosmetics legislation.

Scientific Research on PFAS: Implications for Cosmetics Legislation
Recent scientific studies have underscored alarming health risks associated with chemical exposure, particularly highlighting connections to various cancers and reproductive health issues. Research indicates that hair products marketed towards Black women frequently contain these harmful substances, raising significant safety concerns. Consequently, regulatory agencies are increasingly scrutinizing the safety of these substances in beauty products, resulting in the implementation of more stringent pfas in cosmetics legislation aimed at protecting consumer health. For instance, the Modernization of Cosmetics Regulation Act of 2022 mandates the FDA to evaluate and report on pfas in cosmetics legislation by December 29, 2025, reflecting a growing commitment to transparency and safety within the sector.
Manufacturers are adapting to these evolving regulations by reformulating their products to eliminate PFAS and other harmful ingredients. Numerous leading beauty brands are actively investing in research and development to create safer alternatives that adhere to the new safety standards. This shift aligns with while ensuring compliance with the increasingly complex regulatory environment.
The implications of these studies extend beyond individual products; they signal a broader movement towards enhanced safety regulations in the cosmetics industry. As consumer awareness of the risks associated with PFAS continues to rise, companies must prioritize safety and transparency in their formulations to maintain consumer trust and comply with pfas in cosmetics legislation. The Personal Care Products Council (PCPC), representing approximately 600 member companies and accounting for around 90% of the U.S. beauty industry, highlights the scale of compliance efforts required. Furthermore, the Safer Beauty Bill Package has garnered support from over 150 companies and organizations committed to environmental and consumer health, showcasing the industry's unified push for safer offerings. As Susan Little from EWG asserts, 'It’s time for stronger enforcement to hold companies accountable and ensure our communities are protected.' Despite these legislative efforts, over 170 personal care products in California still contain toxic chemicals banned under state law, underscoring persistent challenges in ensuring product safety. The Supply Chain Transparency Act for beauty products, which mandates suppliers to disclose ingredient safety information, further emphasizes the necessity for openness and adherence in the beauty industry.
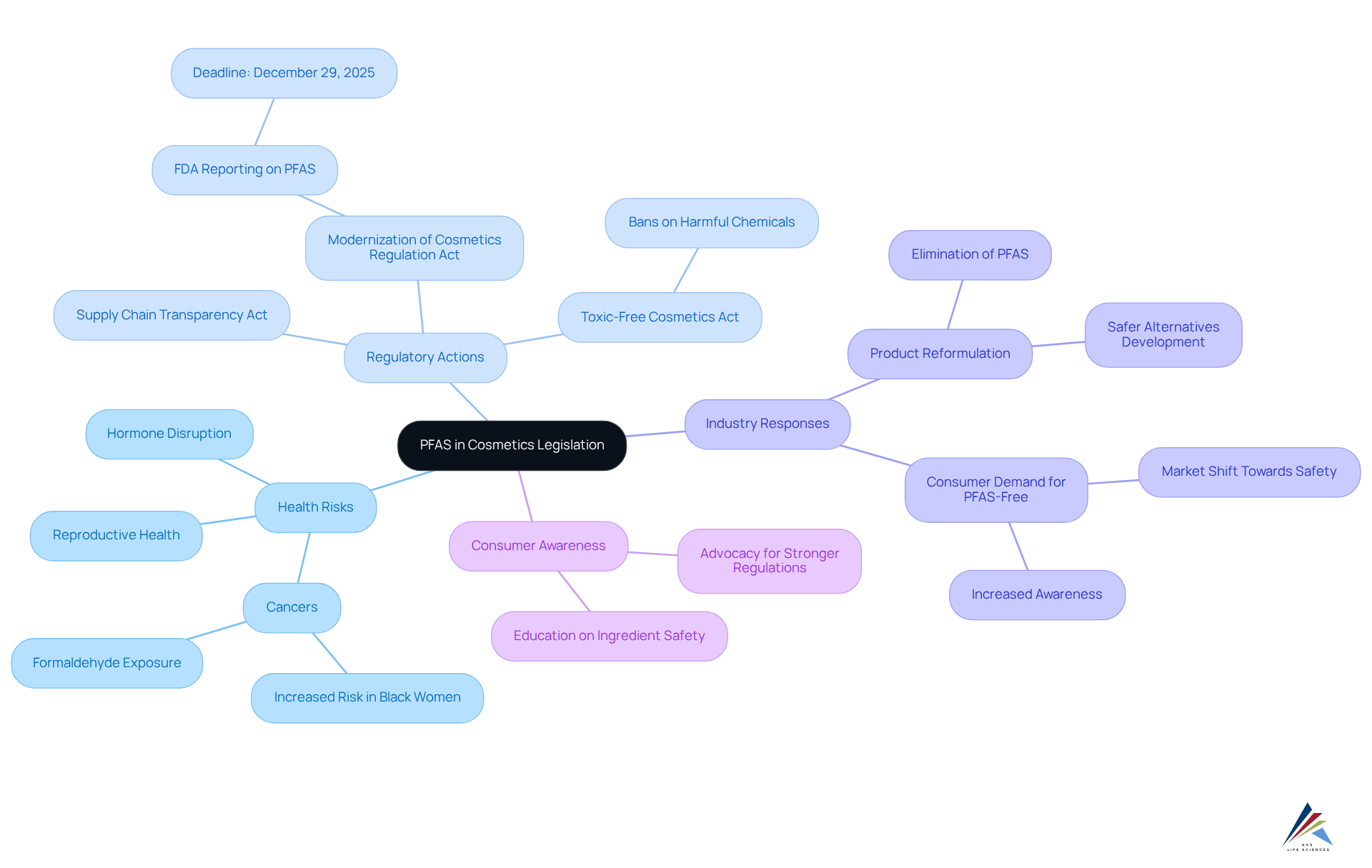
Future Trends in PFAS Legislation: What to Expect
Upcoming trends in chemical regulation are set to implement more extensive prohibitions and rigorous enforcement actions. With increasing public awareness of the health dangers linked to per- and polyfluoroalkyl substances (PFAS), lawmakers are expected to broaden to encompass a wider range of consumer items, extending beyond beauty products.
For instance, Illinois has enacted the Reduction Act, effective January 1, 2032, which prohibits deliberately included substances in:
- Beauty items
- Dental floss
- Youth items
- Menstrual supplies
- Intimate clothing
Similarly, New Mexico's Protection Act regarding PFAS in cosmetics legislation, effective January 1, 2028, phases out intentionally added substances in cosmetics and feminine hygiene products. These legislative actions reflect a nationwide shift towards stricter controls, particularly concerning PFAS in cosmetics legislation.
Manufacturers are advised to proactively adapt to these changes by investing in research and development of safer alternatives. This involves improving adherence frameworks to align with evolving regulatory demands. Regulatory specialists anticipate that by 2025, businesses will have to navigate a landscape where PFAS in cosmetics legislation is not only stricter but also more broadly enforced, necessitating a robust strategy for formulation and safety evaluations. As noted by a regulatory expert, "Without intervention, water utilities faced an estimated $389 million in capital upgrades and millions more in maintenance," underscoring the financial implications of non-compliance.
AVS Life Sciences can play a pivotal role in assisting manufacturers to navigate these changes effectively. By leveraging their expertise in quality management and regulatory adherence solutions, AVS Life Sciences can support companies in enhancing their regulatory frameworks and developing safer product formulations. As the legislative environment continues to evolve, staying ahead of these trends will be crucial for maintaining compliance and ensuring consumer safety.
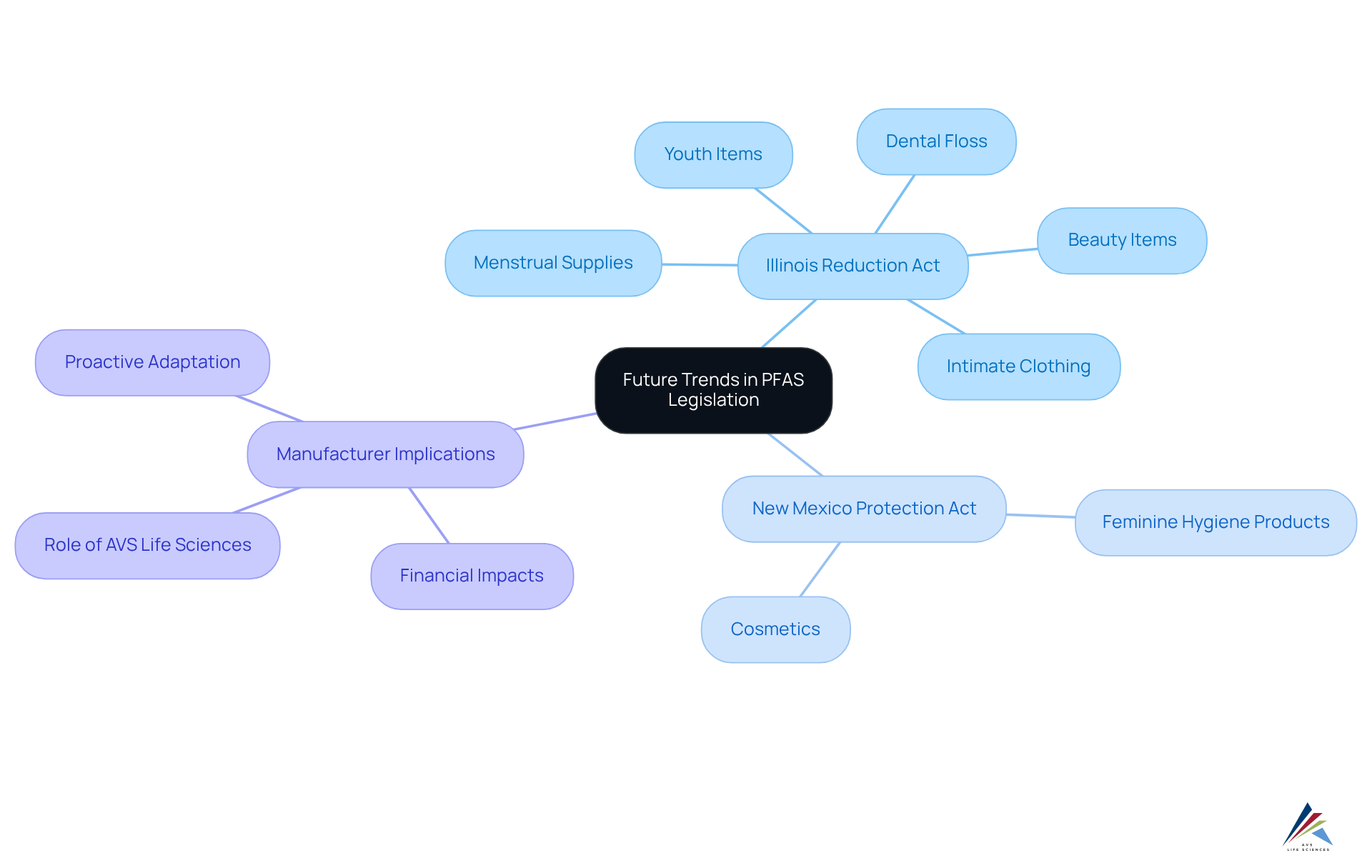
State-Level Regulations: The Patchwork of PFAS Laws Across the U.S.
The regulatory landscape for PFAS in cosmetics legislation presents a fragmented array of state-level laws, each defined by unique requirements. Some states have enacted extensive prohibitions on these substances through PFAS in cosmetics legislation, while others are still developing their legislative frameworks. This inconsistency in PFAS in cosmetics legislation creates substantial regulatory challenges for manufacturers operating across various jurisdictions, as they must navigate a maze of differing rules to ensure compliance with each state's specific mandates.
Furthermore, the essential-use concept for per- and polyfluoroalkyl substances emphasizes that these materials should only be employed when necessary for health or safety, adding another layer of complexity to regulatory strategies. Statistics reveal that approximately 22% of REACH authorizations are still for chrome plating with hexavalent chromium, underscoring the urgency for manufacturers to adapt to evolving regulations.
As Martin Scheringer observed, 'The complexity of these regulations requires customized adherence strategies to effectively handle the diverse requirements set by each state.' This patchwork approach not only complicates compliance efforts but also raises concerns about the overall in safeguarding public health and the environment.
Impact of PFAS Legislation on the Cosmetics Industry: A Comprehensive Overview
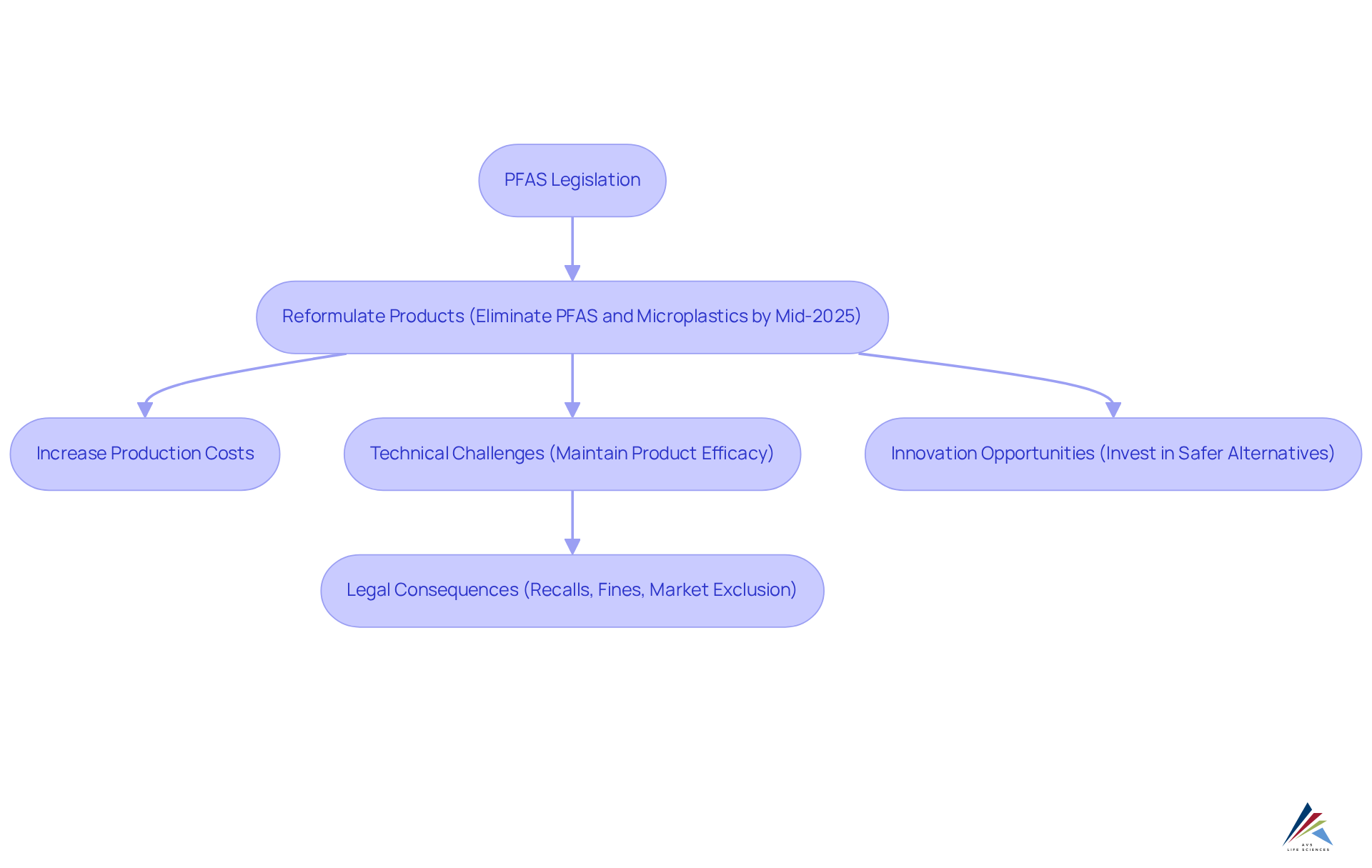
The impact of PFAS in cosmetics legislation on the cosmetics sector is considerable, transforming formulation and marketing approaches. As regulatory frameworks tighten, manufacturers are mandated to reformulate items to eliminate PFAS in cosmetics legislation, microplastics, and other restricted substances by mid-2025. This requirement leads to increased production costs and potential disruptions in supply chains. The transition poses technical challenges as companies strive to maintain product efficacy while adhering to new safety standards. Non-adherence can result in significant legal consequences, including recalls, fines, and market exclusion, which directly affect revenue and brand reputation. This situation underscores the and regulatory records.
Despite these challenges, the evolving landscape opens avenues for innovation. Brands that proactively invest in safer alternatives not only comply with regulations but also distinguish themselves in a competitive market. Industry leaders assert that the 2025 regulatory wave, influenced by PFAS in cosmetics legislation, is not merely another compliance hurdle; it represents a complete reset for personal care manufacturing. As the demand for health-conscious products rises—evidenced by PFAS detections in products like cosmetics and textiles doubling every four years—companies that adapt effectively can leverage this trend to strengthen their market position and foster consumer loyalty.
Conclusion
The evolving landscape of PFAS in cosmetics legislation highlights an urgent imperative for manufacturers to adapt to stringent regulatory requirements designed to protect consumer health. With the implementation of new laws, such as the Toxic-Free Cosmetics Act, the beauty industry confronts significant challenges and responsibilities in reformulating products to eliminate harmful substances. This transformation not only enhances consumer safety but also mirrors a rising public demand for transparency and accountability in personal care products.
Recent developments underscore the necessity of robust compliance strategies that navigate the complexities of state-level regulations, the influential role of advocacy groups in driving change, and the critical need for manufacturers to invest in safer alternatives. With over 350 PFAS-related bills introduced and heightened public awareness, the pressure on companies to ensure their products are devoid of toxic chemicals is more pronounced than ever. Collaborating with experts like AVS Life Sciences can provide essential support in navigating these regulatory changes and maintaining compliance.
Ultimately, the ramifications of PFAS regulations on the cosmetics industry extend beyond mere compliance; this represents a pivotal moment for innovation and consumer trust. As the industry adapts to these changes, the commitment to producing safer, non-toxic products will not only fulfill regulatory demands but also resonate with a growing consumer base that prioritizes health and sustainability. Embracing these shifts is essential for manufacturers aiming to thrive in a market increasingly characterized by safety and transparency.
Frequently Asked Questions
What is AVS Life Sciences and what do they specialize in?
AVS Life Sciences provides customized regulatory solutions focused on quality control and compliance in the cosmetics sector, helping clients navigate the challenges posed by emerging chemical regulations, particularly those related to PFAS.
What is the Toxic-Free Cosmetics Act and when does it take effect?
The Toxic-Free Cosmetics Act, enacted on May 15, 2023, prohibits the production, sale, and distribution of beauty products containing intentionally added PFAS, starting January 1, 2025. It aims to enhance consumer safety by eliminating harmful chemicals from personal care products.
Why is the Toxic-Free Cosmetics Act significant?
The Act is significant because it promotes safer alternatives in cosmetic formulations, protects consumers from toxic chemicals, and aligns with public health advocacy for non-toxic personal care products, particularly for vulnerable populations.
What recent trends have emerged regarding PFAS regulations in cosmetics?
Recent trends show a shift towards stricter enforcement and broader bans on PFAS in cosmetics, with over 350 PFAS-related bills introduced across various states. Manufacturers need to adapt quickly to avoid penalties and maintain market access.
What specific regulatory initiative has Minnesota enacted regarding PFAS?
Minnesota has implemented a regulatory initiative requiring manufacturers to disclose intentionally incorporated PFAS substances in products sold within the state. The reporting deadline has been extended to July 1, 2026, to give manufacturers more time to comply.
How can manufacturers effectively navigate the evolving PFAS regulations?
Manufacturers can navigate the evolving regulations by engaging with stakeholders, utilizing technology solutions for tracking compliance obligations, and seeking consulting services, such as those offered by AVS Life Sciences, to develop compliance matrices and update protocols.
What is the anticipated timeline for changes in PFAS regulations?
The next 12 to 24 months are crucial as state and federal authorities enhance oversight of PFAS in cosmetics legislation, making it essential for manufacturers to stay informed and proactive in adapting to these changes.
