7 Key Insights on CSV Full Form in Pharma Compliance
Overview
This article underscores the critical importance of Computer System Validation (CSV) in the pharmaceutical industry, highlighting its essential role in ensuring compliance with regulatory standards and safeguarding data integrity. Effective CSV practices are not merely beneficial; they are vital for enhancing product quality, mitigating risks associated with data manipulation, and adapting to emerging technologies. These elements are crucial for navigating the intricate landscape of pharmaceutical compliance. By understanding and implementing robust CSV strategies, organizations can not only meet regulatory demands but also position themselves favorably in a competitive market.
Introduction
The pharmaceutical industry navigates a complex web of regulations, where the integrity of computerized systems is essential for ensuring compliance and patient safety. Computer System Validation (CSV) is not merely a regulatory requirement; it is a strategic necessity that can significantly enhance product quality and operational efficiency. As organizations face the challenges of evolving technologies and increasing compliance demands, what key insights can guide them through the intricacies of CSV? This article explores seven essential aspects of CSV in pharma, demonstrating how effective validation practices can transform compliance challenges into opportunities for growth and innovation.
AVS Life Sciences: Comprehensive CSV Solutions for Pharmaceutical Compliance
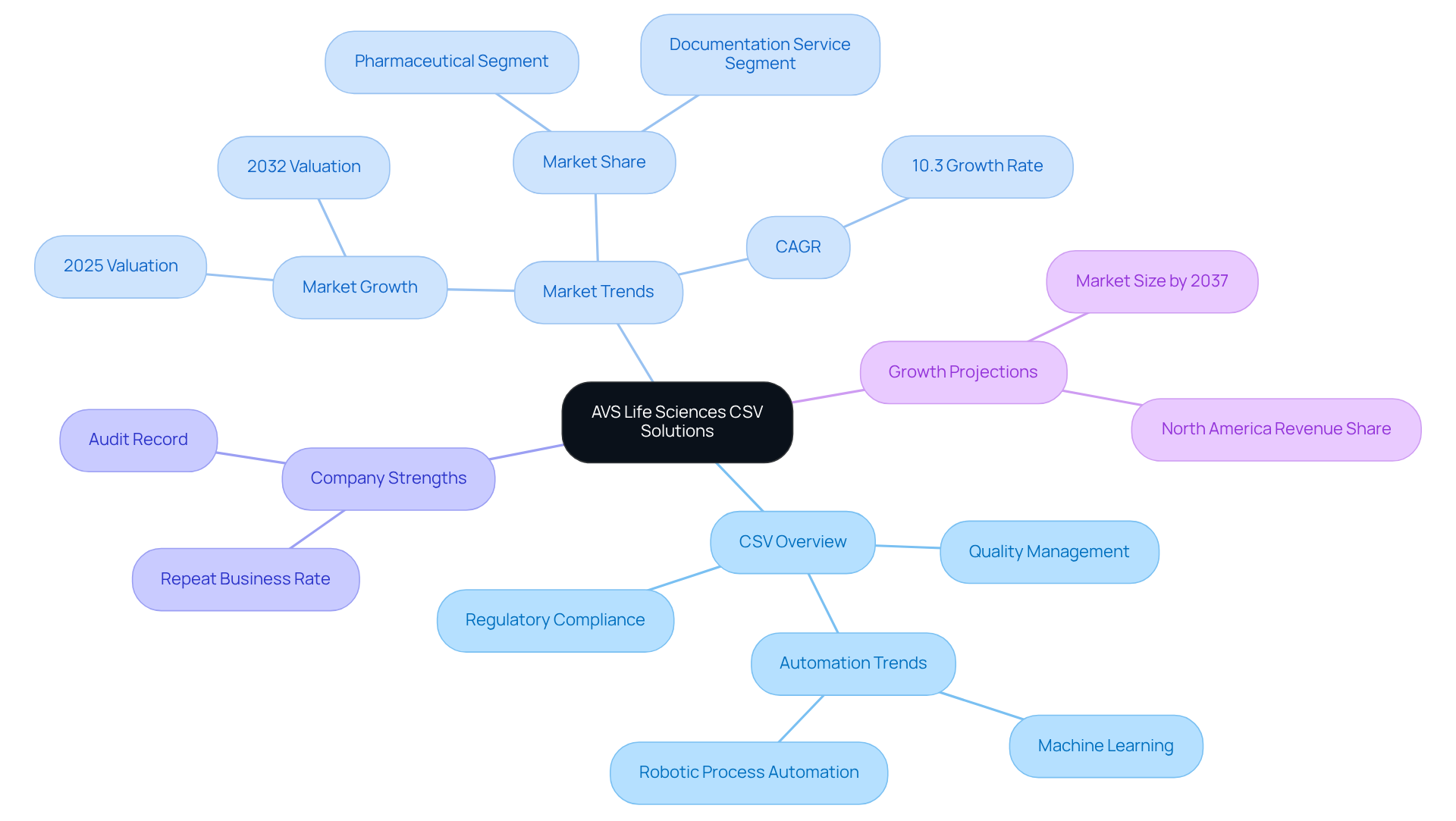
AVS Life Sciences provides a comprehensive suite of Computer System Validation (CSV) solutions, which embody the csv full form in pharma, specifically tailored for the pharmaceutical industry. By emphasizing quality management and regulatory compliance, AVS enables clients to establish effective CSV processes that reflect the csv full form in pharma, aligning with rigorous industry standards, including GXP and FDA regulations. With a dedicated team of over 300 seasoned professionals, the company delivers practical consulting that streamlines validation efforts and ensures compliance throughout the entire product lifecycle.
Recent trends reveal a notable shift towards automation in CSV processes, propelled by advancements in technologies such as machine learning and robotic process automation. These innovations enhance efficiency and accuracy, allowing organizations to navigate the complexities of regulatory requirements with greater effectiveness. The global CSV market is anticipated to expand from USD 3.39 billion in 2025 to USD 7.33 billion by 2032, representing a compound annual growth rate (CAGR) of approximately 10.3%. This growth highlights the rising demand for specialized CSV services within the pharmaceutical sector, projected to capture 40% of the market share during this timeframe.
In this competitive landscape, AVS Life Sciences distinguishes itself through rapid value creation and meticulous project execution. The company's dedication to excellence is reflected in an impressive 80% repeat business rate and a flawless audit record with zero findings. By leveraging its extensive expertise in quality management systems development, compliance audits, and regulatory adherence, AVS Life Sciences not only meets but exceeds client expectations, establishing itself as a trusted partner in the life sciences consulting arena.
CSV Full Form: Understanding Computer System Validation in Pharma
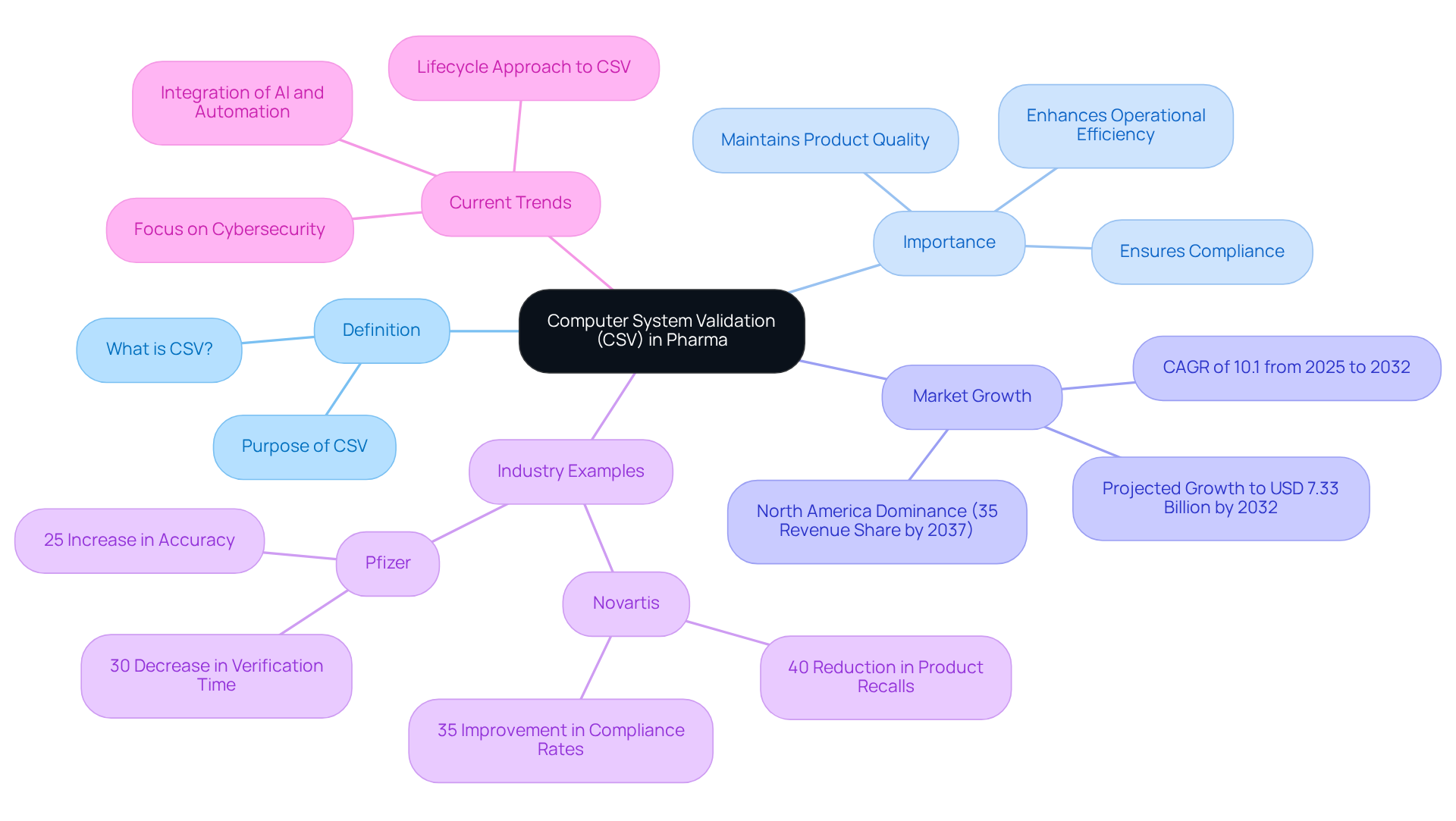
The csv full form in pharma, which stands for Computer System Validation, is a critical process in the pharmaceutical industry, ensuring that computerized systems function correctly and comply with rigorous regulatory standards. This assessment necessitates comprehensive documentation to verify that systems fulfill their intended use and operate consistently—an essential factor for maintaining product quality and safety. With the global CSV market projected to grow significantly, reaching USD 7.33 billion by 2032, the importance of CSV in ensuring compliance with Good Manufacturing Practices (GMP) and other regulations is paramount.
Efficient CSV procedures are exemplified by industry leaders such as Novartis, which implemented thorough verification strategies that resulted in a 40% reduction in product recalls and a 35% improvement in compliance rates. Similarly, Pfizer's integration of AI-driven anomaly detection systems led to a 30% decrease in verification time and a 25% increase in accuracy, showcasing how innovative strategies can enhance CSV efforts.
Current trends indicate a shift towards a lifecycle approach in CSV, integrating verification processes throughout a system's lifespan. This evolution is fueled by the growing complexity of governance landscapes and the rising adoption of cloud computing and automation technologies. As organizations face escalating demands for advanced validation solutions, the focus on continuous validation practices is expected to replace traditional one-time validations, ensuring sustained compliance and data integrity.
In conclusion, understanding the csv full form in pharma is not merely a compliance obligation; it is a strategic necessity for pharmaceutical companies striving to improve product quality and operational efficiency. As the industry evolves, the role of CSV in addressing compliance challenges will remain crucial.
Importance of CSV: Ensuring Quality and Compliance in Pharmaceuticals
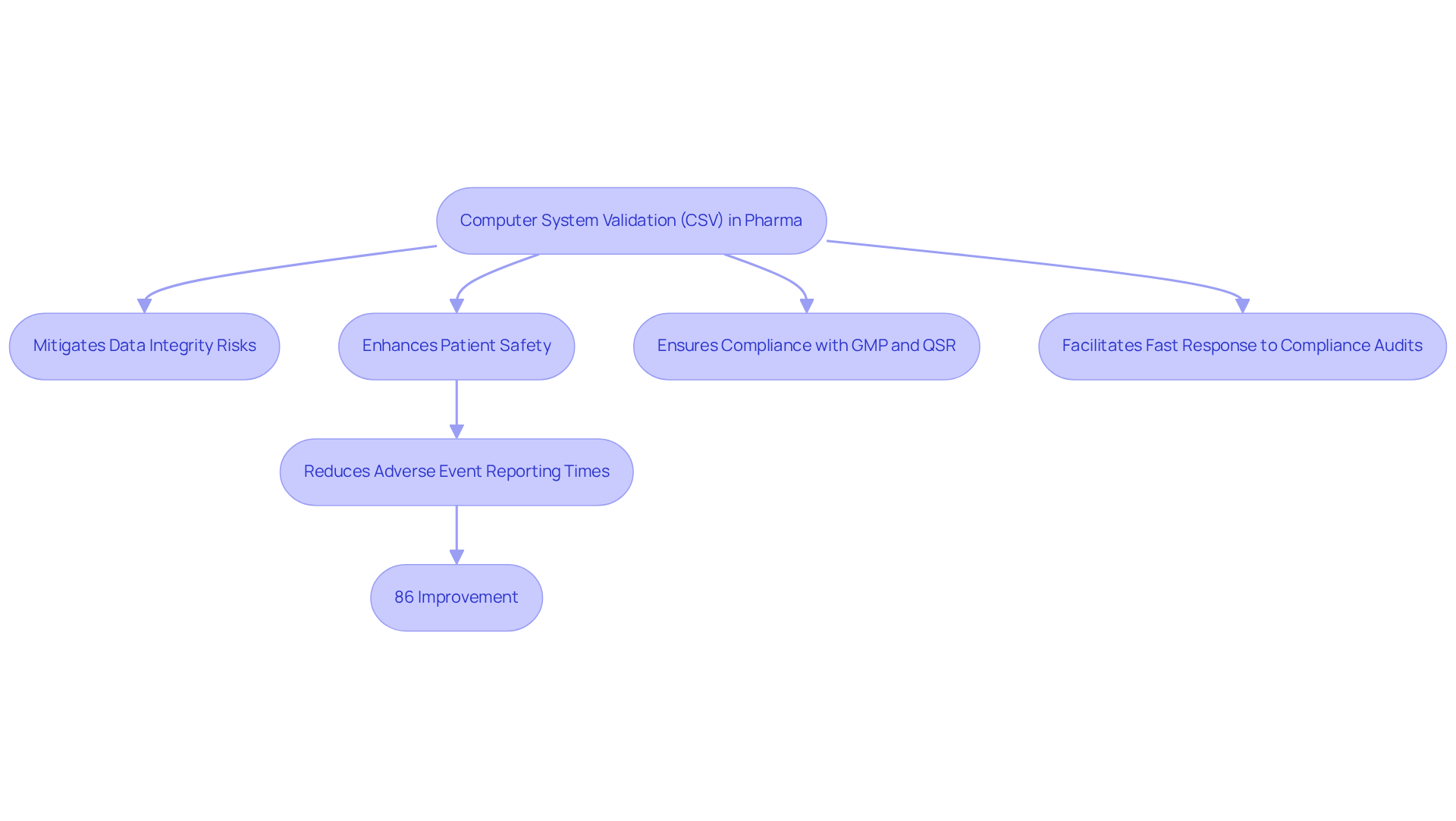
The significance of the csv full form in pharma, specifically Computer System Validation, cannot be overstated. The csv full form in pharma ensures that all computerized systems involved in drug development, manufacturing, and quality control are not only reliable but also comply with stringent standards. By rigorously validating these systems, pharmaceutical companies effectively mitigate data integrity risks, which are essential for maintaining accurate and trustworthy data. This process is crucial for preventing costly non-compliance penalties and safeguarding patient safety.
CSV plays a vital role in enhancing patient safety by ensuring that the data generated throughout the drug development lifecycle is of high quality and fully traceable. Effective CSV practices have been shown to reduce adverse event reporting times significantly, from an average of 16.2 days to just 2.3 days—an impressive 86% improvement. This swift response capability allows for faster oversight actions, ultimately enhancing patient safety outcomes.
Moreover, the implementation of robust CSV procedures strengthens compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR), ensuring that computerized systems positively contribute to overall quality objectives. By creating comprehensive documentation and audit trails, CSV services enable companies to respond promptly to compliance audits, minimizing delays and potential penalties.
In summary, the csv full form in pharma has a profound impact on drug development and patient safety. It not only streamlines inspections but also fosters a culture of quality and compliance within pharmaceutical organizations. As the industry evolves, investing in reliable CSV solutions is a strategic imperative for maintaining trust and ensuring the safety of pharmaceutical products.
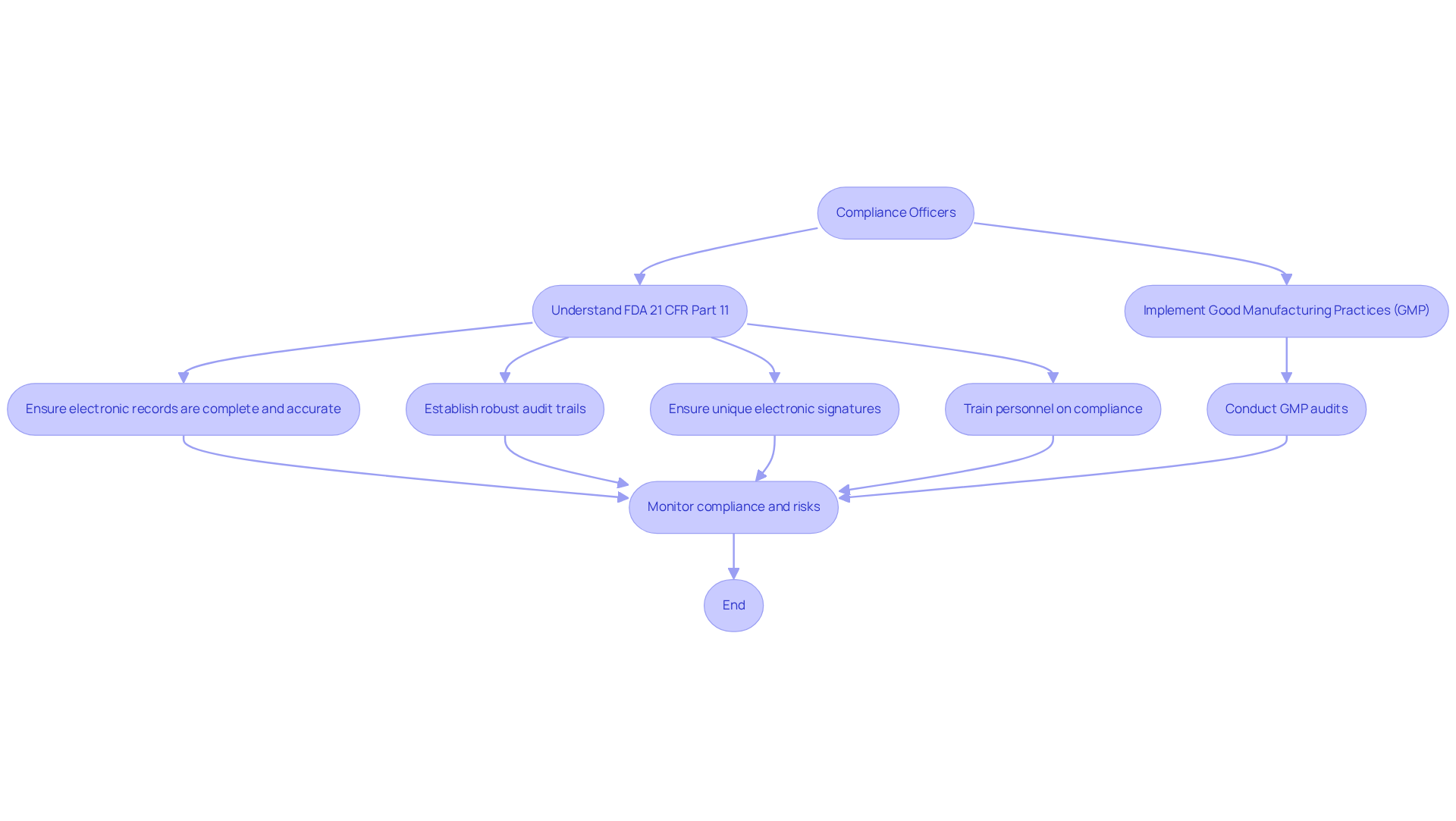
Regulatory Requirements for CSV: Key Guidelines for Compliance Officers
Compliance officers face the formidable challenge of navigating a complex environment of legal requirements when implementing the csv full form in pharma, known as Computerized System Validation. At the heart of this framework are the FDA 21 CFR Part 11 guidelines, which govern electronic records and signatures, ensuring their integrity and reliability. This regulation mandates that electronic records must be complete, accurate, and verifiable, with no missing data, and requires robust audit trails that document all changes, including timestamps and user details. Furthermore, the guidelines stipulate that electronic signatures must be unique to individuals and include the date and time of signing, reinforcing accountability in record-keeping.
Good Manufacturing Practices (GMP) further complement these regulations by establishing quality standards for pharmaceutical products. Compliance with GMP ensures that products are consistently produced and controlled according to quality standards, which is critical for patient safety. AVS Life Sciences provides extensive GXP oversight services, including GMP audits, to guarantee adherence in APIs, drug products, and testing facilities. Recent statistics indicate that non-compliance with 21 CFR Part 11 can lead to significant repercussions, including fines and product recalls, underscoring the necessity for thorough validation processes.
Organizations that successfully adhere to these regulations implement comprehensive strategies related to the CSV full form in pharma, enhancing operational efficiency and bolstering market reputation. By emphasizing adherence, they mitigate hazards linked to enforcement measures. As the pharmaceutical landscape evolves, staying informed about the latest regulatory requirements concerning the csv full form in pharma is essential for oversight officials aiming to uphold the highest standards of quality and safety. Moreover, the FDA highlights that staff must be sufficiently trained to review electronic data and regulated systems, underscoring the essential role of training in regulatory efforts. Addressing frequent issues in adherence, such as misinterpreting regulations or insufficient system verification, is crucial for establishing robust adherence frameworks.
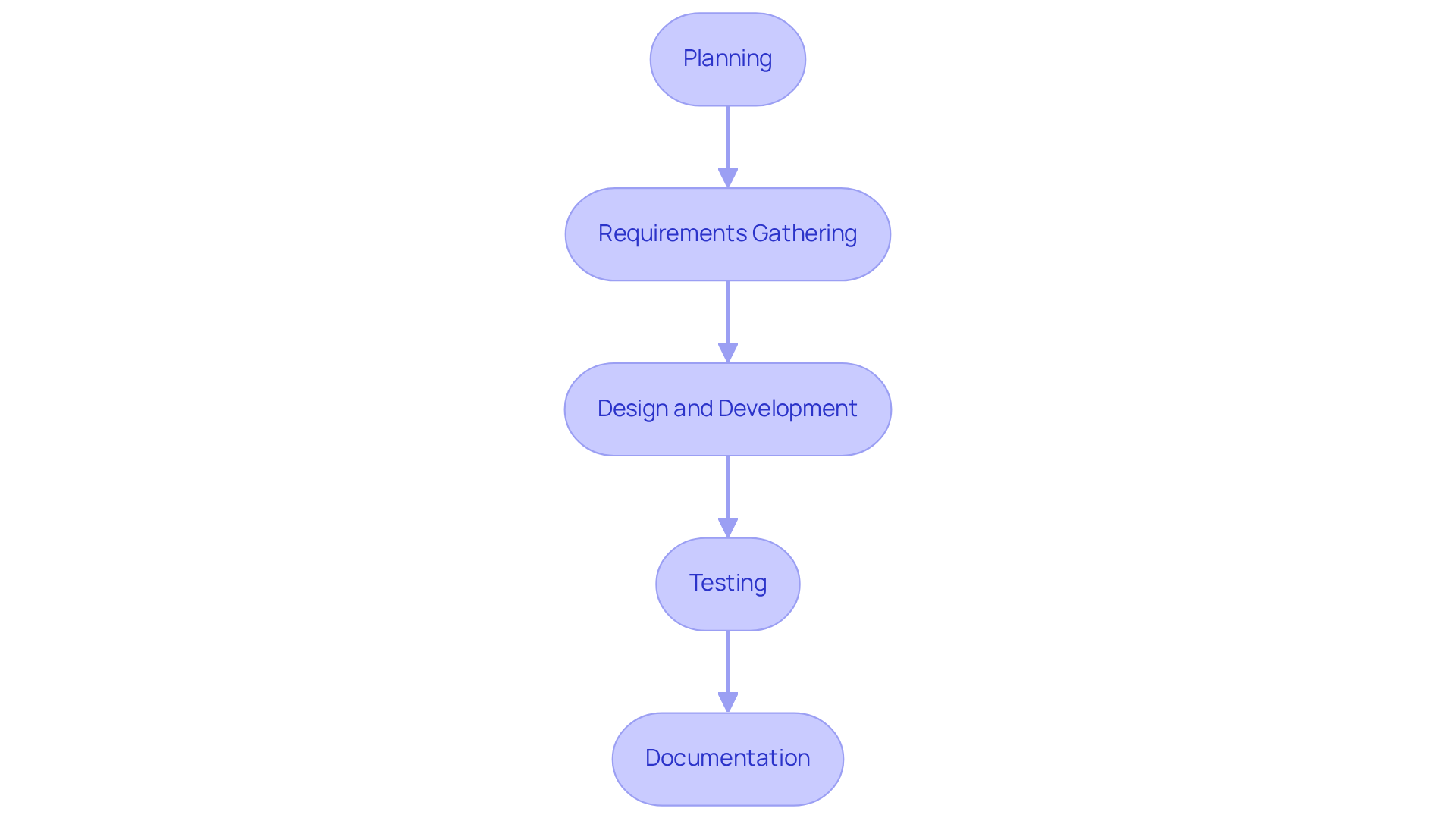
Phases of CSV: A Step-by-Step Guide for Pharmaceutical Validation
The Computer System Validation (CSV) process encompasses several essential phases that ensure compliance and operational integrity in the pharmaceutical sector:
-
Planning: This initial phase defines the objectives and scope of the assessment effort, laying the groundwork for subsequent activities. As Ahmed Hasham emphasizes, "Without adequate planning and preparation, computer system verification is highly dependent upon assurance formal Development Cycle, tasks cycle."
-
Requirements Gathering: During this stage, User Requirement Specifications (URS) are developed to capture the needs and expectations of users, ensuring that all functionalities are addressed.
-
Design and Development: This phase focuses on creating the system in accordance with the specified requirements, ensuring it is capable of performing its intended functions.
-
Testing: The system undergoes rigorous assessment to confirm it meets its intended use, which includes various testing methodologies such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each of these testing phases is essential for confirming that the system functions as expected and can endure challenging conditions, thus guaranteeing patient safety and adherence to established standards.
-
Documentation: Detailed documentation is created to furnish proof of adherence and validation activities, which is essential for inspections and audits. This corresponds with the statistic that typical obstacles encountered during the implementation of the CSV full form in pharma involve insufficient documentation and planning, which can result in regulatory issues.
Each of these phases plays a vital role in ensuring that the system operates as intended, thereby maintaining product quality and regulatory compliance. By adopting a systematic method, organizations can reduce these risks and improve their verification processes. The V-Model, as outlined in the Good Automated Manufacturing Practices (GAMP) 5 Guide, serves as a framework for this process, ensuring quality checks at each stage. Furthermore, the FDA defines software validation as "Confirmation, by inspection and the presentation of tangible evidence, that the software specifications comply with the purposes and planned uses of the user and that the specific requirements implemented through software can consistently meet their obligations." AVS Life Sciences is dedicated to delivering expert quality solutions and has established excellence in life sciences consulting, ensuring that the CSV full form in pharma processes adhere to the highest standards of regulations and quality assurance.
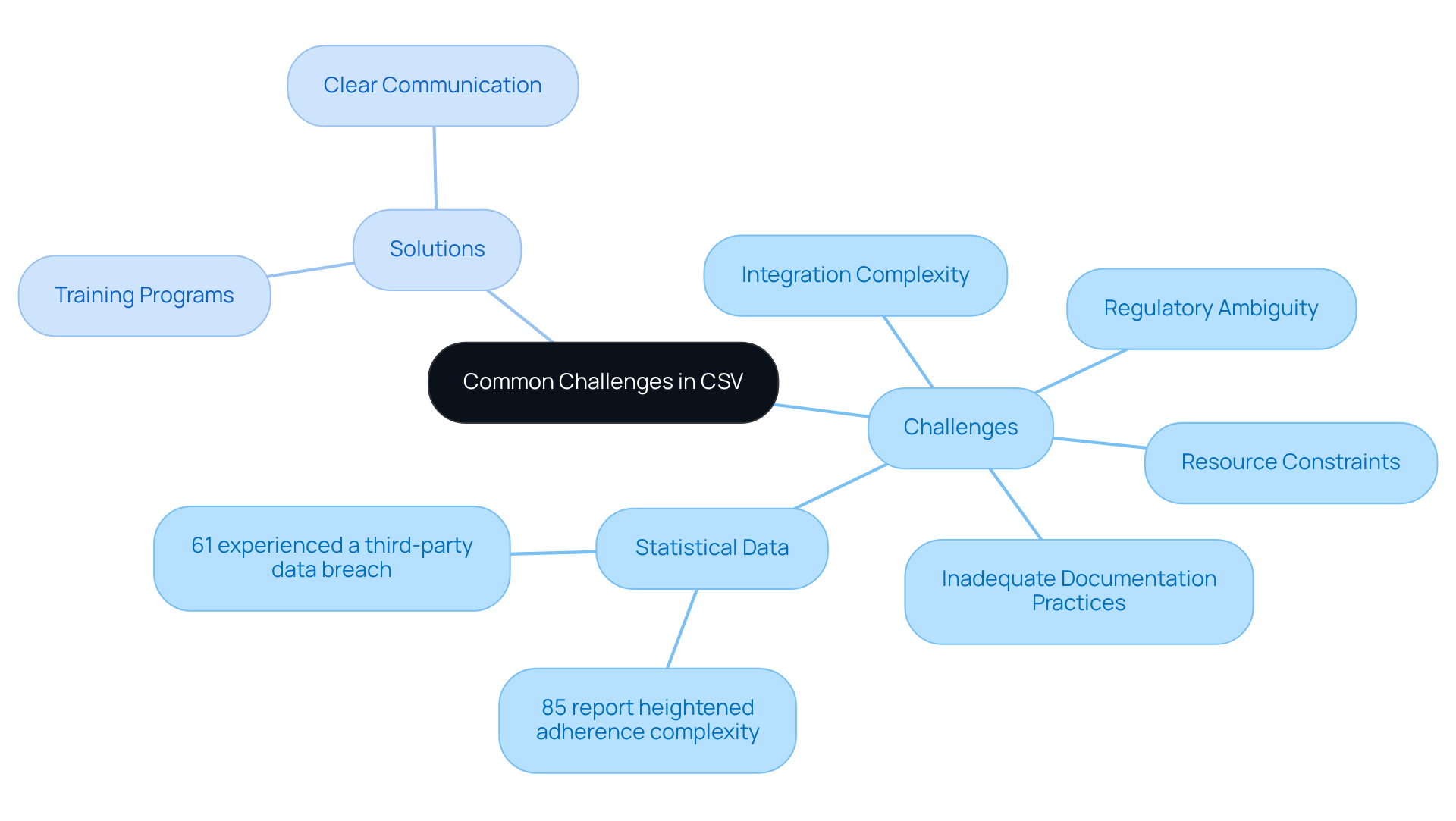
Common Challenges in CSV: Navigating Compliance Obstacles
Organizations frequently encounter significant challenges when implementing Computer System Validation (CSV), primarily due to regulatory ambiguity, inadequate documentation practices, and the complexities associated with integrating legacy systems. A staggering 85% of organizations report heightened adherence complexity, which can obstruct efficient verification efforts. Resource constraints further exacerbate these issues, making it difficult to allocate sufficient time and personnel for thorough validation processes. Notably, 61% of organizations have experienced a third-party data breach, underscoring the vulnerabilities that can arise from insufficient oversight in regulatory practices.
To navigate these obstacles, a proactive approach is essential. This includes:
- Investing in extensive training programs to ensure all stakeholders are well-informed about regulatory requirements and best practices.
- Clear communication among team members to facilitate a smoother implementation process, ultimately enhancing the success rates of CSV initiatives.
Organizations that prioritize these strategies not only bolster their adherence stance but also cultivate a culture of accountability and transparency, which is vital in today’s evolving regulatory landscape.
To further support organizations in their CSV initiatives, AVS Life Sciences offers a comprehensive checklist detailing essential steps in the verification process, ensuring thorough adherence and efficient execution. By leveraging expert advice and assistance from AVS Life Sciences, organizations can effectively tackle these challenges and guarantee robust compliance with CSV requirements.
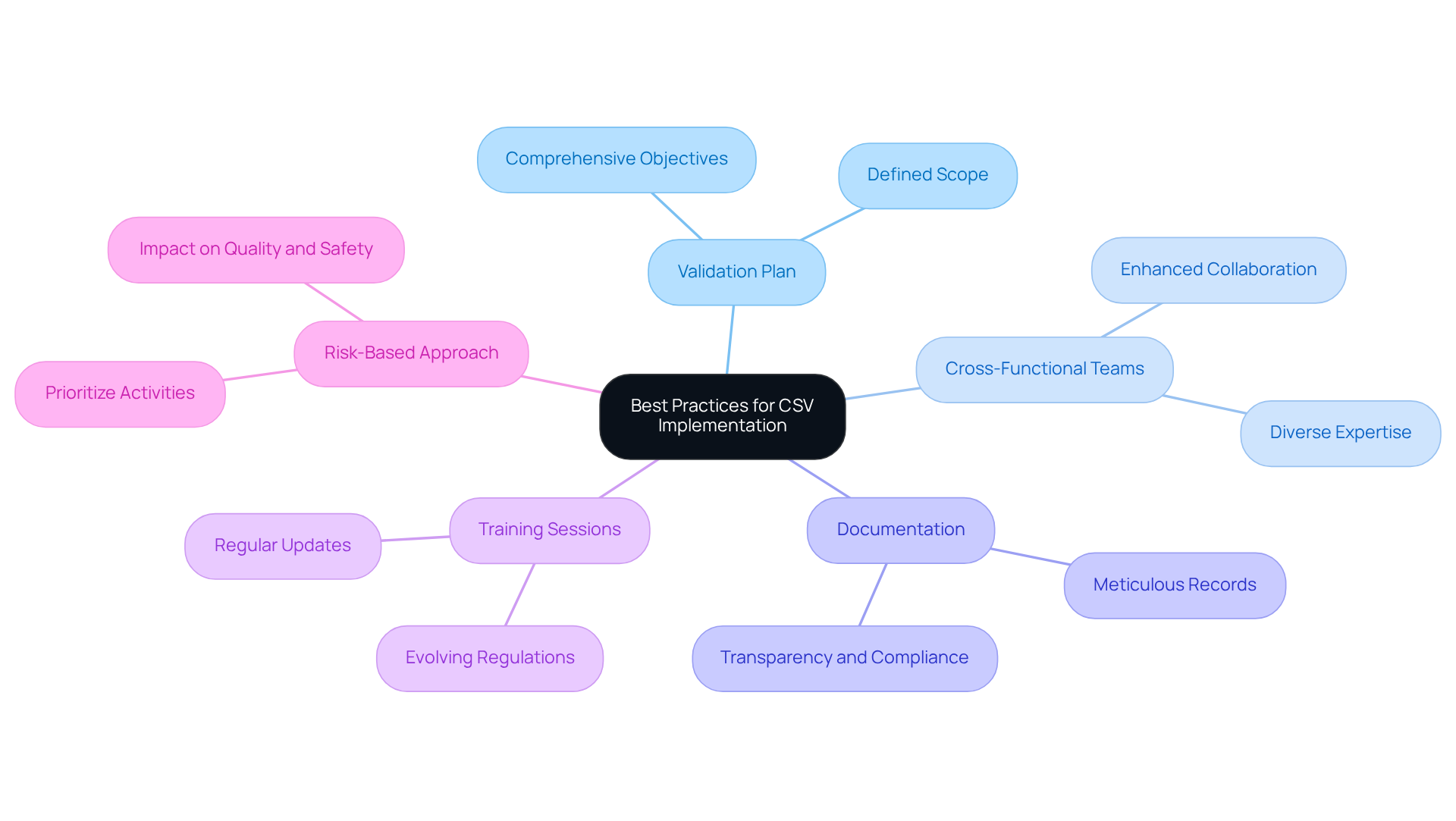
Best Practices for CSV Implementation: Strategies for Compliance Success
The successful execution of Computer System Validation (CSV full form in pharma) in pharmaceutical organizations relies on several best practices:
- Develop a comprehensive validation plan that clearly defines objectives and scope.
- Engage cross-functional teams to enhance collaboration and communication across departments.
- Maintain meticulous documentation throughout the validation lifecycle to ensure transparency and adherence to standards.
- Conduct regular training sessions to keep staff informed about evolving regulatory requirements.
- Implement a risk-based approach to prioritize validation activities based on their potential impact on product quality and patient safety.
By adopting these strategies, organizations can significantly enhance their adherence stance and operational efficiency.
Real-World CSV Examples: Learning from Industry Applications
Multiple pharmaceutical firms have effectively adopted Computer System Validation (CSV), known as the csv full form in pharma, to enhance their regulatory frameworks. For instance, a leading biopharmaceutical firm utilized the CSV full form in pharma to validate its clinical trial management system, resulting in improved data integrity and streamlined regulatory submissions. Another example involves a medical device producer that implemented the CSV full form in pharma to ensure adherence to FDA regulations, leading to a notable decrease in audit findings. These cases illustrate the tangible benefits of effective practices related to the CSV full form in pharma.
We urge regulatory officers to utilize our extensive checklist as a reference for their upcoming validation projects. Should you require assistance or more information on the CSV process, AVS Life Sciences stands ready to support you with expert guidance and skilled professionals.
CSV and Data Integrity: Ensuring Compliance in Pharmaceutical Operations
Data integrity is fundamental to the csv full form in pharma, ensuring that all data generated and managed by computerized systems is accurate, reliable, and secure. Robust processes that explain the csv full form in pharma are essential for mitigating risks associated with data manipulation and unauthorized access, which have become increasingly critical in light of recent FDA warnings regarding data integrity deficiencies. For instance, between 2017 and 2022, the FDA issued over 160 Warning Letters citing such deficiencies, underscoring the need for stringent data governance.
By prioritizing data integrity, pharmaceutical companies can significantly enhance their compliance stance, which includes understanding the csv full form in pharma, thereby maintaining the trust of oversight bodies and stakeholders. Organizations that implement effective practices related to the CSV full form in pharma not only safeguard their data but also position themselves favorably during audits. This is evidenced by AVS Life Sciences' commitment to zero findings during inspections, a testament to their dedication to data integrity. Industry insights stress that a well-prepared Registration Dossier is essential for obtaining Marketing Authorization from oversight bodies.
Examples of organizations successfully ensuring data integrity through CSV include those that have adopted comprehensive audit trails and data logging systems, which are critical for accountability and traceability. Such measures not only comply with regulatory requirements but also foster a culture of continuous improvement in data management practices. Ultimately, this enhances the overall quality and reliability of pharmaceutical operations.
Future Trends in CSV: Preparing for the Next Phase of Pharmaceutical Compliance
The future of Computer System Validation (CSV full form in pharma) in the pharmaceutical industry is on the brink of substantial transformation, driven by the increasing integration of automation and artificial intelligence (AI). These technologies streamline verification processes, enhance efficiency, and mitigate the potential for human error. For instance, AI-driven automation adeptly manages repetitive tasks, allowing assessment teams to focus on more strategic activities, thereby optimizing resource allocation.
Moreover, the adoption of machine learning and predictive analytics is anticipated to refine validation quality by identifying patterns and potential issues early in the process. This proactive approach not only bolsters adherence to legal standards but also ensures data integrity and security—critical elements in the highly regulated pharmaceutical sector.
The incorporation of blockchain technology further enhances CSV by facilitating secure and transparent data sharing, essential for maintaining traceability and compliance with regulations. As organizations navigate these advancements, they must remain agile and proactive, continuously adapting their validation strategies to align with evolving technologies and compliance requirements.
Additionally, AVS Life Sciences stands at the forefront of these innovations, leveraging AI and automation to elevate their CSV processes. By implementing sophisticated quality management solutions, AVS Life Sciences guarantees that their clients meet regulatory standards while improving operational efficiency.
In summary, embracing automation and AI in processes related to the csv full form in pharma transcends mere trendiness; it is a necessity for pharmaceutical companies striving to ensure compliance and operational success in a rapidly changing environment.
Conclusion
Understanding the full form of CSV in pharma—Computer System Validation—is crucial for pharmaceutical companies aiming to ensure their systems operate effectively while complying with stringent regulatory standards. This process transcends mere compliance; it embodies a strategic approach to enhancing product quality and operational efficiency in an industry where safety and reliability are non-negotiable.
Key insights from the article underscore the significance of robust CSV practices in mitigating risks associated with data integrity and regulatory compliance. With the global CSV market poised for substantial growth, organizations must adopt comprehensive validation strategies, leverage innovative technologies, and adhere to regulatory guidelines to adeptly navigate the complexities of pharmaceutical compliance.
As the industry evolves, the integration of automation and AI into CSV processes will become increasingly essential. Companies are urged to embrace these advancements to bolster compliance, enhance data integrity, and optimize operational success. By prioritizing effective CSV implementation, organizations can not only safeguard patient safety but also position themselves as leaders in the competitive pharmaceutical landscape.
Frequently Asked Questions
What does CSV stand for in the pharmaceutical industry?
CSV stands for Computer System Validation, which is a critical process ensuring that computerized systems function correctly and comply with regulatory standards.
Why is Computer System Validation important in pharmaceuticals?
CSV is essential for ensuring that computerized systems involved in drug development, manufacturing, and quality control are reliable and compliant with stringent standards, thus mitigating data integrity risks and enhancing patient safety.
How does AVS Life Sciences support CSV in the pharmaceutical industry?
AVS Life Sciences provides a comprehensive suite of CSV solutions tailored for the pharmaceutical industry, focusing on quality management and regulatory compliance, and helping clients establish effective CSV processes.
What are some recent trends in Computer System Validation?
Recent trends include a shift towards automation in CSV processes, with advancements in technologies such as machine learning and robotic process automation, enhancing efficiency and accuracy.
What is the projected growth of the global CSV market?
The global CSV market is anticipated to grow from USD 3.39 billion in 2025 to USD 7.33 billion by 2032, representing a compound annual growth rate (CAGR) of approximately 10.3%.
How do effective CSV practices impact patient safety?
Effective CSV practices improve the quality and traceability of data generated during drug development, significantly reducing adverse event reporting times and enhancing patient safety outcomes.
What role does documentation play in Computer System Validation?
Comprehensive documentation is necessary to verify that systems fulfill their intended use and operate consistently, which is crucial for maintaining product quality and safety.
How do leading pharmaceutical companies implement CSV?
Companies like Novartis and Pfizer have implemented thorough verification strategies and AI-driven systems that have resulted in significant improvements in compliance rates and reductions in verification time.
What is the significance of continuous validation practices?
Continuous validation practices are becoming essential as they ensure sustained compliance and data integrity throughout a system's lifespan, replacing traditional one-time validations.
How does AVS Life Sciences differentiate itself in the CSV market?
AVS Life Sciences distinguishes itself through rapid value creation, meticulous project execution, an impressive 80% repeat business rate, and a flawless audit record with zero findings.
