7 Key Differences Between FDA Cleared vs Approved Products

Overview
This article elucidates the critical distinctions between FDA cleared and FDA approved products, emphasizing the differing regulatory pathways, evidence requirements, and post-market obligations that characterize each category. It clarifies that FDA clearance generally entails a less rigorous process via the 510(k) pathway, whereas FDA approval necessitates comprehensive clinical trial data and is designated for higher-risk products. Understanding these differences is paramount for effective compliance within the life sciences industry, driving the need for informed decision-making and strategic engagement with compliance solutions.
Introduction
Understanding the nuances between FDA cleared and FDA approved products is essential for stakeholders in the life sciences industry. While both terms are often used interchangeably, they represent distinct regulatory pathways that carry different implications for safety, efficacy, and market access. This article delves into the seven key differences between these two classifications, illuminating the critical aspects that manufacturers must navigate to ensure compliance and successful product development.
What are the fundamental distinctions that could impact a product's journey to market, and why does this knowledge matter in an increasingly competitive landscape? As we explore these differences, it becomes clear that a comprehensive understanding is not just beneficial—it is vital for navigating the complexities of compliance in today's market.
AVS Life Sciences: Expert Guidance on FDA Clearance and Approval Processes
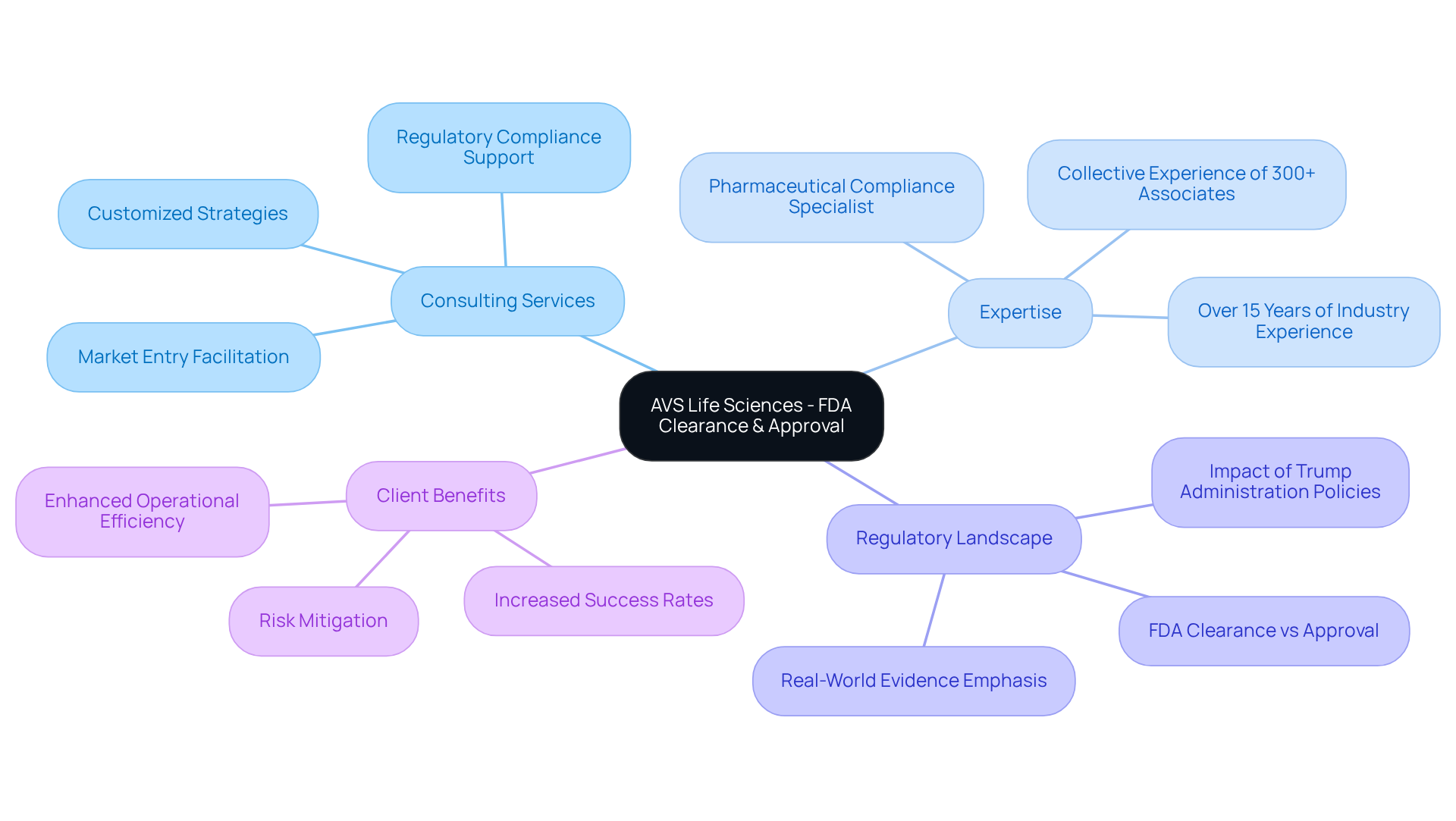
AVS Life Sciences provides expert consulting services to assist clients in navigating the complex landscape of FDA cleared vs approved. Our dedicated team includes a Pharmaceutical Compliance Specialist with over 15 years of industry experience, ensuring that AVS develops customized strategies that meet regulatory standards across biopharmaceuticals, medical instruments, and nutraceuticals. This tailored approach streamlines the product development lifecycle, significantly increasing the chances of achieving FDA cleared vs approved status.
Recent trends show that companies utilizing robust consulting services experience higher success rates in managing the complexities of FDA regulations, ultimately facilitating more efficient market entry. As the FDA evolves its procedures, placing greater emphasis on Real-World Evidence and patient-centric data, AVS remains at the forefront, equipping clients to adapt and thrive in this dynamic regulatory environment.
In a landscape where compliance challenges are ever-present, partnering with AVS Life Sciences not only mitigates risks but also enhances your organization’s ability to succeed in a competitive market. Engage with us today to explore how our expertise can empower your compliance journey.
Understanding FDA Clearance: Definition and Process
Understanding FDA Clearance: Definition and Process
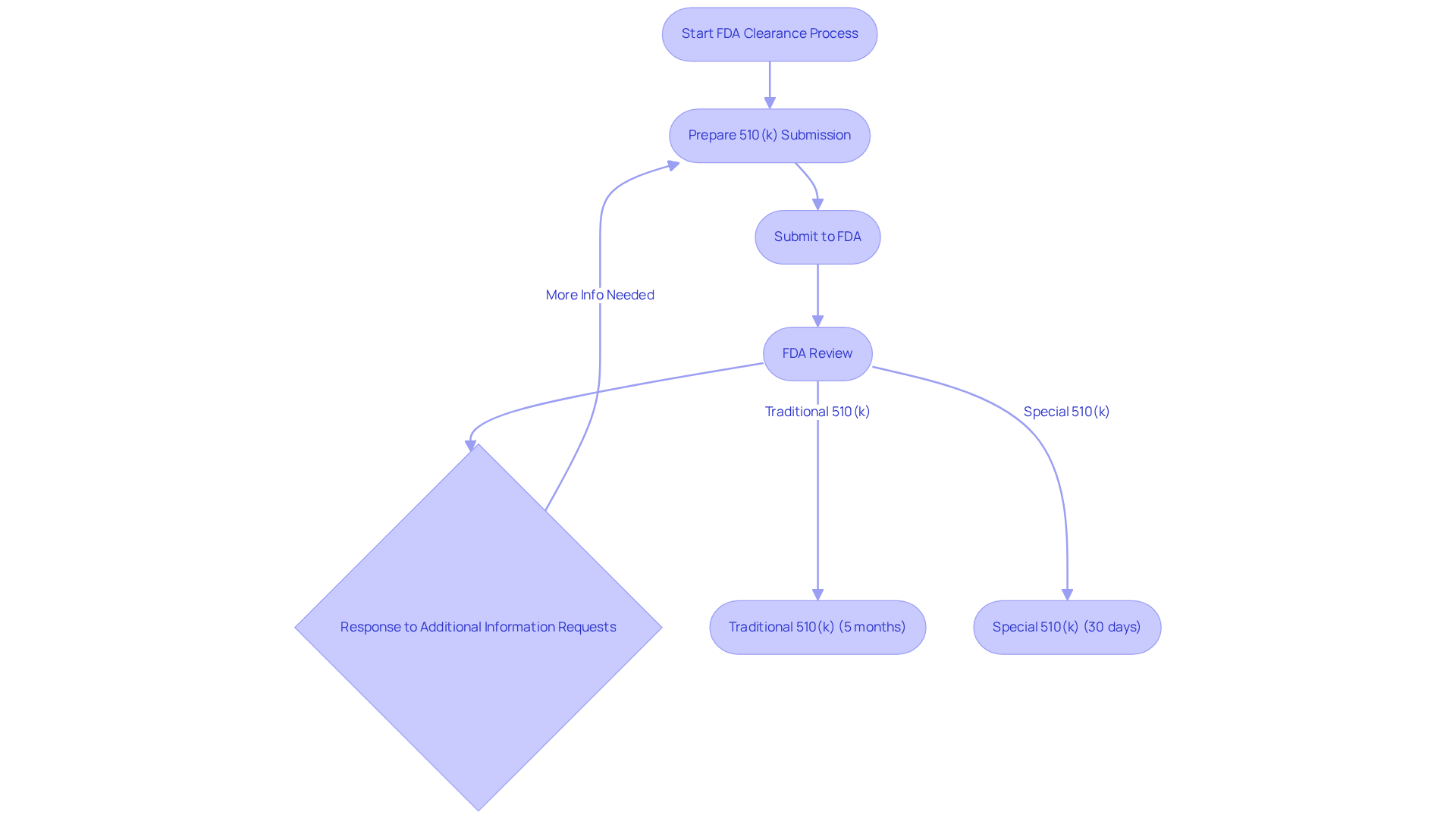
FDA clearance is the process through which a medical device is recognized as safe and effective for use, based on its substantial equivalence to a legally marketed device. The predominant pathway for clearance, specifically in the context of FDA cleared vs approved, is the 510(k) submission, which mandates that manufacturers demonstrate their product's safety and effectiveness relative to an existing one. This procedure typically involves providing comprehensive information regarding the design, intended application, and performance of the device, which the FDA scrutinizes to ensure compliance with regulatory standards.
At AVS Life Sciences, we grasp the complexities surrounding FDA approval and offer tailored consulting solutions to help navigate these challenges effectively. Our team of industry-leading professionals is prepared to assist you in crafting thorough submissions, ensuring compliance with regulatory requirements, and boosting your chances of successful clearance.
In recent years, the success rates of 510(k) submissions have exhibited variability. Statistics reveal that approximately 67% of submissions necessitated additional information requests during the initial review cycle. This underscores the critical importance of meticulous preparation and a comprehensive understanding of submission requirements. The typical evaluation duration for a Traditional 510(k) is about five months, while the Special 510(k) route—designed for modifications to existing devices—can expedite the timeline to an average of just 30 days.
Significant similarity is a pivotal concept in the 510(k) process, allowing manufacturers to compare their new product with a legally marketed one, thereby demonstrating adherence to safety and effectiveness standards, particularly when discussing FDA cleared vs approved. For example, if a new device employs established assessment methods, it may qualify for the Special 510(k) Program, which streamlines the review process.
Expert insights emphasize the necessity of a robust Quality Management System (QMS) to adeptly handle the complexities of the 510(k) submission. By adhering to best practices and leveraging available resources, manufacturers can enhance their probability of successful clearance, ultimately facilitating quicker market access for innovative medical products. Contact AVS Life Sciences today to discover how we can assist you on your FDA clearance journey.
Defining FDA Approval: Key Aspects and Implications
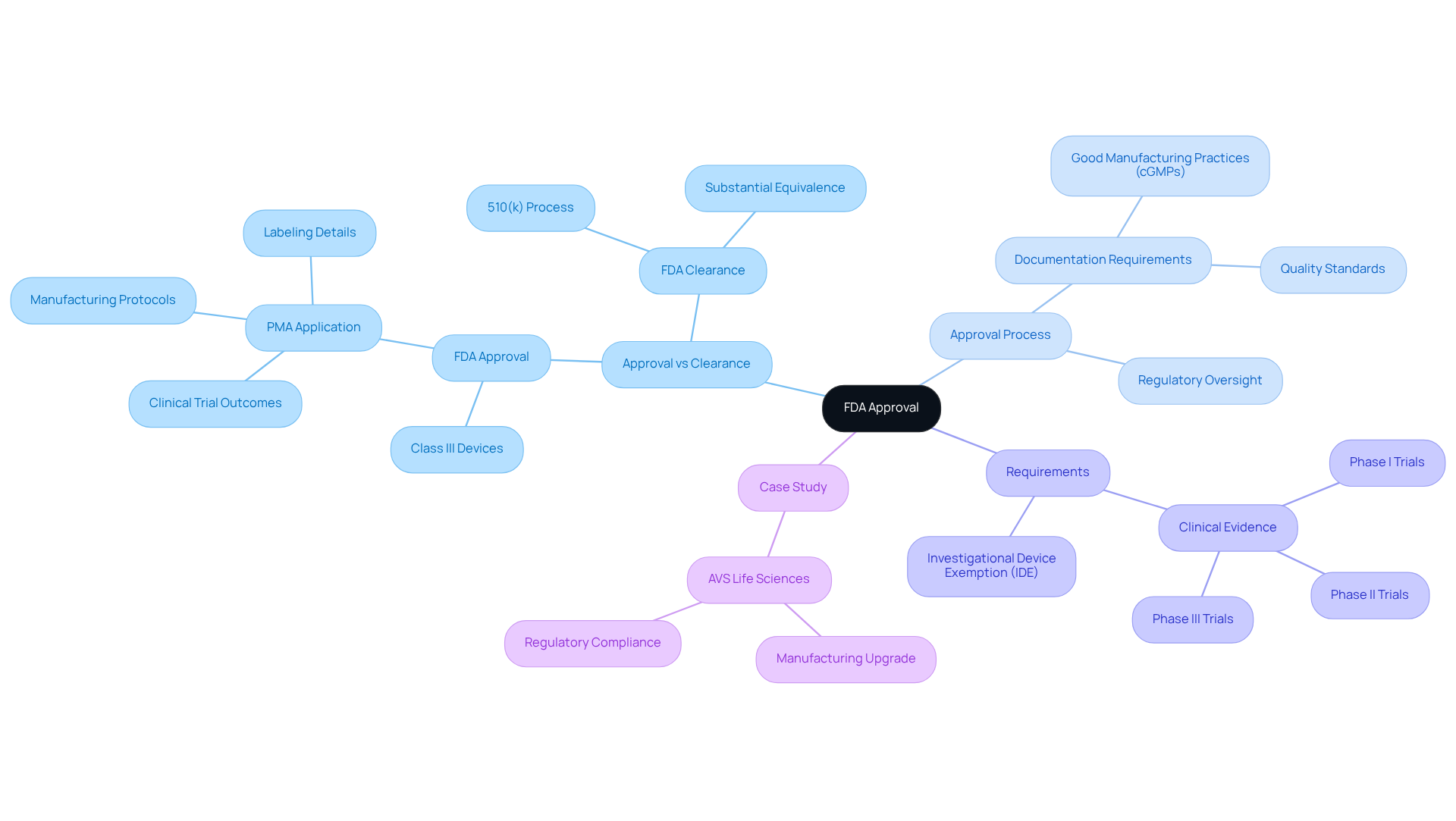
When discussing FDA cleared vs approved, it is important to note that FDA endorsement signifies a more stringent procedure than clearance, primarily relevant to Class III medical devices and new pharmaceuticals. This procedure necessitates a thorough evaluation of clinical data to confirm the product's safety and effectiveness for its intended use. Typically, the FDA certification process can span several months to years, demanding comprehensive documentation that includes:
- Clinical trial outcomes
- Manufacturing protocols
- Labeling details
The implications of obtaining FDA endorsement are profound; it allows the product to be marketed and sold in the U.S., while also subjecting it to continuous regulatory oversight. For instance, manufacturers must submit a Premarket Approval (PMA) application for Class III products, which can exceed 1,000 pages in length, detailing extensive clinical evidence. Furthermore, compliance with Good Manufacturing Practices (cGMPs) guarantees that drug products are consistently manufactured and regulated, underscoring the significance of quality standards in the approval procedure.
A transformative case study exemplifying this is AVS Life Sciences' collaboration with a leading biotechnology company in San Francisco. Here, AVS successfully upgraded their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade not only adhered to stringent quality assurance standards but also demonstrated AVS's commitment to regulatory compliance, ultimately allowing the client to focus on developing life-saving medicines.
In contrast, the distinction between FDA cleared vs approved is important, as FDA approval is frequently obtained via a Premarket Notification (510(k)) when an item is determined to be substantially equivalent to an already authorized product, significantly simplifying the procedure. Understanding these distinctions, along with the critical aspects of GXP, SOPs, and data integrity, is crucial for navigating the complexities of regulatory compliance in the life sciences sector.
Requirements for FDA Clearance: What You Need to Know
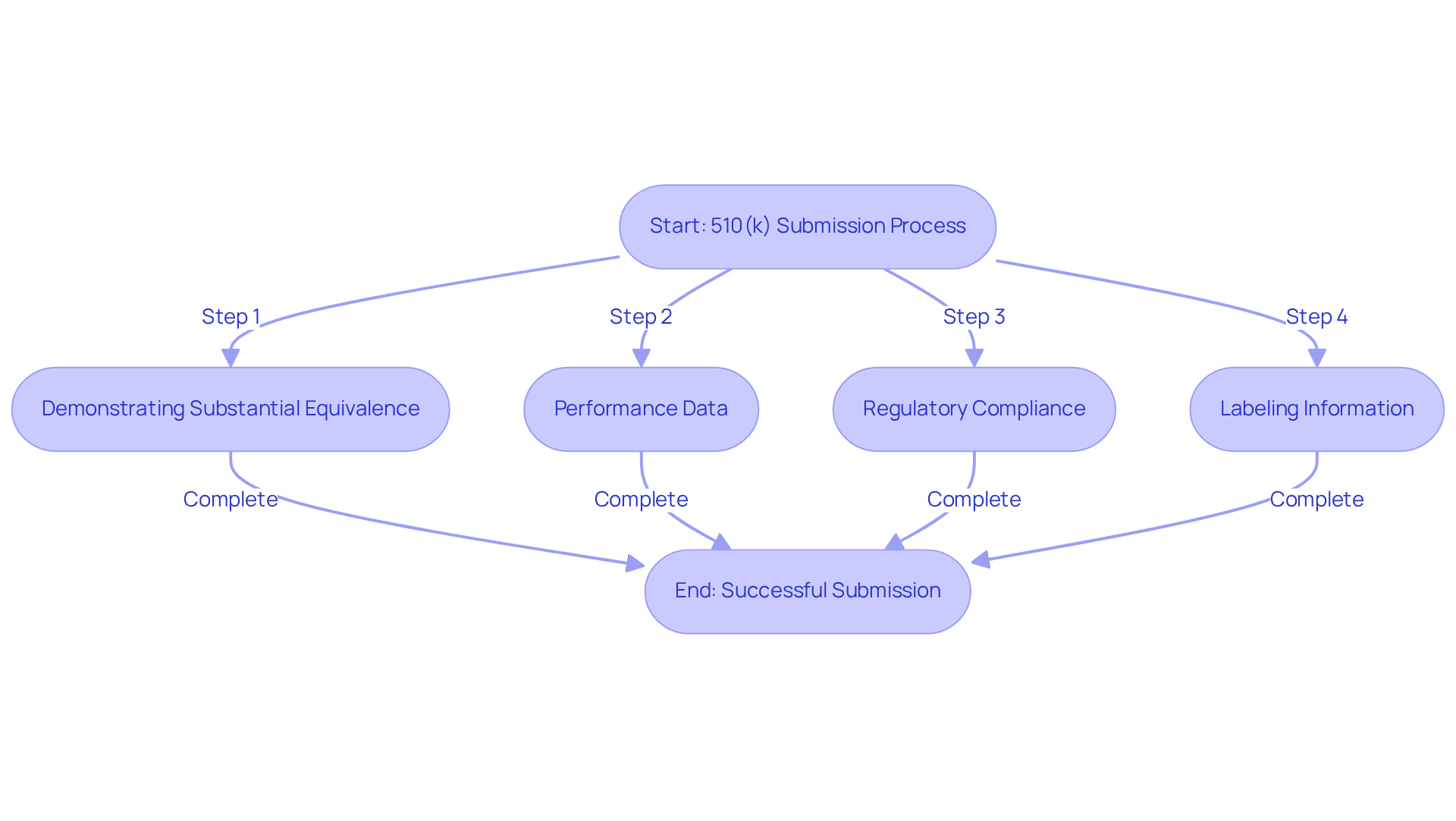
To understand the difference between FDA cleared vs approved, manufacturers must submit a 510(k) application that outlines comprehensive details about the product, including its intended use, technological characteristics, and performance data. The key requirements for this process include:
- Demonstrating Substantial Equivalence: Manufacturers must show that their device is substantially equivalent to a legally marketed predicate device. This involves a thorough comparison of intended use, technological characteristics, and performance regarding FDA cleared vs approved.
- Performance Data: Supplying information from bench tests is crucial, and in some instances, clinical studies may also be necessary to confirm the safety and effectiveness of the apparatus.
- Regulatory Compliance: Ensuring adherence to applicable FDA regulations and standards is critical throughout the development and submission phase.
- Labeling Information: Submissions must include labeling that meets FDA requirements, clearly outlining the device's intended use and instructions for use.
Manufacturers should also foresee potential inquiries from the FDA during the evaluation, which may necessitate additional information or clarification. In recent years, the number of successful 510(k) submissions has shown a steady increase, which reflects the industry's ability to navigate the complexities of FDA cleared vs approved requirements effectively. For example, in 2023, there were more than 4,000 successful submissions, highlighting the significance of careful preparation and comprehension of the clearance procedure.
To navigate the complexities of FDA clearance effectively, consider engaging with AVS Life Sciences for tailored consulting solutions in quality management and regulatory compliance. Our group of top-tier experts is prepared to help you ensure compliance and enhance your submission process.
Requirements for FDA Approval: A Comprehensive Overview
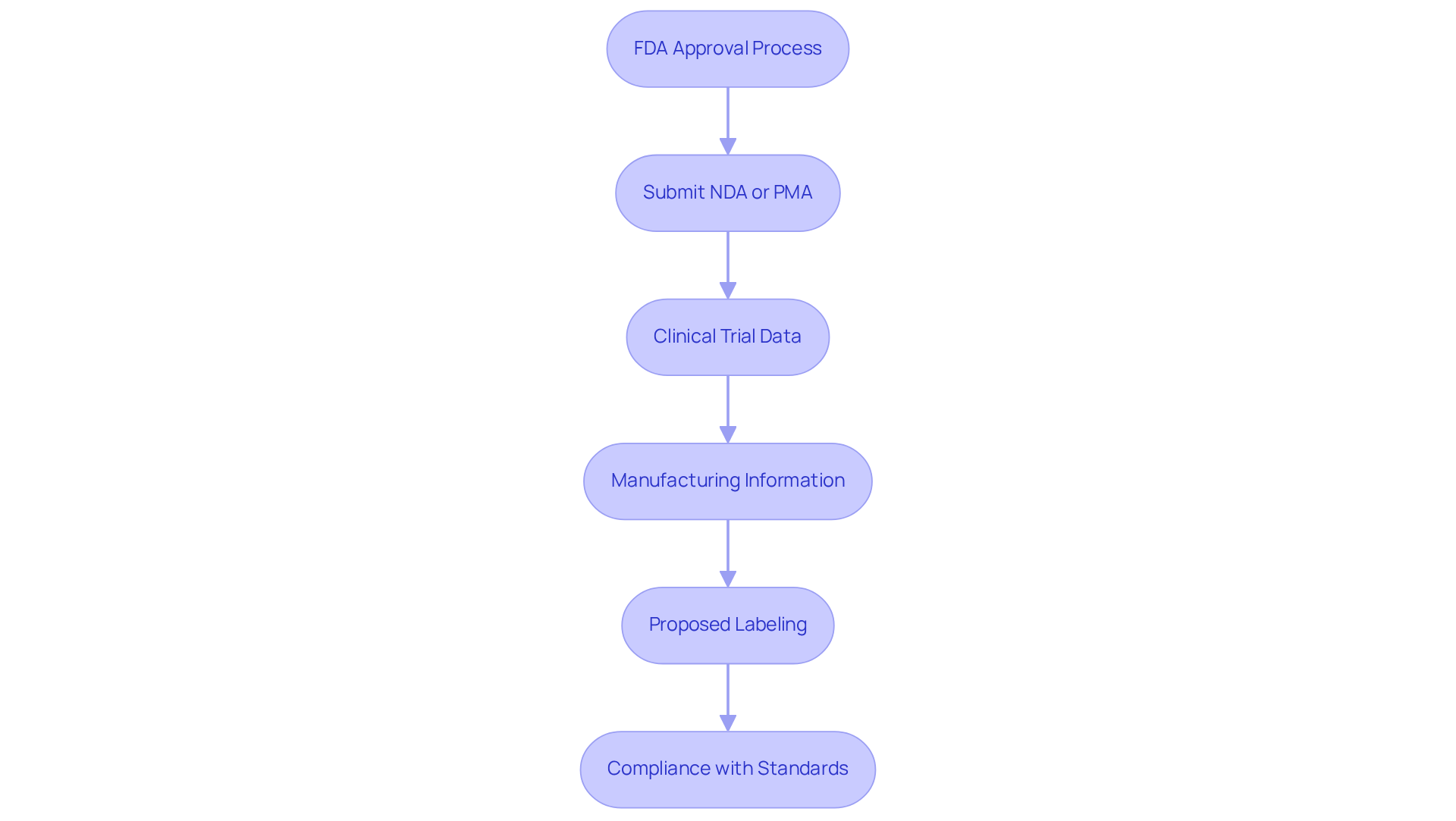
The FDA evaluation procedure is governed by stringent criteria that vary based on the product category, particularly in terms of FDA cleared vs approved, especially regarding whether a New Drug Application (NDA) or a Premarket Approval (PMA) is submitted. Key requirements include:
- Submission of an NDA or PMA application, tailored to the nature of the product.
- Comprehensive clinical trial data demonstrating both safety and efficacy, focusing on Level I or II evidence for most new Class III devices.
- Detailed manufacturing information, encompassing quality control measures to ensure compliance with Good Manufacturing Practices (GMP).
- Proposed labeling that accurately reflects the product's intended use and associated risks.
- Compliance with regulatory standards, including continuous communication with the FDA during the authorization phase.
The PMA method, in particular, is recognized for its rigor, often taking an average of 3 to 7 years to finalize, compared to the longer durations usually associated with drug authorizations, which can extend up to 12 years. Significantly, producers of Class III preamendments products must submit a PMA within 30 months following the final classification regulation announcement. Recent statistics indicate that only 18% of devices authorized conditionally by the FDA completed the necessary post-market studies within five years, underscoring the importance of comprehensive clinical trial data in the evaluation process. Furthermore, the FDA typically adds 3 to 6 months to the timeline for clinical studies, impacting overall schedules. Industry leaders stress that effective communication with the FDA, including early contact and collaboration through pre-submission meetings, is vital for navigating these complexities and understanding FDA cleared vs approved to achieve successful outcomes.
AVS Life Sciences offers expert consulting services to assist clients in navigating FDA authorization, ensuring adherence to regulatory standards and enhancing quality management practices. Our team is dedicated to providing thorough support throughout the authorization process, helping clients achieve positive results.
The Role of Clinical Evidence in FDA Approval
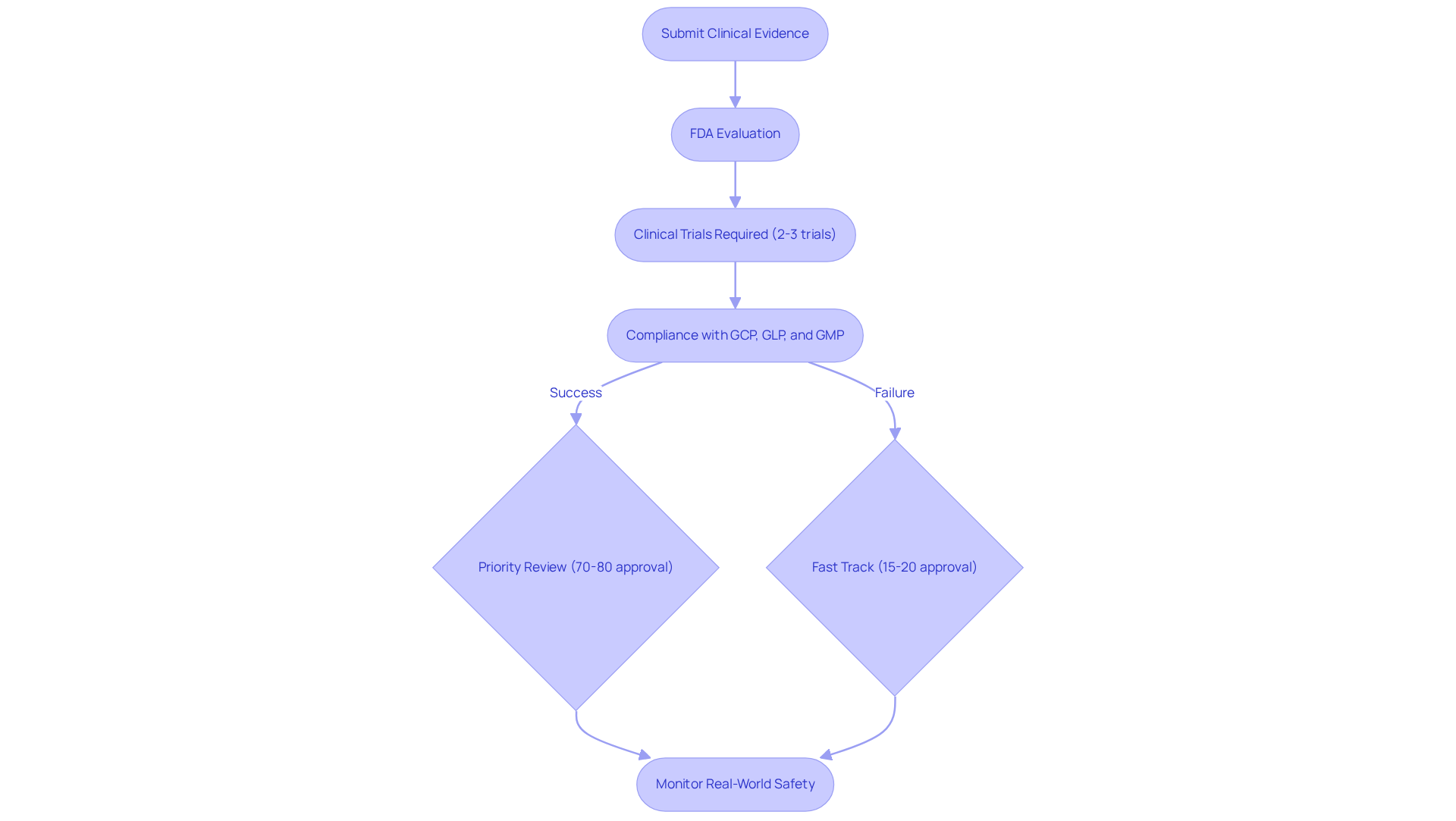
Clinical evidence plays a crucial role in the FDA evaluation process, particularly for new medications and high-risk medical devices. The FDA requires comprehensive data from well-designed clinical trials to assess the safety and efficacy of a product. This evidence must convincingly demonstrate that the benefits of the product outweigh its associated risks. The trials must adhere to strict protocols and comply with Good Clinical Practice (GCP) guidelines, ensuring the integrity and reliability of the data collected.
AVS Life Sciences emphasizes the significance of robust documentation practices and Standard Operating Procedures (SOPs) in maintaining compliance with FDA regulations, including GCP, GLP, and GMP, while also addressing Data Integrity Deviations. The quality and reliability of clinical evidence can significantly influence the timeline and result of the approval process. For instance, approximately 70%-80% of drugs that receive FDA Priority Review are approved, while Fast Track-designated drugs have a success rate of around 15%-20%. This highlights the varying success rates of different designations. Moreover, the typical number of clinical trials needed for FDA authorization usually varies from two to three, illustrating the stringent criteria established by the agency.
Recent updates to clinical trial requirements emphasize the need for robust pharmacovigilance programs to monitor real-world safety and encourage the reporting of side effects, ensuring ongoing compliance with regulatory expectations. As Russell Katz from the FDA states, 'A drug product must be found to be effective and safe before it may be approved for general marketing.' This underscores the importance of rigorous trial designs in the approval process.
AVS Life Sciences stands as a leading provider of quality management and regulatory compliance solutions, ensuring that clinical trials meet the highest standards of integrity and oversight. By engaging with AVS Life Sciences, organizations can navigate the complexities of compliance effectively and enhance their chances of successful FDA approvals.
Implications of FDA Clearance for Medical Devices
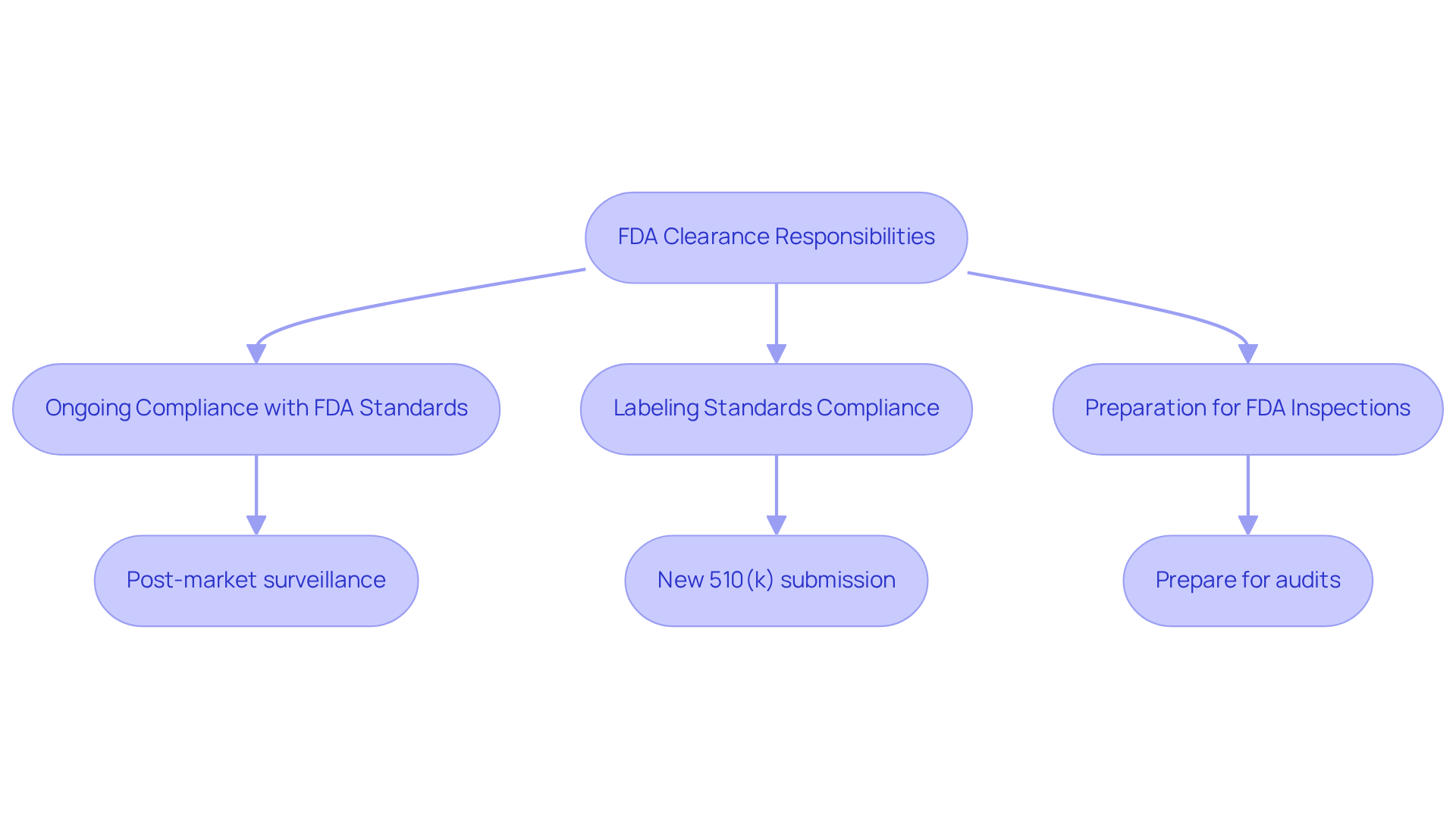
Understanding the difference between FDA cleared vs approved is a pivotal step for manufacturers aiming to market their medical devices in the U.S. However, it comes with several critical responsibilities:
- Manufacturers must ensure ongoing compliance with FDA standards and regulations, which includes rigorous post-market surveillance to monitor device performance and promptly report any adverse events.
- Compliance with labeling standards is crucial; any alterations to the apparatus may necessitate a new 510(k) submission to ensure adherence.
- Additionally, manufacturers should be prepared for potential FDA inspections and audits, which serve to verify adherence to regulatory standards.
Data indicates that FDA inspections of cleared devices are thorough, focusing on compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR). These inspections can lead to significant findings if manufacturers fail to meet established guidelines, underscoring the importance of maintaining high-quality standards throughout the product lifecycle.
For tailored consulting solutions in quality management and regulatory compliance, engage with AVS Life Sciences to ensure your organization meets all necessary standards effectively.
Implications of FDA Approval: Ensuring Product Safety and Efficacy
The distinction of FDA cleared vs approved signifies that a product has undergone rigorous evaluation, affirming its safety and efficacy for its intended use. The implications of this approval are significant:
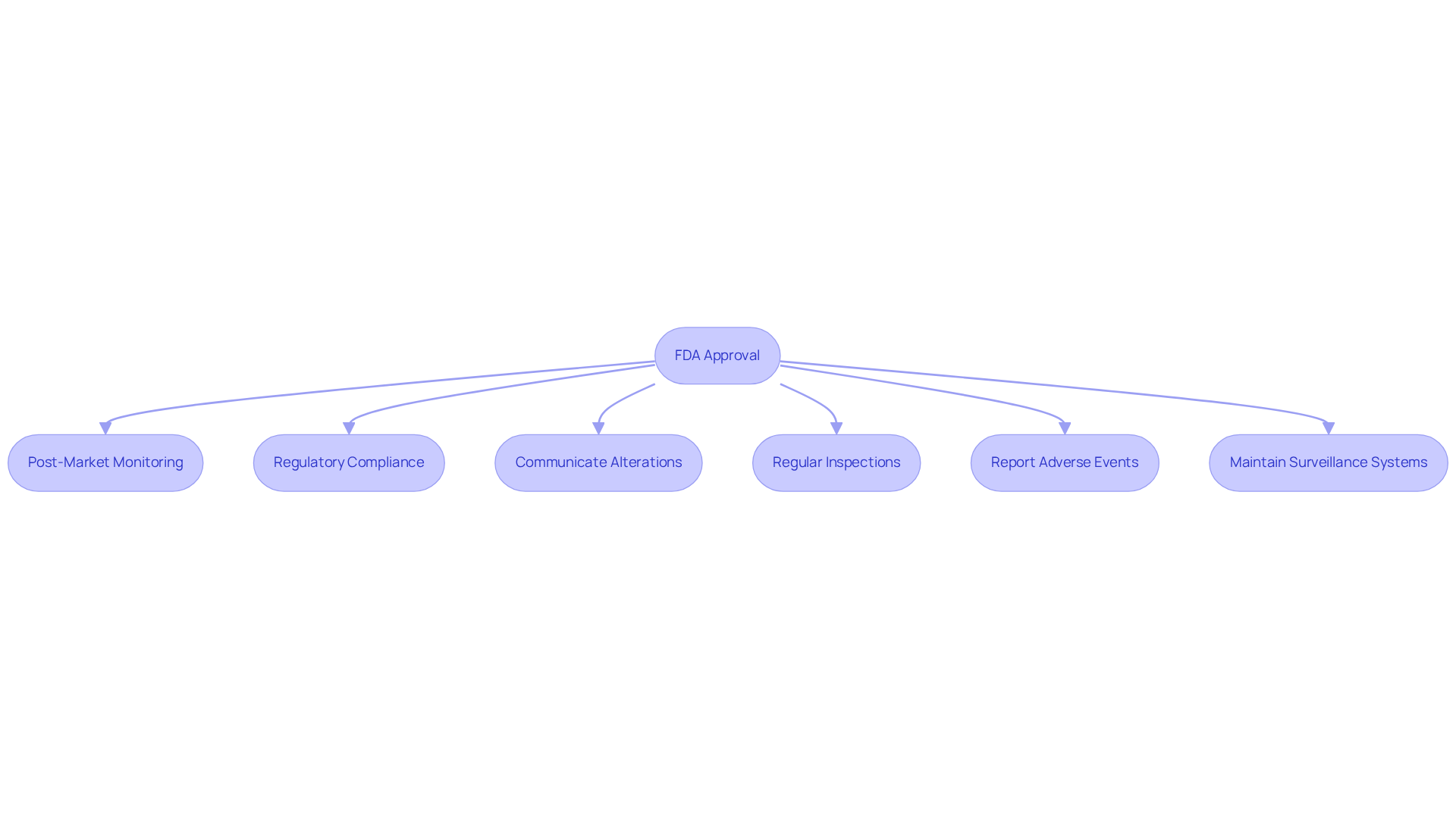
- Manufacturers are obligated to comply with stringent post-market monitoring and reporting requirements, as delineated in 21 CFR Part 822, which governs post-market surveillance activities.
- They bear the responsibility of ensuring that the product remains compliant with FDA regulations throughout its lifecycle, with nearly 60% of FDA citations between FY22 and FY24 associated with post-market surveillance issues.
- Any alterations to the product or its labeling must be communicated to the FDA, and in certain circumstances, this may necessitate a new authorization process.
- The FDA conducts regular inspections to ensure ongoing adherence to manufacturing and quality standards, with 2,439 inspections of medical equipment establishments recorded in 2024 alone.
- Compliance challenges frequently emerge post-fda cleared vs approved, particularly in sustaining effective post-market surveillance systems that can quickly identify and address potential safety issues, thus enabling manufacturers to effectively respond to user feedback and adverse events.
- Manufacturers are mandated to report any device-related adverse events to the FDA, including those that may have resulted in death or severe injury, within thirty days, underscoring the critical nature of timely reporting in maintaining regulatory compliance.
Common Misconceptions About FDA Clearance vs. Approval
Misunderstandings surrounding FDA cleared vs approved are prevalent in the medical equipment sector. A common misconception is the belief that FDA cleared vs approved are synonymous; however, FDA cleared usually involves a less rigorous process than approved. For instance, FDA approval is achieved through the 510(k) pathway, which requires manufacturers to demonstrate that their product is significantly comparable to an already marketed item. Conversely, FDA approval is essential for Class III products, necessitating a more comprehensive review process that includes clinical evidence of safety and effectiveness.
Another misconception is the assumption that all FDA-cleared products are inherently safe. While these devices meet specific standards, they still necessitate ongoing monitoring to ensure continued compliance with safety regulations. Furthermore, many individuals believe that FDA endorsement guarantees a product's effectiveness. Although endorsement indicates that an apparatus has been evaluated for safety and effectiveness based on available data, its actual performance in real-world scenarios may differ.
Lastly, there is a prevalent belief that once a product is cleared or approved, it is free from regulatory oversight. In reality, manufacturers are required to comply with FDA regulations throughout the product's lifecycle, including post-market surveillance and reporting any adverse events. Understanding these distinctions is crucial for navigating the complexities of FDA cleared vs approved regulations and ensuring compliance in the medical equipment market.
Key Differences Between FDA Clearance and Approval: A Summary
Understanding the key differences between FDA cleared vs approved is essential for stakeholders in the life sciences industry.
Regulatory Pathway: Clearance typically follows the 510(k) process, which allows manufacturers to demonstrate that a new device is substantially equivalent to an already marketed device. In contrast, approval involves a more rigorous Premarket Approval (PMA) or New Drug Application (NDA) process, requiring extensive clinical trial data.
Evidence Requirements: For clearance, manufacturers must provide evidence of substantial equivalence, while approval necessitates comprehensive clinical trial data to demonstrate safety and effectiveness. This distinction is crucial, particularly in the context of FDA cleared vs approved, as the evidentiary burden varies significantly depending on the classification of the equipment.
Risk Classification: Clearance is generally applicable to Class I and Class II products, regarded as low to moderate risk. Approval, however, is reserved for Class III equipment and new medications, which present higher risks and require more stringent evaluation.
Post-Market Obligations: Both clearance and approval require ongoing compliance with FDA regulations, but the level of scrutiny and reporting obligations can differ. Class III devices, for instance, face more rigorous post-market surveillance compared to Class I and II devices.
Understanding the differences of FDA cleared vs approved is vital for manufacturers to navigate the complexities of regulatory pathways effectively. Misinterpreting terminology can result in substantial regulatory challenges, as emphasized by industry leaders who underline the necessity of clarity in communication about FDA procedures. This knowledge not only aids in compliance but also enhances the ability to manage product development and market strategies effectively.
AVS Life Sciences, with its 80% repeat business rate, exemplifies credibility and expertise in navigating these regulatory pathways. Engaging with AVS Life Sciences can provide tailored consulting solutions that enhance understanding and compliance with FDA processes.
Conclusion
Understanding the differences between FDA cleared and approved products is crucial for stakeholders in the life sciences sector. The nuances of these terms highlight the varying levels of scrutiny and regulatory requirements that medical devices and pharmaceuticals must navigate. Clarity in these distinctions not only aids in compliance but also enhances strategic decision-making throughout the product development lifecycle.
This article has detailed the key distinctions between FDA clearance and approval, emphasizing the regulatory pathways, evidence requirements, risk classifications, and post-market obligations associated with each. It has explored how FDA clearance typically involves a less rigorous process compared to the comprehensive evaluations required for FDA approval, particularly for Class III devices and new drugs. Furthermore, the importance of ongoing compliance and the implications of these processes on product safety and market access have been underscored.
Ultimately, engaging with expert consulting services, such as those provided by AVS Life Sciences, can significantly streamline the journey through the complexities of FDA regulations. By leveraging specialized knowledge and resources, manufacturers can enhance their understanding of FDA processes, mitigate compliance risks, and ultimately ensure that their products meet the highest standards of safety and efficacy in the marketplace.
Frequently Asked Questions
What services does AVS Life Sciences provide?
AVS Life Sciences offers expert consulting services to help clients navigate the FDA clearance and approval processes, focusing on biopharmaceuticals, medical instruments, and nutraceuticals.
What is the difference between FDA clearance and FDA approval?
FDA clearance is a process that recognizes a medical device as safe and effective based on its substantial equivalence to a legally marketed device, typically through a 510(k) submission. In contrast, FDA approval involves a more stringent evaluation of clinical data, primarily for Class III medical devices and new pharmaceuticals.
What is the 510(k) submission process?
The 510(k) submission process requires manufacturers to demonstrate their product's safety and effectiveness compared to an existing legally marketed device. This involves providing detailed information about the device's design, intended use, and performance.
How long does the evaluation process for a 510(k) submission typically take?
The typical evaluation duration for a Traditional 510(k) submission is about five months, while the Special 510(k) route, designed for modifications to existing devices, can reduce the timeline to an average of 30 days.
What are the implications of obtaining FDA approval?
Obtaining FDA approval allows a product to be marketed and sold in the U.S. and subjects it to continuous regulatory oversight. This process requires extensive documentation, including clinical trial outcomes and manufacturing protocols.
What is the significance of having a Quality Management System (QMS) in the 510(k) submission process?
A robust Quality Management System (QMS) is essential for managing the complexities of the 510(k) submission process, as it helps ensure compliance with regulatory requirements and enhances the chances of successful clearance.
Can you provide an example of AVS Life Sciences' impact on a client's regulatory compliance?
AVS Life Sciences collaborated with a biotechnology company to upgrade their manufacturing facility from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, ensuring adherence to quality assurance standards and allowing the client to focus on developing life-saving medicines.
How does AVS Life Sciences stay current with FDA regulatory changes?
AVS Life Sciences stays at the forefront of FDA regulatory changes by emphasizing Real-World Evidence and patient-centric data, equipping clients to adapt and thrive in a dynamic regulatory environment.
