7 Key Differences Between FDA Approved vs FDA Cleared

Overview
This article delineates the critical distinctions between FDA approved and FDA cleared products, emphasizing the unique processes and their implications for product safety and market access.
FDA approval entails a comprehensive evaluation of safety and efficacy, particularly for high-risk devices, which underscores the rigorous standards set by regulatory authorities.
Conversely, FDA clearance facilitates expedited market entry by demonstrating substantial equivalence to existing products.
This distinction is vital for stakeholders in the life sciences sector, as understanding these differences is essential for navigating compliance challenges and ensuring successful market strategies.
Introduction
Understanding the nuances between FDA approval and FDA clearance is crucial for stakeholders in the life sciences industry. These two pathways dictate the regulatory landscape and significantly influence product development timelines and market strategies. As companies navigate the complexities of compliance, a pressing question arises: how do these distinctions impact the safety and efficacy of products reaching consumers? This article delves into the key differences between FDA approved and FDA cleared, offering insights that empower professionals to make informed decisions in a rapidly evolving regulatory environment.
AVS Life Sciences: Expert Guidance on FDA Approval and Clearance Processes
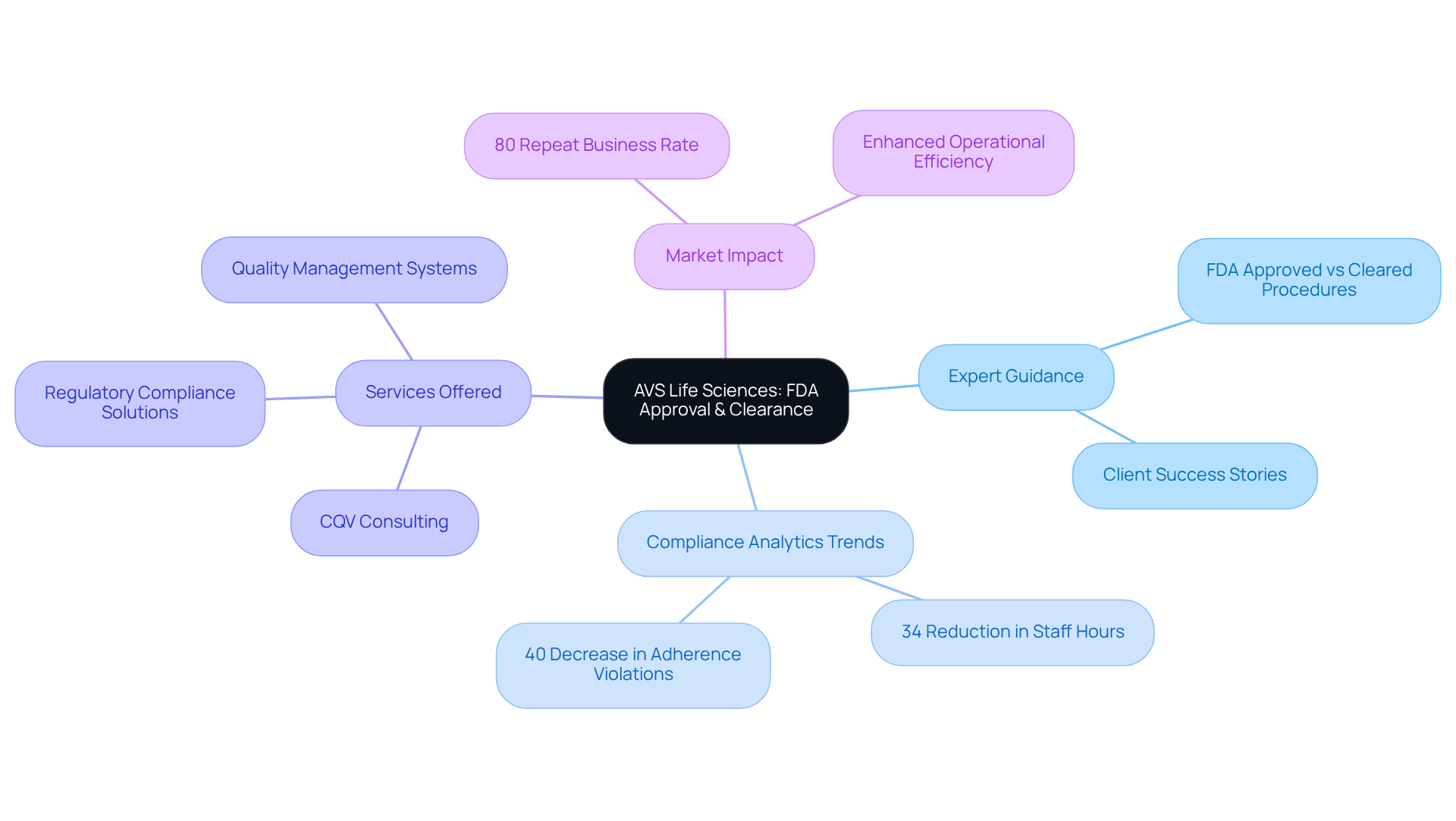
AVS Life Sciences offers unparalleled support in navigating the differences between FDA approved vs FDA cleared procedures, ensuring clients are well-informed and prepared to meet compliance requirements. As a dedicated partner providing comprehensive oversight, CQV, and quality solutions across biopharmaceuticals, medical devices, in-vitro diagnostics, over-the-counter products, cosmetics, and food & nutraceuticals, AVS delivers tailored strategies that align with the unique needs of . This enables a smoother journey through the complexities of FDA guidelines.
Recent trends reveal that organizations utilizing compliance analytics solutions have achieved:
- A 34% reduction in staff hours spent on reporting tasks
- A 40% decrease in adherence violations
This highlights the critical role of expert guidance in sustaining compliance. Furthermore, the evolving FDA landscape in 2025, which will include updates to oversight frameworks, necessitates a proactive approach to compliance challenges, making AVS's extensive consulting services indispensable.
As Mark Toland, Chairman of the Board of AVS, noted, the IDE approval for the Pulse IVL™ System marks a significant clinical milestone, demonstrating the company's commitment to excellence in navigating the complexities of FDA approved vs FDA cleared processes. This dedication not only boosts operational efficiency but also positions clients for success in a competitive market, as demonstrated by AVS's remarkable 80% repeat business rate.
To capitalize on these insights, pharmaceutical compliance officers should integrate AVS's services into their regulatory strategies.
Understanding FDA Approval vs. FDA Clearance: Core Definitions
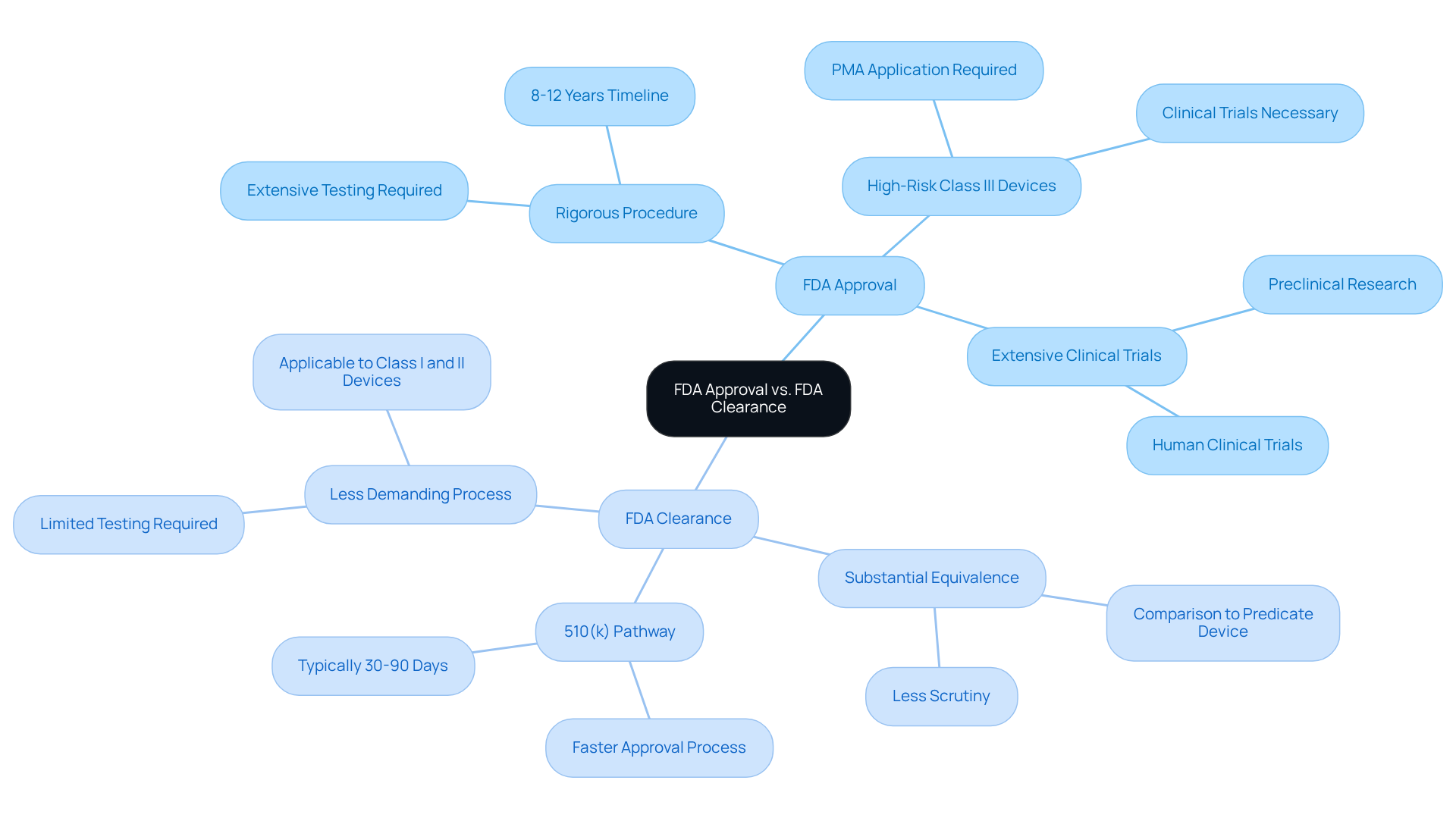
FDA approval represents a rigorous procedure through which the FDA evaluates and authorizes new drugs or medical devices for market entry, grounded in comprehensive evidence that demonstrates their safety and effectiveness. This procedure is particularly stringent for high-risk Class III medical devices, which necessitate extensive clinical trials and documentation. In contrast, FDA clearance permits the marketing of devices deemed substantially equivalent to existing, legally marketed devices, typically under the 510(k) pathway. This pathway is less demanding, often requiring only proof of equivalence rather than extensive testing. Notably, approximately half of all medical devices in the U.S. are approved through the 510(k) pathway, a process that can span from a few months to several years.
Understanding these definitions is crucial for stakeholders in the life sciences sector, as the implications of FDA approved vs FDA cleared can significantly influence product development timelines and market strategies. While products that are FDA approved undergo rigorous testing, the distinction between indicates that FDA-cleared devices may not face the same level of scrutiny, potentially leading to safety concerns. As highlighted by industry experts, the distinction between FDA approved vs FDA cleared processes is paramount, particularly since FDA clearance does not guarantee safety; some products that are cleared have been associated with significant health issues.
AVS Life Sciences plays a pivotal role in assisting stakeholders as they navigate these complex compliance landscapes. Through comprehensive quality management and regulatory adherence solutions, AVS Life Sciences ensures that products meet FDA standards, thereby enhancing safety and efficacy. This understanding empowers stakeholders to effectively manage their compliance strategies and adeptly navigate the intricacies of FDA regulations.
Navigating the FDA Approval Process: Key Pathways and Requirements
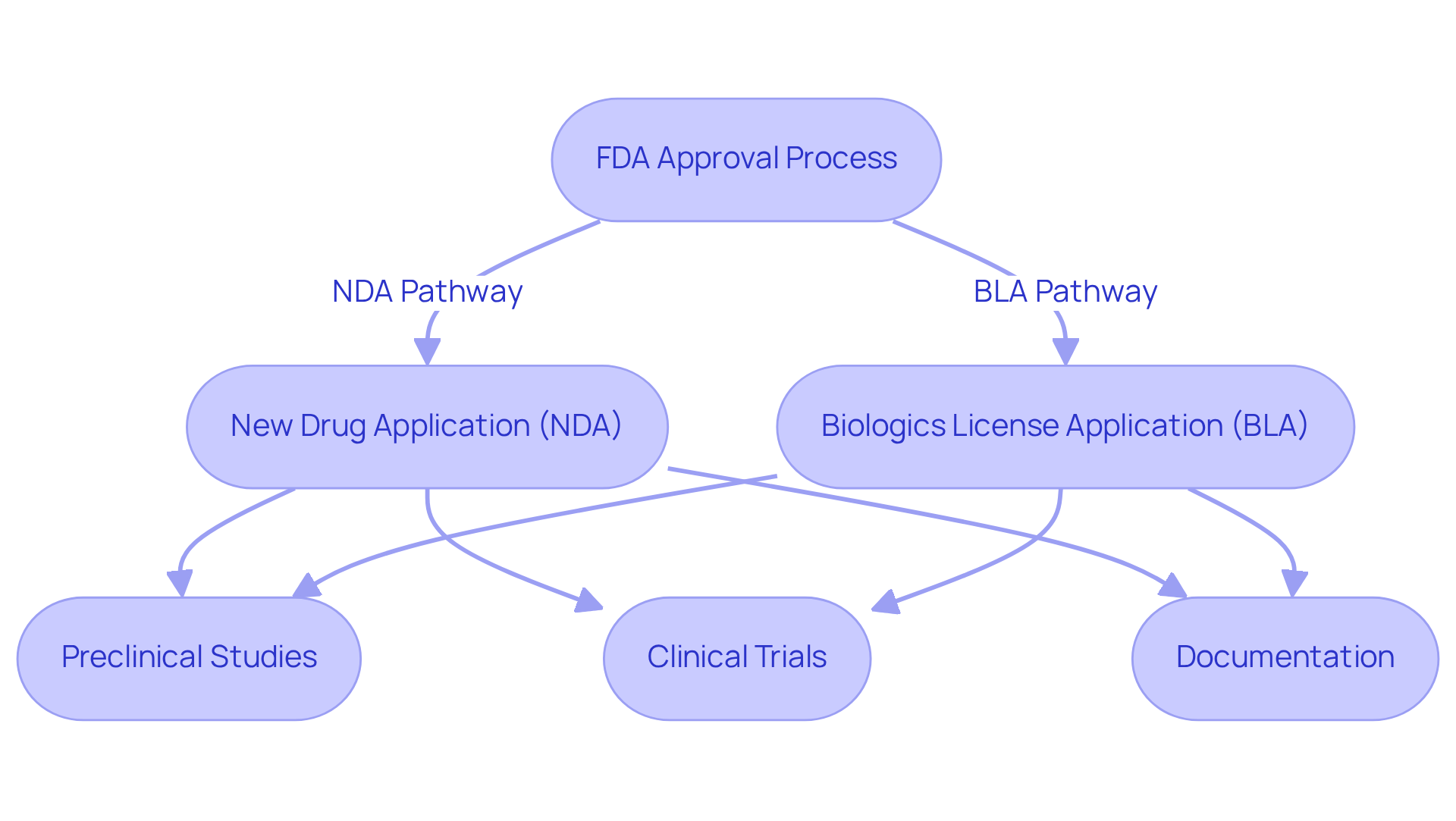
The FDA approval procedure encompasses several essential pathways, especially when comparing FDA approved vs FDA cleared options, such as the New Drug Application (NDA) for pharmaceuticals and the Biologics License Application (BLA) for biologics. Each pathway, including FDA approved vs FDA cleared, imposes specific requirements, such as preclinical studies, clinical trials, and extensive documentation. An NDA, for example, must demonstrate the drug's safety and efficacy, supported by data from clinical trials, whereas a BLA emphasizes the quality and safety of biologics, which can be understood in the context of FDA approved vs FDA cleared. Recent updates to NDA requirements underscore the importance of robust evidence to substantiate claims of safety and effectiveness, highlighting the differences between FDA approved vs FDA cleared and reflecting the FDA's unwavering commitment to patient safety.
Successful NDA case studies exemplify the potential for , especially when discussing FDA approved vs FDA cleared products, as priority review items are granted a six-month timeline for approval, significantly compressing the standard review period. Conversely, the BLA method has exhibited a success rate that underscores the importance of thorough preparation and adherence to regulatory standards, particularly in the context of FDA approved vs FDA cleared. Regulatory consultants play a pivotal role in navigating these pathways, often emphasizing that effective communication with the FDA is critical for understanding FDA approved vs FDA cleared in order to achieve timely approvals. They assist in compiling and formatting submissions in the required eCTD format, ensuring all documentation aligns with the differences between FDA approved vs FDA cleared expectations. This strategic approach not only supports compliance but also enhances the likelihood of favorable outcomes in the competitive landscape of drug development, especially in the context of FDA approved vs FDA cleared.
At AVS Life Sciences, we provide tailored consulting solutions designed to help clients navigate the complexities of the processes associated with FDA approved vs FDA cleared. Our team of top professionals is dedicated to delivering the assistance you need in quality management and compliance, particularly in understanding the differences between FDA approved vs FDA cleared. Contact us today to discover how we can support you in achieving your regulatory goals.
Exploring the FDA Clearance Process: What You Need to Know
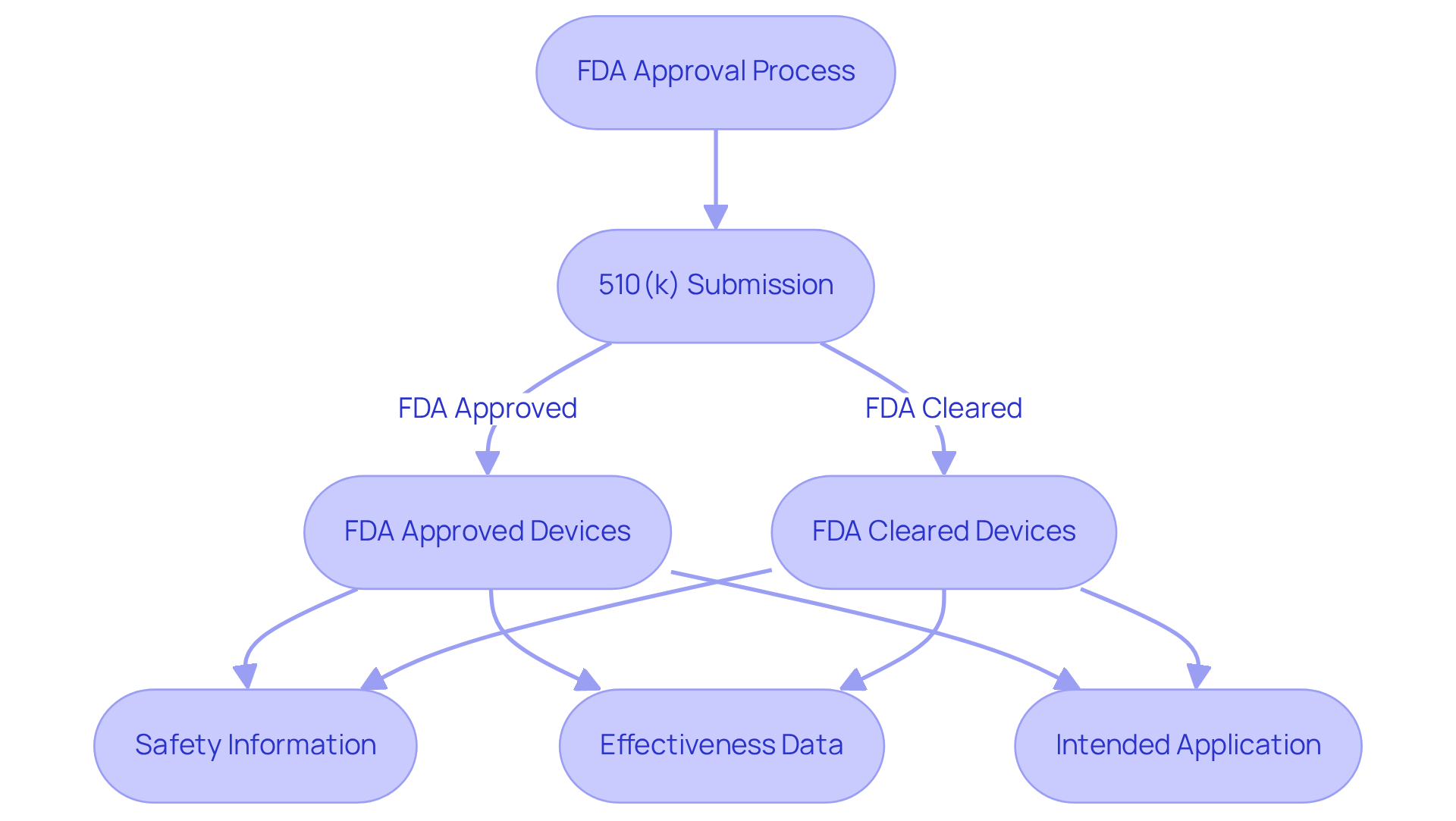
The FDA approval procedure primarily involves the 510(k) submission, which illustrates the distinction between FDA approved vs FDA cleared devices by demonstrating that a new device is significantly comparable to an existing, legally marketed device. This procedure requires the submission of , alongside comprehensive descriptions of its intended application. Recognizing these requirements is crucial for successful market entry.
In navigating the complexities of FDA compliance, organizations must adopt a strategic approach to ensure adherence to regulatory standards, ultimately facilitating a smoother path to market.
Consumer Implications of FDA Approval and Clearance: What It Means for You
The distinction between FDA approved vs FDA cleared carries substantial implications for consumers, ensuring that products adhere to rigorous safety and efficacy standards. The distinction between FDA approved vs FDA cleared drugs and devices is that both undergo comprehensive evaluations, instilling confidence in their use.
At AVS Life Sciences, we understand the critical nature of these processes and offer extensive quality management and regulatory adherence solutions that facilitate navigation through the complexities of FDA regulations. By grasping these implications, consumers are empowered to make informed decisions regarding the products they utilize, backed by AVS Life Sciences' .
Products Requiring FDA Approval vs. Clearance: A Comparative Overview
When discussing , it is important to note that the former generally encompasses new pharmaceuticals, biologics, and high-risk medical devices classified as Class III. These products necessitate extensive clinical data to validate safety and efficacy. Conversely, lower-risk medical devices, typically classified as Class I or II, may only need to be considered under the distinction of FDA approved vs FDA cleared via the 510(k) pathway, which assesses substantial equivalence to existing products.
The FDA usually determines substantial equivalence within 90 days of the 510(k) submission, providing a more defined timeline for the clearance process when looking at the differences between FDA approved vs FDA cleared, as the approval journey for drugs can span 12 to 15 years. Regulatory specialists emphasize that understanding these classifications is vital for navigating the complex regulatory landscape and ensuring compliance with safety standards.
The FDA's classification system categorizes devices by risk, with Class III devices subjected to the most rigorous requirements. This knowledge not only facilitates product development but also aids in aligning strategies for successful market entry.
As Dr. Sanket S. Dhruva highlighted, many physicians and patients misconstrue the implications of FDA approval, underscoring the necessity for enhanced communication regarding the approval process. Furthermore, concerns about expedited pathways and their potential impact on patient safety further highlight the importance of comprehending the distinctions between FDA approved vs FDA cleared and compliance.
Debunking Myths: Common Misunderstandings About FDA Approval and Clearance
Widespread fallacies concerning FDA approved vs FDA cleared frequently lead to misunderstandings about product safety and the stringency of oversight. A common misconception is that all items that are FDA approved are inherently safe; however, the reality is that the differences between FDA approved vs FDA cleared procedures involve extensive assessments to ensure safety and efficacy.
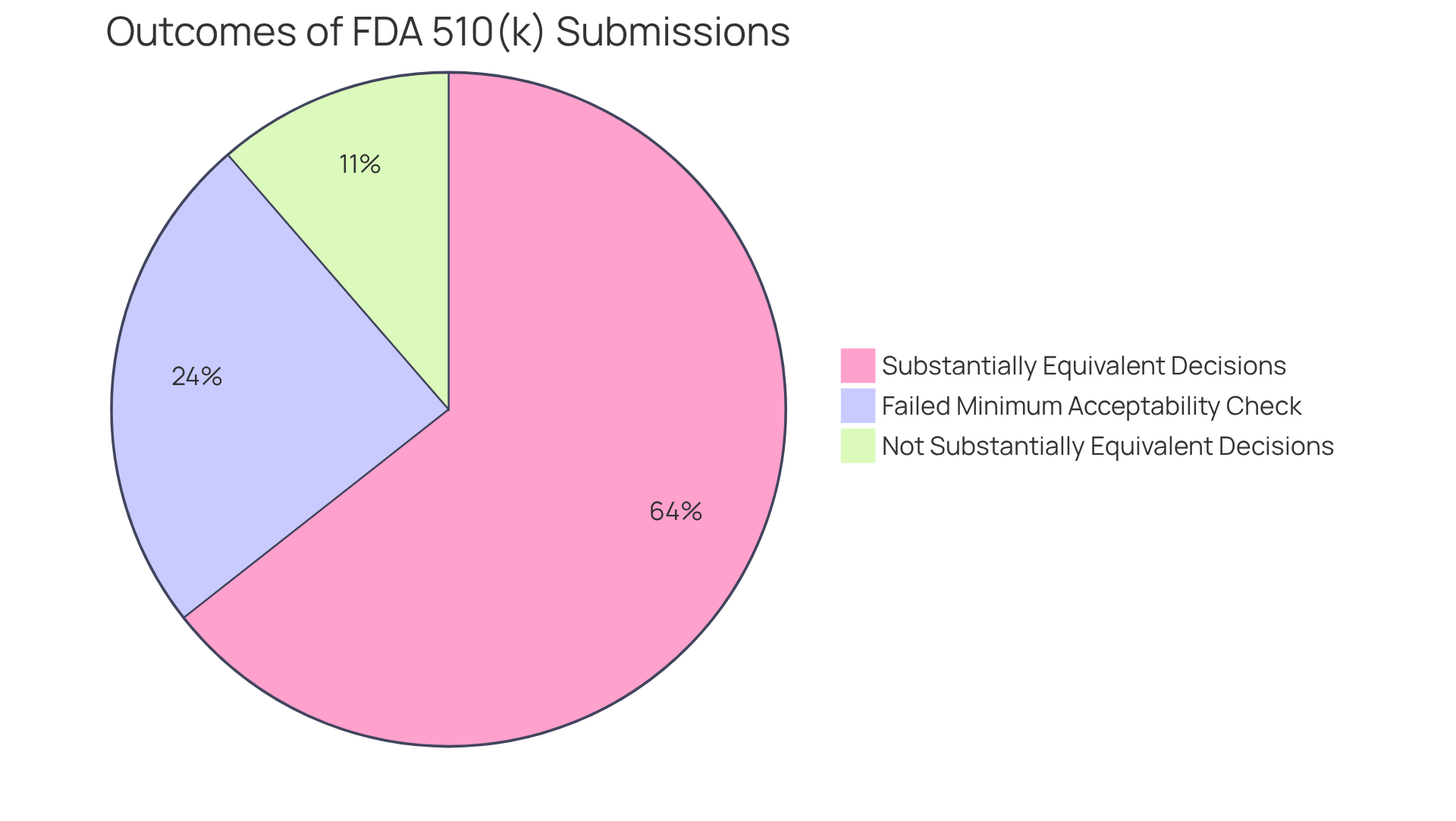
For instance, while 85 percent of FDA 510(k) applications received a Substantially Equivalent decision, 15 percent did not, underscoring the challenges in meeting regulatory standards. Additionally, the typical duration for a 510(k) decision is around five months, indicating a systematic review rather than a mere formality.
It is also a misconception that the processes of FDA approved vs FDA cleared indicate that clearance is a less demanding procedure; in fact, both pathways require continuous safety evaluations throughout the product lifecycle. Regulatory professionals, such as those at AVS Life Sciences, emphasize the importance of to foster a realistic perspective on FDA regulations.
AVS Life Sciences provides expert guidance to help stakeholders navigate these complexities, ensuring informed decision-making in the life sciences sector. Addressing these myths is essential for stakeholders to navigate the intricacies of compliance and ensure informed decision-making in the life sciences sector. Furthermore, it is noteworthy that nearly 32 percent of FDA 510(k) submissions failed the minimum acceptability check, further illustrating the complexities involved in the submission process.
The Role of FDA Regulations in Medical Device Approval and Clearance
FDA regulations play a crucial role in determining the differences between FDA approved vs FDA cleared medical devices, establishing rigorous standards for safety, effectiveness, and quality. Compliance with these regulations is not merely a formality; it is essential for manufacturers seeking market access and striving to maintain consumer trust. The compliance environment is complex, making it imperative for successful product development to grasp these regulations.
Recent developments underscore the evolving nature of FDA regulations. For instance, the agency's recent regulatory freeze has sparked concerns regarding its implications for medical device standards and patient safety. Such changes can significantly influence how devices are developed and introduced to the market, as manufacturers must adapt to new requirements and ensure adherence to Good Manufacturing Practices (GMP) and Quality System Regulations (QSR).
Case studies vividly illustrate the real-world effects of these regulations. For example, AVS Life Sciences adeptly assisted a pharmaceutical manufacturer in upgrading their facility from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project involved:
- Performing a gap analysis to identify discrepancies
- Acquiring new equipment
- Executing comprehensive validation procedures (IQ/OQ/PQ) to ensure compliance
This transformative facility upgrade was completed on time and within budget, showcasing AVS's proficiency in managing intricate compliance requirements. Similarly, Cohera Medical, Inc. faced significant funding challenges after its second product line failed to obtain FDA approval, emphasizing the high stakes involved in navigating regulatory requirements. The difficulties encountered by LifeHealth, LLC in further highlight the critical need for compliance-driven strategies in product development.
Moreover, the FDA's emphasis on safety and effectiveness is evident in its stringent standards, which mandate thorough documentation and validation processes. Companies like AVS Life Sciences are pivotal in assisting clients to meet these standards, offering tailored consulting expertise that enhances compliance and accelerates market readiness. With an impressive 80% repeat business rate, AVS Life Sciences exemplifies the value of strategic compliance guidance within the life sciences sector.
In summary, the impact of FDA regulations on medical device approval and clearance, especially regarding the differences between FDA approved vs FDA cleared, is profound, influencing market access, product development, and ultimately, patient safety. Staying informed about the latest compliance changes and understanding their implications is crucial for manufacturers aiming to thrive in this highly regulated sector.
Ensuring Compliance with FDA Standards: Best Practices for Life Sciences
To ensure adherence to FDA standards, organizations must adopt a multifaceted approach that includes regular audits, meticulous documentation, and continuous staff training. Creating a culture of adherence is essential for fulfilling standards and reducing risks. A transformative case study exemplifying this is AVS Life Sciences' successful upgrade of a biotechnology GMP facility. We assisted a leading San Francisco-based biotechnology company in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, showcasing the significance of detailed documentation and quality assurance in meeting regulatory standards.
During the upgrade, we encountered challenges, such as issues with barcode scanner cameras being installed upside down, which led to anomalies in test results. This oversight stemmed from the barcode scanner returning sets of values that could yield identical results, highlighting the need for sufficient testing to challenge the system. The lessons learned from this experience prompted the QC laboratory team and Quality team to evaluate their business processes, identifying gaps that allowed for unreliable test results. This collaboration empowered our client to concentrate on what they do best—developing medicines to enhance their target patient’s quality of life.
Furthermore, the frequency of audits plays a crucial role in achieving regulatory success. Entities that perform regular internal audits are better equipped to recognize and address regulatory gaps before they escalate into major problems. The FDA's recent guidance on Remote Regulatory Assessments (RRAs) underscores the necessity for companies to adapt to new oversight methods, which may involve digital tools for evaluation. By embracing these changes and ensuring that documentation is thorough and current, organizations can enhance their preparedness for both routine and unforeseen assessments.
Integrating input from regulatory officers emphasizes the effectiveness of these strategies. Many highlight that cultivating a setting where adherence is prioritized results in enhanced outcomes during inspections and official interactions. The insights gained from the AVS case study, particularly concerning the assessment of business processes and the , further demonstrate how open conversations about responsibilities can bolster adherence efforts. As the landscape continues to evolve, staying updated on FDA policies and maintaining adaptable strategies will be crucial for success in the life sciences sector.
Staying Informed: The Importance of Understanding FDA Regulations for Professionals
For experts in the life sciences sector, staying abreast of FDA regulations is essential for ensuring compliance and adapting to changes in the regulatory landscape. Continuous education, such as participating in webinars like the FDA's Data Integrity Guidance, offers invaluable insights into the implications of data integrity for business decision-making and compliance. Furthermore, engaging in industry conferences and empowers professionals to remain informed and effective in their roles, thereby reinforcing their commitment to excellence in compliance.
Conclusion
Understanding the distinctions between FDA approved and FDA cleared products is essential for stakeholders in the life sciences sector. FDA approval signifies a comprehensive evaluation of safety and efficacy, whereas FDA clearance permits the marketing of devices deemed substantially equivalent to existing products. This difference is crucial not only for manufacturers navigating the regulatory landscape but also for consumers relying on these products for health and safety.
The rigorous requirements of the FDA approval process, including extensive clinical trials for high-risk devices, contrast sharply with the relatively streamlined 510(k) clearance pathway. The implications for product development timelines and market strategies are significant. Organizations must grasp these processes to ensure compliance and maintain consumer trust. Moreover, the importance of expert guidance, such as that provided by AVS Life Sciences, cannot be overstated. Such expertise helps companies navigate these complexities and enhance their operational efficiency.
In light of evolving FDA regulations and the increasing importance of compliance, stakeholders are urged to actively engage with resources and expertise that support their understanding and adherence to these standards. By doing so, they not only safeguard their products but also contribute to a healthcare environment where safety and efficacy remain paramount. Embracing a proactive approach to FDA regulations is not merely advisable; it is essential for thriving in the competitive landscape of the life sciences industry.
Frequently Asked Questions
What is the difference between FDA approval and FDA clearance?
FDA approval involves a rigorous evaluation process where the FDA assesses new drugs or medical devices for safety and effectiveness, particularly for high-risk Class III devices that require extensive clinical trials. In contrast, FDA clearance allows marketing of devices that are substantially equivalent to existing ones, typically through the less demanding 510(k) pathway, which often requires only proof of equivalence.
Why is understanding the distinction between FDA approved and FDA cleared important?
The distinction is crucial as it significantly influences product development timelines and market strategies. FDA approved products undergo rigorous testing, while FDA cleared devices may not face the same level of scrutiny, potentially leading to safety concerns.
What role does AVS Life Sciences play in the FDA approval and clearance processes?
AVS Life Sciences provides expert guidance and comprehensive support to help clients navigate the complexities of FDA regulations, ensuring compliance and enhancing safety and efficacy of products. They offer tailored strategies for pharmaceutical and medical device companies.
What are some recent trends in compliance analytics solutions mentioned in the article?
Organizations using compliance analytics solutions have reported a 34% reduction in staff hours spent on reporting tasks and a 40% decrease in adherence violations, highlighting the importance of expert guidance in maintaining compliance.
What are the key pathways involved in the FDA approval process?
Key pathways include the New Drug Application (NDA) for pharmaceuticals and the Biologics License Application (BLA) for biologics. Each pathway has specific requirements, such as preclinical studies and clinical trials, and emphasizes the importance of robust evidence for safety and effectiveness.
How does AVS Life Sciences assist clients in navigating the FDA approval process?
AVS Life Sciences offers tailored consulting solutions, helping clients understand the differences between FDA approved and FDA cleared products, ensuring compliance with regulatory standards, and improving the likelihood of favorable outcomes in drug development.
What is the significance of the IDE approval for the Pulse IVL™ System mentioned in the article?
The IDE approval for the Pulse IVL™ System represents a significant clinical milestone for AVS Life Sciences, showcasing their commitment to excellence in navigating FDA processes and enhancing operational efficiency for clients.
How can pharmaceutical compliance officers benefit from AVS Life Sciences' services?
Pharmaceutical compliance officers can integrate AVS's services into their regulatory strategies to better manage compliance challenges and enhance their understanding of FDA regulations, ultimately positioning their products for success in the market.
