7 Key Changes in Cosmetic Industry Regulation Reform

Overview
The article "7 Key Changes in Cosmetic Industry Regulation Reform" primarily focuses on outlining significant updates in regulations that impact the cosmetic industry. It elaborates on how these reforms, including the introduction of the Safer Beauty Bill Package and the implementation of stricter safety standards, compel manufacturers to adapt their compliance strategies. This adaptation is essential to ensure product safety and transparency, which in turn fosters consumer trust and enhances market competitiveness.
Introduction
The cosmetic industry stands at a pivotal crossroads, grappling with a wave of regulatory reforms designed to enhance safety and transparency for consumers. As the landscape shifts with the introduction of stringent guidelines—such as the Safer Beauty Bill Package—companies must adapt swiftly to maintain compliance and consumer trust.
What strategies can brands employ to navigate this evolving regulatory maze while seizing opportunities in the booming clean beauty market? This article delves into seven key changes reshaping the cosmetic industry's regulatory framework, offering insights into how businesses can thrive amidst these challenges.
AVS Life Sciences: Comprehensive Regulatory Solutions for Cosmetic Compliance
AVS Life Sciences presents a comprehensive suite of meticulously designed for the , ensuring strict adherence to the rapidly evolving regulatory landscape. With extensive expertise in and regulatory submissions, AVS empowers clients to adeptly navigate the intricacies of cosmetic industry regulation reform, from initial product development to market entry. By harnessing profound industry insights, AVS aids companies in establishing robust compliance strategies that align with , Data Integrity, and Standard Operating Procedures (SOPs).
This strategic approach not only enhances product safety but also positions brands to seize opportunities within the burgeoning , currently valued at $11.6 billion. As intensifies—especially with the introduction of the Safer Beauty Bill Package, which bans 18 harmful chemicals, including mercury and formaldehyde—AVS's unwavering commitment to becomes vital for brands striving to uphold consumer trust and comply with cosmetic industry regulation reform.
Furthermore, with 39% of regulatory professionals already leveraging AI-driven solutions for compliance, AVS stands at the forefront of innovative strategies, assisting clients in mitigating risks associated with legal scrutiny concerning 'clean beauty' claims. To ensure compliance and maintain product integrity, brands are urged to conduct thorough ingredient audits and invest in staff training regarding compliance updates. This proactive stance not only protects consumer health but also bolsters brand reputation in an increasingly competitive market.
North America: Key Regulatory Updates Impacting the Cosmetic Industry
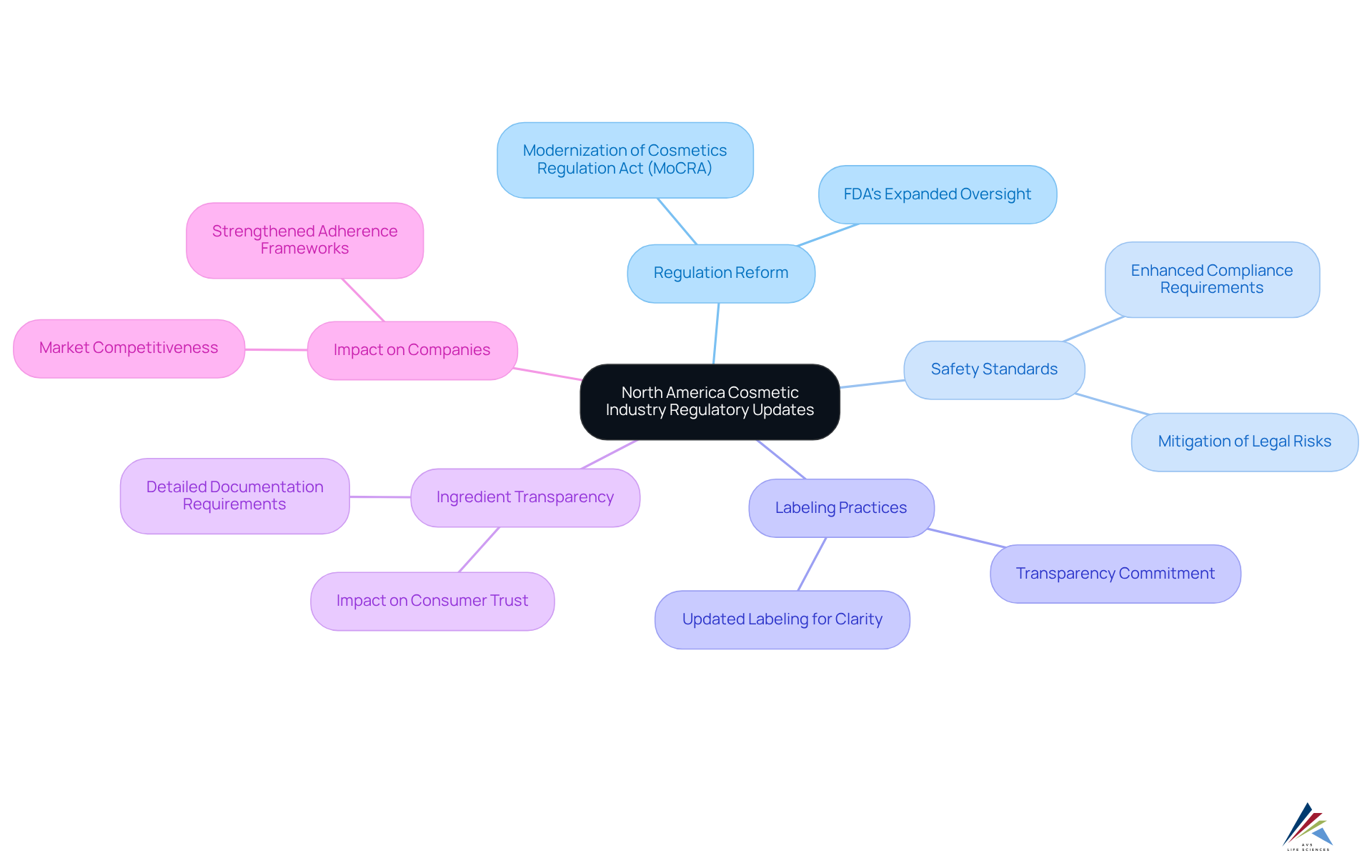
Recent policy updates in North America have significantly tightened guidelines surrounding , particularly in terms of safety and labeling. The FDA's emphasis on ingredient clarity has heightened the need for cosmetic industry regulation reform, requiring manufacturers to provide . This change compels companies to strengthen their adherence frameworks due to the cosmetic industry regulation reform, ensuring that all products conform to the new to mitigate the risk of legal consequences. Notably, numerous prominent cosmetic brands have proactively updated their labeling practices in light of cosmetic industry regulation reform to , demonstrating a and . As these regulations evolve, the focus on not only influences compliance strategies but also shapes in the realm of cosmetic industry regulation reform.
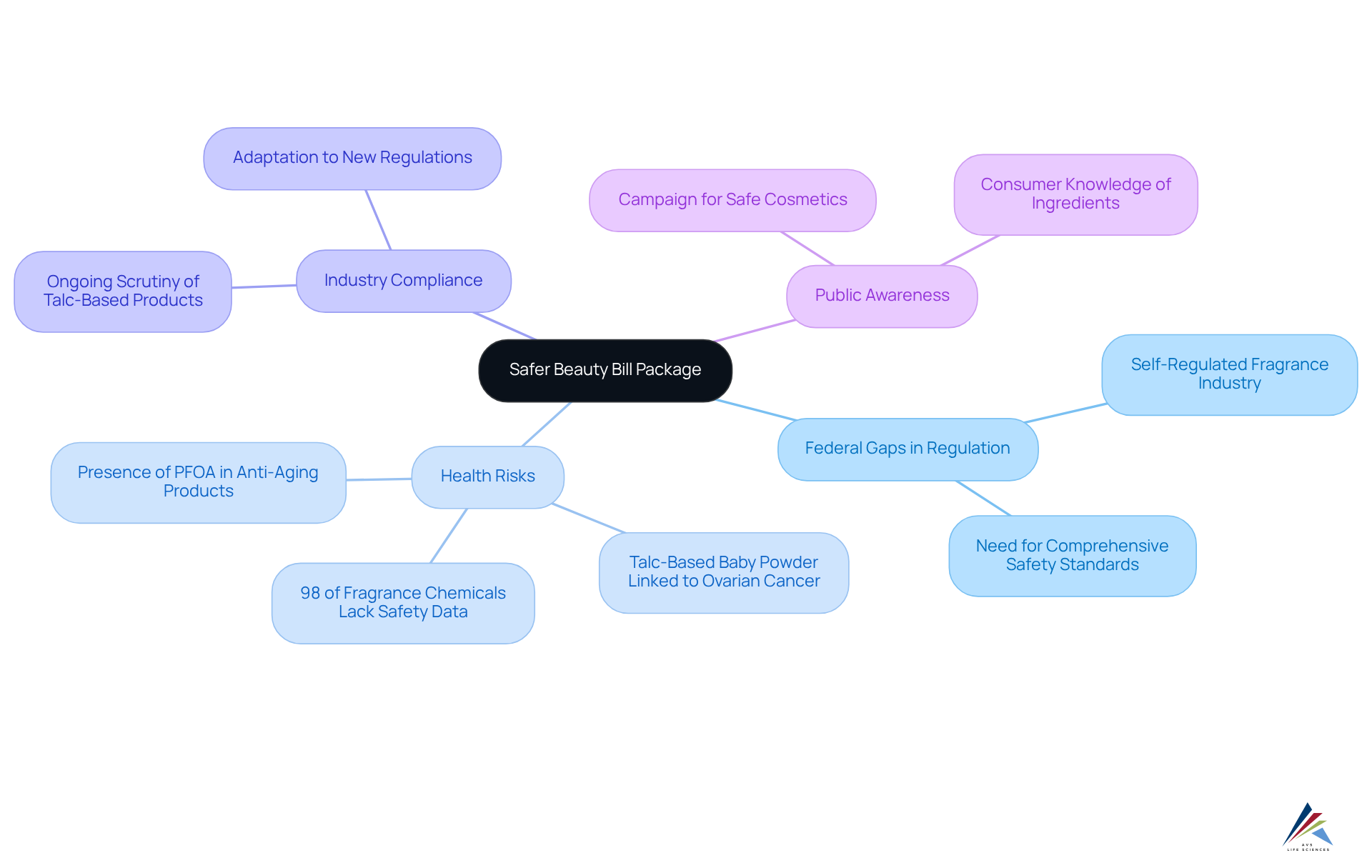
Safer Beauty Bill Package: Addressing Federal Gaps in Cosmetics Safety
The represents a significant leap forward in cosmetics safety and is a vital aspect of the , directly addressing in regulation. This comprehensive legislation mandates , compelling manufacturers to prioritize .
As highlighted by Breast Cancer Prevention Partners, "the report reveals , $50 billion fragrance sector, where harmful chemicals are frequently utilized in perfumes, personal care, beauty, and cleaning items—without the awareness or approval of the public."
Alarmingly, over 98% of fragrance chemicals lack fundamental safety data or are classified as high concern, underscoring the . The absence of safety data is linked to severe , including cancer and reproductive harm, emphasizing the stakes involved in these regulatory changes.
Businesses must remain vigilant in adapting to these developments to ensure compliance and uphold public trust. The ongoing scrutiny of Johnson & Johnson's talc-based baby powder further illustrates the real-world implications of regulations and the pressing need for cosmetic industry regulation reform.
As the industry evolves, proactive engagement with these legislative developments will be essential for maintaining a competitive edge and safeguarding public health.
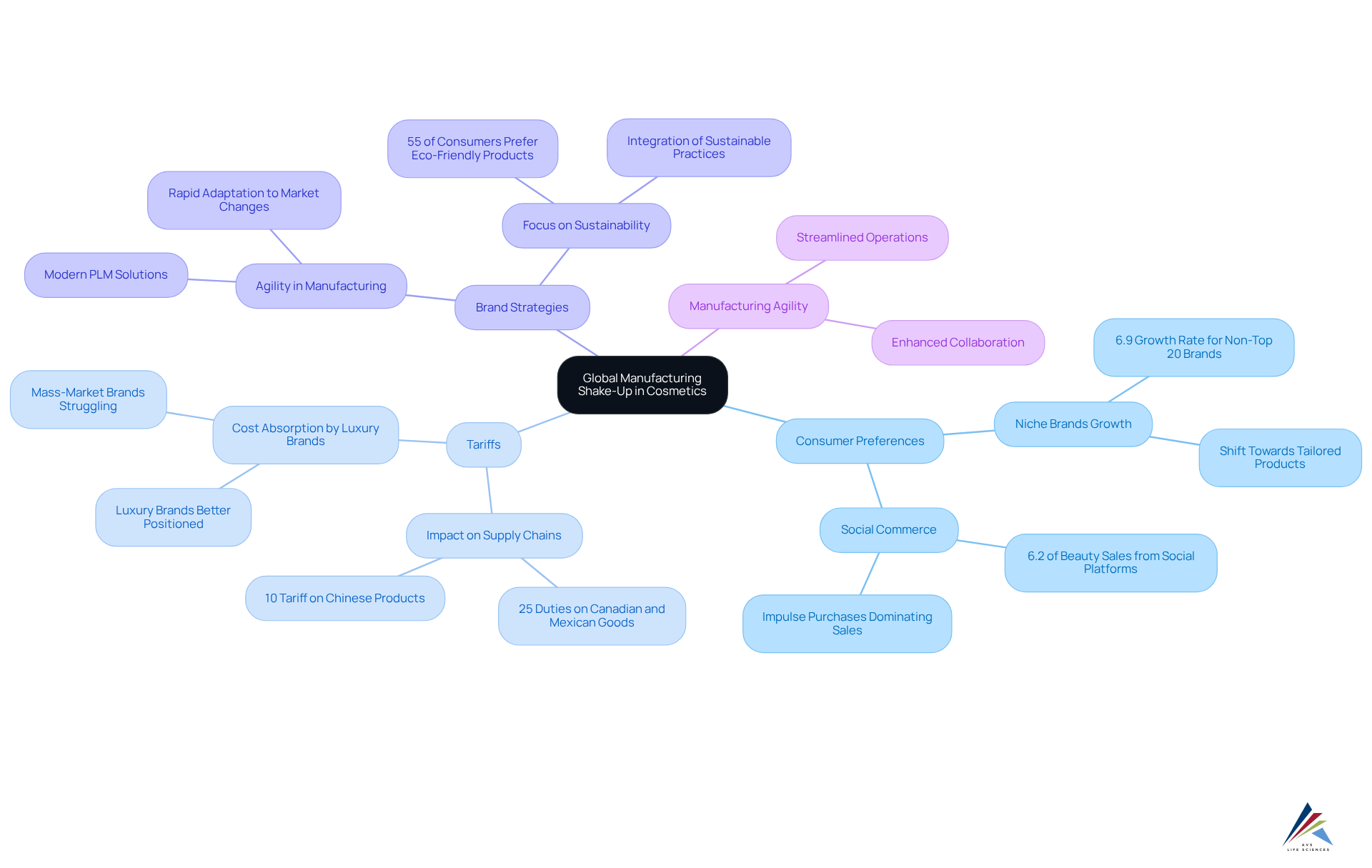
Global Manufacturing Shake-Up: Tariffs and Consumer Shifts in Cosmetics
The cosmetic industry is undergoing a significant and evolving consumer preferences. As brands adapt to these changes, they increasingly seek while ensuring . This dual focus is essential for maintaining integrity and market competitiveness.
In 2025, are shifting towards , with a notable 6.9% growth rate for companies outside the top 20 beauty brands, which experienced a growth of 2.7% in 2024. This trend reflects a desire for tailored and distinctive items that resonate with individual values. Furthermore, the emergence of social commerce platforms, which currently represent 6.2% of beauty sales, signifies a change in how consumers interact with beauty items, with more than two-thirds of purchases being impulse-driven.
Companies are also facing challenges due to tariffs, which can disrupt supply chains and increase costs. For example, , together with an extra 10% tariff on goods from China, are encouraging brands to reevaluate their . Luxury brands, in particular, are better positioned to absorb these costs compared to mass-market brands, allowing them to maintain their market presence despite rising expenses.
Industry analysts highlight the significance of . Contemporary lifecycle management (PLM) solutions are becoming essential for allowing companies to adjust rapidly to market shifts and customer needs. By leveraging these technologies, brands can streamline their operations and enhance collaboration, ensuring they remain competitive in a rapidly evolving landscape.
As Patrick Overall pointed out, "Tariffs can influence costs of goods sold (COGS), supply chains, and buyer behavior," emphasizing the urgency of these challenges. As the sector continues to address these challenges, a strong adherence plan will be essential for ensuring that products not only satisfy legal requirements but also align with the changing tastes of today's shoppers, particularly in the context of .
Consumer Fragmentation: Navigating Regulatory Compliance in Cosmetics
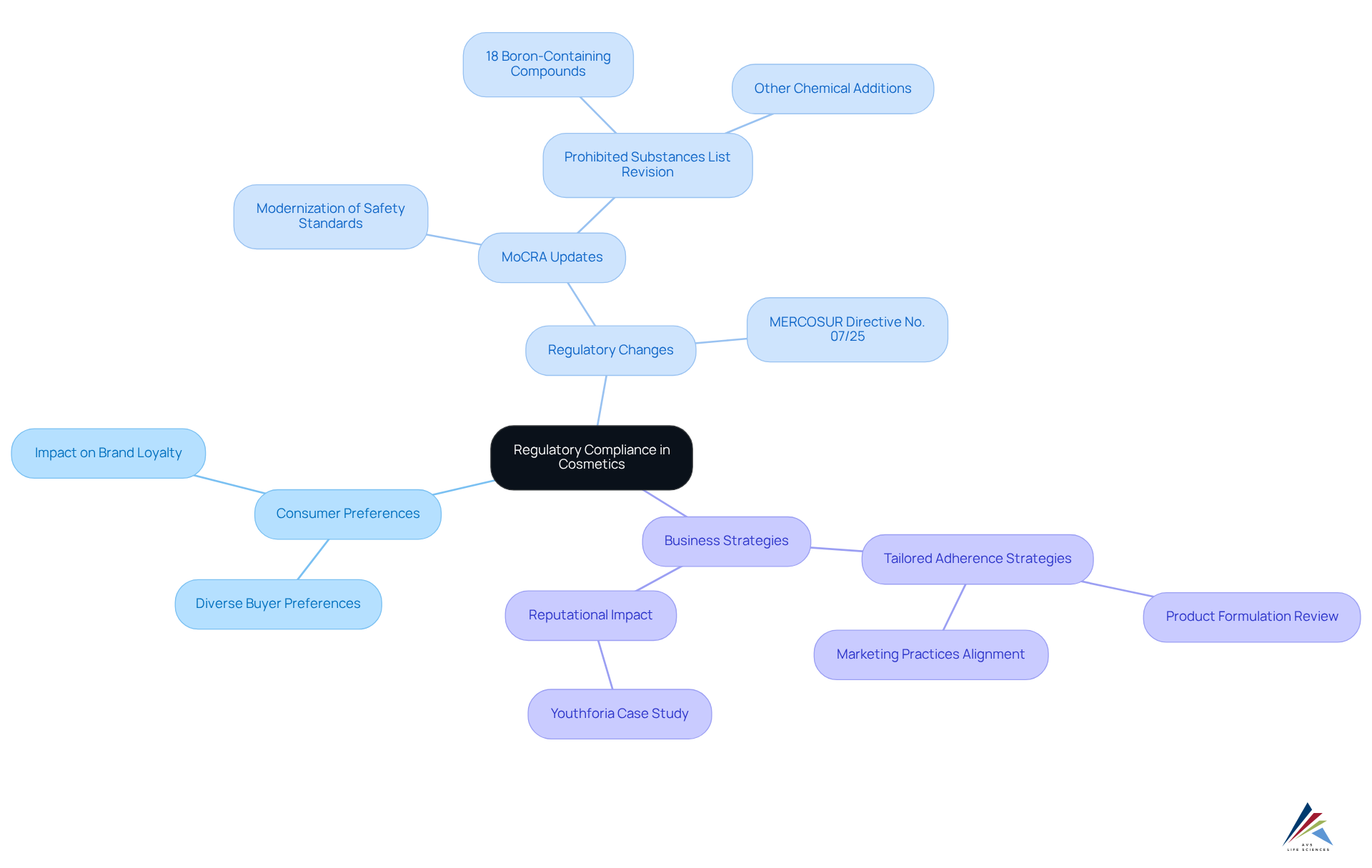
As buyer preferences become increasingly diverse, cosmetic producers encounter significant challenges in maintaining due to the need for across various markets. To effectively navigate this landscape, businesses must develop tailored adherence strategies that address the unique demands of different client groups while adhering to overarching regulatory obligations. For instance, the recent updates to the restricted substances list, including the addition of 18 boron-containing compounds, necessitate that brands reassess their formulations to .
This strategic approach not only enhances adherence but also fosters brand loyalty among consumers. Companies that successfully align their offerings with while catering to specific consumer preferences are more likely to forge lasting connections with their clientele. The impact of is evident in the cases of firms that have proactively adapted to changes, such as those responding to the cosmetic industry regulation reform, specifically the , which aims to update safety standards in the U.S. market.
Moreover, as illustrated by the recent shutdown of Youthforia in response to backlash from a controversial launch, inadequate management of regulations can lead to significant reputational damage. Thus, cosmetic brands must prioritize in light of cosmetic industry regulation reform to not only meet legal requirements but also engage with their target audiences, ultimately nurturing brand loyalty and ensuring long-term success.
To begin in light of , brands should conduct a comprehensive review of their and marketing practices, ensuring alignment with the latest regulations and consumer expectations.
AI and Automation: Transforming Compliance in Beauty Manufacturing
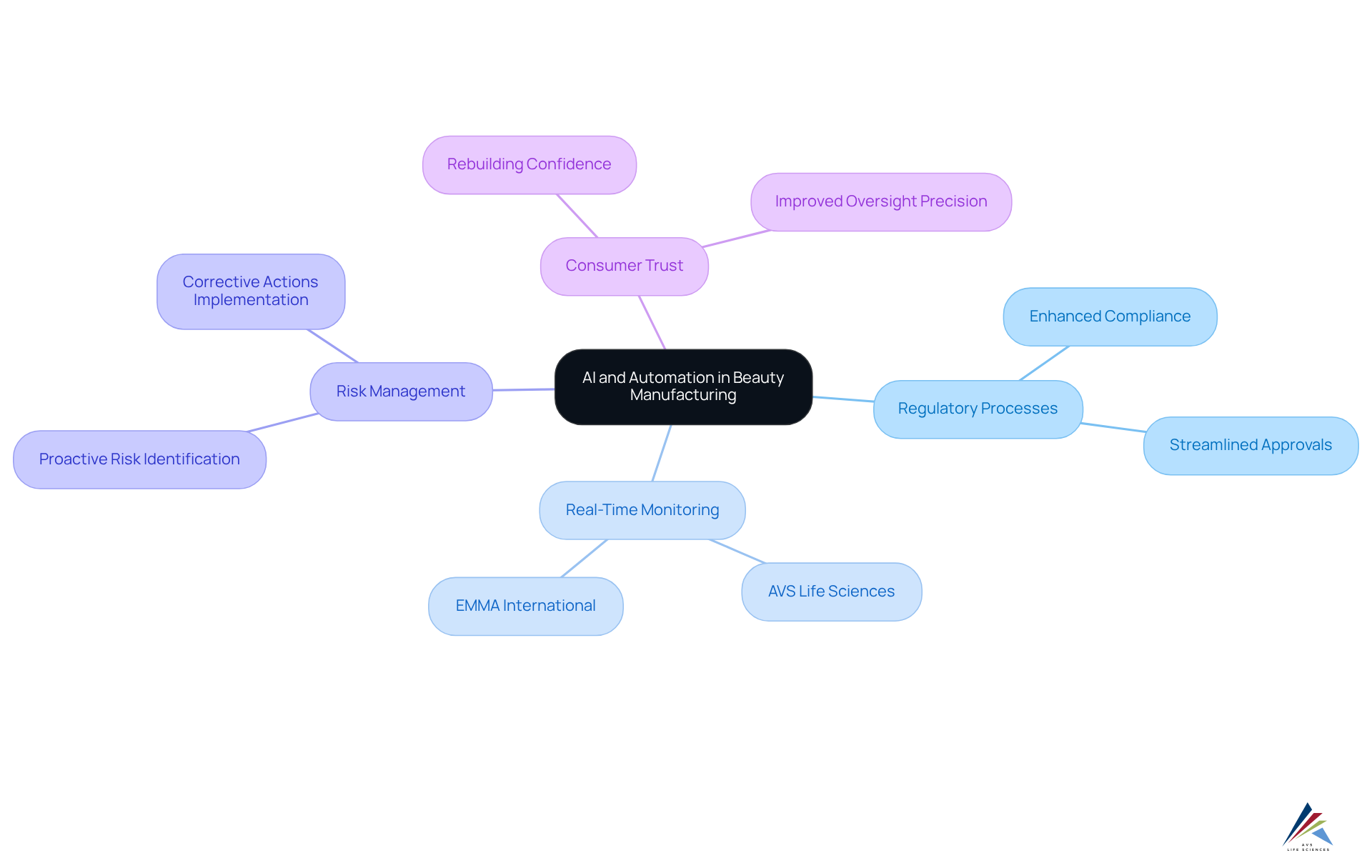
The incorporation of AI and automation in beauty manufacturing is revolutionizing regulatory processes as part of the , markedly enhancing both accuracy and efficiency. , a leading provider of , exemplifies how advanced technologies facilitate of production processes, ensuring stringent adherence to standards.
By leveraging , businesses can proactively identify risks and swiftly implement corrective actions, thereby diminishing the likelihood of legal infractions. For example, EMMA International is utilizing AI tools to automate submissions and optimize , demonstrating how technology can expedite traditionally sluggish processes.
As the beauty sector increasingly embraces these innovations, the anticipated cosmetic industry regulation reform is expected to improve oversight precision, fostering greater consumer trust and compliance with standards. Given that consumer confidence is currently at a historic low, is crucial for rebuilding trust.
Furthermore, AI's capabilities in identifying patient eligibility and monitoring real-time data in clinical trials can be mirrored in beauty manufacturing, underscoring the technology's broader implications. To bolster adherence initiatives, pharmaceutical oversight officers should consider deploying AI tools that facilitate , ensuring their procedures remain robust and in compliance with cosmetic industry regulation reform.
AVS Life Sciences' expertise in , and positions them as an invaluable partner in navigating these complexities.
Evolving Marketing Landscape: Compliance Challenges in the Cosmetic Sector
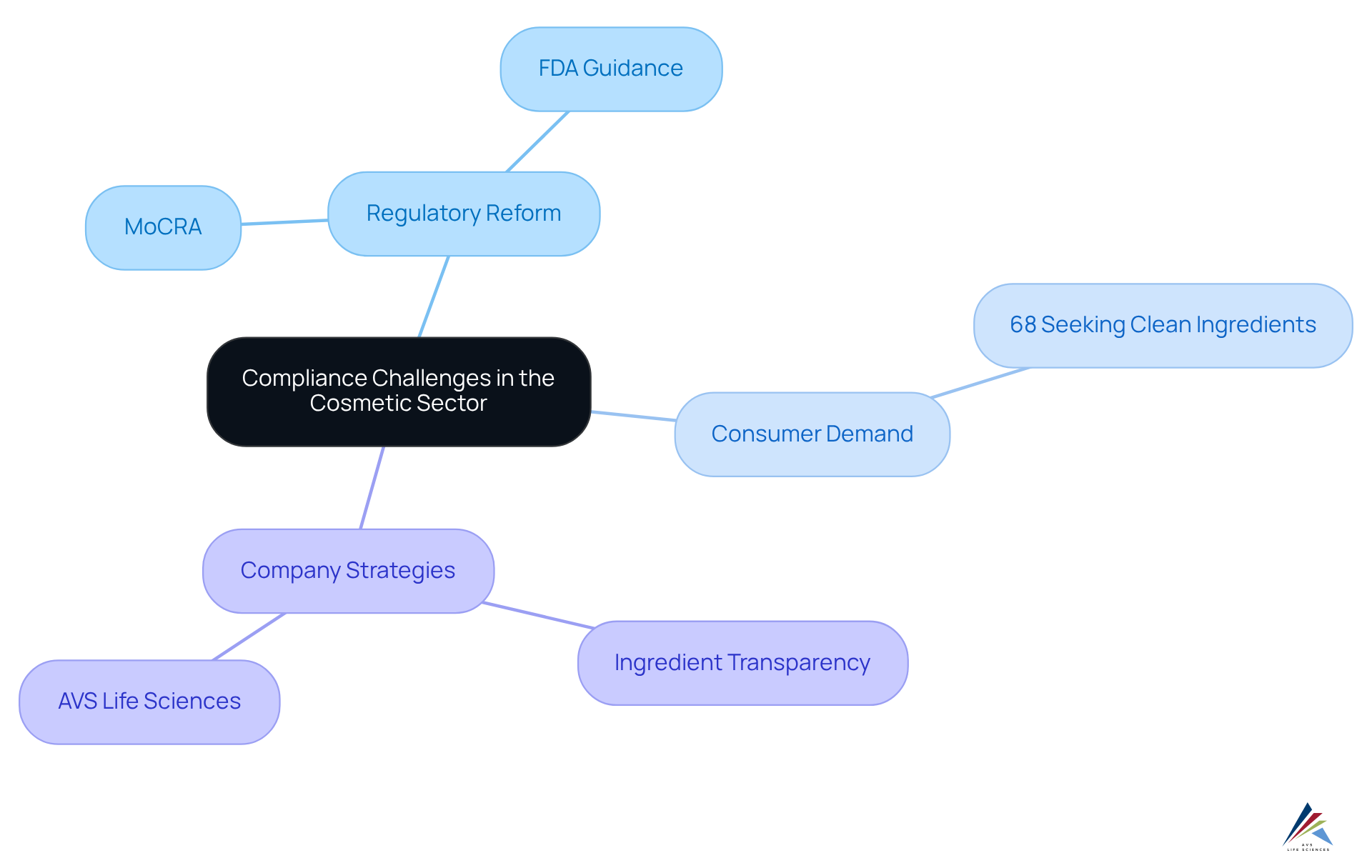
The dynamic marketing landscape presents significant compliance challenges for cosmetic companies, particularly in light of concerning advertising claims and . As the demand for comprehensive safety and effectiveness information from buyers increases, manufacturers must align their marketing practices with the cosmetic industry regulation reform and . This necessitates to avoid misleading consumers and mitigate potential legal risks. Notably, recent data indicates that , underscoring the necessity for brands to transparently communicate their ingredient sourcing and formulation methods.
Companies that prioritize ingredient transparency, such as , are better positioned to and contribute to cosmetic industry regulation reform while fostering trust with their customers. AVS Life Sciences helps cosmetic companies , particularly with the implementation of the and the FDA's draft guidance on cosmetic product facility registrations. is crucial for maintaining brand integrity and consumer trust within the cosmetic sector, especially with the ongoing cosmetic industry regulation reform.
Regional Growth Trends: Impact of Regulatory Reforms on the Beauty Industry
is significantly shaping regional growth trends within the beauty industry. As nations implement stricter safety and regulatory standards, manufacturers face the critical challenge of adapting their strategies to comply with cosmetic industry regulation reform.
to support companies navigating these complexities through its expert consulting in validation, quality assurance, and governance strategies. This adaptability not only ensures compliance but also empowers businesses to seize new market opportunities driven by consumer demand for safer and more transparent products.
By leveraging AVS Life Sciences' , organizations can enhance their operational strategies and maintain a competitive edge in this dynamic landscape.
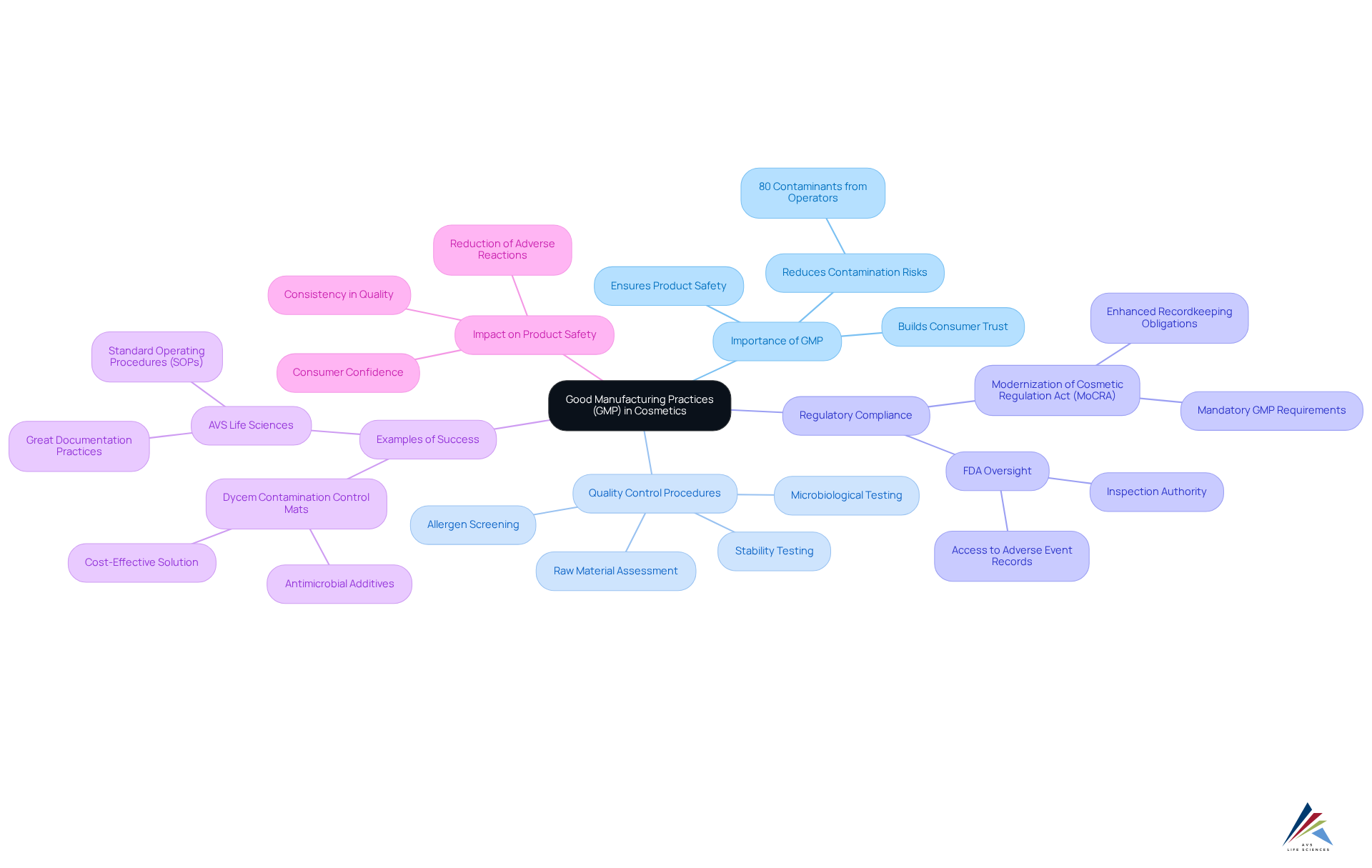
Good Manufacturing Practices (GMP): Ensuring Compliance in Cosmetics
are essential for ensuring compliance within the , which includes a variety of quality control measures that manufacturers must adopt to guarantee safety and efficacy. By adhering to GMP guidelines, companies can significantly reduce contamination risks—critical, given that 80% of contaminants enter cleanrooms via operators' shoes or equipment wheels. This proactive approach not only supports regulatory standards but also fosters trust in safety.
involve rigorous testing for stability, effectiveness, and microbiological safety, ensuring that all products are safe for public use. For instance, quality control laboratories conduct microbiological testing to identify harmful microorganisms, while allergen screening is implemented to prevent adverse reactions among consumers. These practices are vital, as they ensure that all supporting safety data is derived from scientifically sound methods.
The impact of GMP on product safety is underscored by the recent enactment of the Modernization of Cosmetic Regulation Act (MoCRA), highlighting the necessity for cosmetic industry regulation reform to ensure that all facilities engaged in cosmetic manufacturing comply with stringent . Companies that adhere to these regulations not only avert severe consequences, such as suspended facility registrations and mandatory recalls, but also enhance their market reputation.
Several companies exemplify successful adherence to GMP. For example, organizations utilizing contamination control solutions, such as Dycem mats, have effectively minimized contamination risks while upholding high hygiene standards. These mats, with an average lifespan exceeding three years, incorporate to inhibit the growth of harmful microorganisms, thereby further ensuring product safety.
aids companies in achieving through its Great Documentation Practices and Standard Operating Procedures (SOPs), which are crucial for maintaining high-quality standards in the cosmetic industry.
In summary, the implementation of GMP transcends mere compliance; it represents a commitment to excellence in quality assurance. As the FDA continues to solidify GMP regulations, cosmetic industry regulation reform necessitates that companies like AVS Life Sciences reassess and enhance their practices to align with these evolving standards, ultimately ensuring that consumers can purchase cosmetic products with confidence.
Future Outlook: Anticipating Changes in Cosmetic Industry Regulations
The cosmetic sector is undergoing significant transformation, which is compelling businesses to remain vigilant regarding the and any that may affect their operations. Key trends reveal an intensified emphasis on , which are set to drive .
For example, the expansion of allergen labeling requirements from 24 to 80 substances by 2026 highlights the growing scrutiny on product formulations. Moreover, the impending ban on formaldehyde in cosmetics, effective January 1, 2027, underscores the importance of cosmetic industry regulation reform in promoting user safety and environmental responsibility.
Companies that proactively adapt to the cosmetic industry regulation reform will not only ensure compliance but also in a market that increasingly values transparency and sustainability. Industry analysts emphasize that modern consumers demand comprehensive information about ingredient origins and health implications, urging brands to prioritize ethical sourcing and sustainable practices.
By embracing these trends, manufacturers can navigate the complexities of compliance while fostering trust and loyalty among their customer base. AVS Life Sciences offers , enabling companies to effectively adjust to these changes.
By leveraging the expertise of AVS Life Sciences, manufacturers can ensure and adherence to cosmetic industry regulation reform in the global perfume and cosmetics industry, which are influencing product innovation and brand value, presenting both challenges and opportunities in this dynamic landscape.

Conclusion
The landscape of the cosmetic industry is undergoing a profound transformation, driven by rigorous regulatory reforms aimed at enhancing safety, transparency, and consumer trust. The introduction of key regulations, such as the Safer Beauty Bill Package and the Modernization of Cosmetics Regulation Act, underscores the industry's commitment to prioritizing public health and safety. Companies must adapt to these evolving standards to maintain compliance and leverage opportunities within the growing clean beauty market.
Key insights reveal that:
- The tightening of safety and labeling guidelines
- The integration of AI and automation for compliance
- The shifting consumer preferences towards niche brands
These factors are reshaping how cosmetic manufacturers operate. The importance of Good Manufacturing Practices (GMP) cannot be overstated, as they serve as the backbone for ensuring product safety and quality in an increasingly scrutinized market. Additionally, the challenges posed by tariffs and consumer fragmentation necessitate a proactive approach to regulatory compliance.
In this rapidly evolving environment, cosmetic brands are urged to embrace these regulatory changes not only as a compliance obligation but as a strategic advantage. By prioritizing transparency and sustainability, businesses can foster trust and loyalty among consumers, positioning themselves favorably in a competitive landscape. Engaging with comprehensive regulatory solutions, such as those offered by AVS Life Sciences, will be essential for navigating these complexities and ensuring long-term success in the cosmetic industry.
Frequently Asked Questions
What is AVS Life Sciences and what do they offer to the cosmetic industry?
AVS Life Sciences provides a comprehensive suite of compliance solutions tailored for the cosmetic industry, assisting clients in navigating regulatory reforms and ensuring adherence to quality management, regulatory submissions, and Good Manufacturing Practices (GMP).
How does AVS Life Sciences help brands in the cosmetic industry?
AVS aids companies in developing robust compliance strategies that align with regulatory requirements, enhancing product safety, and positioning brands to capitalize on opportunities in the clean beauty market.
What recent regulatory changes are impacting the cosmetic industry in North America?
Recent updates have tightened guidelines on safety and labeling, requiring manufacturers to provide detailed documentation of formulations and claims, leading to a stronger focus on ingredient transparency and compliance.
What is the Safer Beauty Bill Package and why is it important?
The Safer Beauty Bill Package is legislation aimed at improving cosmetics safety by mandating rigorous testing and reporting of cosmetic ingredients, addressing federal gaps in regulation and prioritizing public safety.
What are the implications of the Safer Beauty Bill Package for manufacturers?
Manufacturers must adapt to new safety standards and testing requirements to ensure compliance, which is crucial for maintaining public trust and mitigating health risks associated with harmful chemicals.
How does the focus on ingredient clarity influence the cosmetic industry?
The emphasis on ingredient clarity enhances compliance strategies, shapes public trust, and increases market competitiveness as brands update their labeling practices to meet new regulatory standards.
What role does AI play in cosmetic industry compliance?
AI-driven solutions are increasingly utilized by regulatory professionals to assist in compliance, helping companies mitigate risks associated with legal scrutiny over 'clean beauty' claims.
Why is it essential for brands to conduct ingredient audits and staff training?
Conducting thorough ingredient audits and investing in staff training on compliance updates is vital for maintaining product integrity, protecting consumer health, and bolstering brand reputation in a competitive market.
