7 Key Benefits of MES Integration for Compliance Officers

Overview
The key benefits of MES integration for compliance officers are significant:
- Enhanced regulatory adherence
- Improved data accuracy
- Streamlined compliance monitoring
MES automates compliance tasks, providing real-time monitoring capabilities that reduce human error. This automation leads to better operational efficiency and minimizes the risk of non-compliance, making it a crucial solution for today’s regulatory landscape.
Introduction
The landscape of regulatory compliance in the life sciences sector is increasingly complex, demanding innovative solutions to ensure adherence to stringent standards. Manufacturing Execution Systems (MES) integration emerges as a pivotal strategy for compliance officers, offering a multitude of benefits that streamline processes and enhance data accuracy.
However, the pressing question remains: how can organizations effectively leverage MES integration not only to meet compliance requirements but also to foster a culture of continuous improvement and operational excellence?
This exploration unveils the key advantages of MES integration, revealing its transformative impact on regulatory compliance and operational efficiency.
AVS Life Sciences: Enhanced Regulatory Compliance through MES Integration
AVS Life Sciences employs Manufacturing Execution Systems (MES) to significantly enhance adherence to regulations. By incorporating mes integration, organizations can automate compliance-related tasks, ensuring strict adherence to Good Manufacturing Practices (GMP) alongside other requirements, including GXP and FDA standards. This mes integration not only streamlines regulatory management but also boosts , allowing companies to concentrate on their core activities while upholding high standards of quality and compliance.
The impact of MES on GMP adherence is profound; it facilitates real-time monitoring and documentation, which are essential for maintaining conformity with regulatory standards. Organizations that adopt MES can achieve a 15-30% reduction in downtime and a 25-40% decrease in defect rates, underscoring the system's effectiveness in improving production quality and reliability. Furthermore, the real-time data provided by MES enables swift corrective actions, thereby ensuring compliance and operational excellence.
Industry leaders emphasize the critical role of MES in regulatory management. A comprehensive framework for implementing electronic signatures and data integrity controls within MES aligns with regulatory standards, ensuring that organizations not only meet compliance benchmarks but also enhance operational efficiency. As highlighted, "Validating the MES and interfacing it with the LIMS, ELN, or ERP allows pharmaceutical and biotechnology companies to gain a competitive advantage with improved quality and better traceability."
Successful MES implementation requires a phased approach, typically spanning 8-12 months, encompassing assessment, design, development, testing, and rollout. This organized strategy minimizes disruption and allows for necessary adjustments during the integration process. Organizations can adeptly navigate the complexities of regulatory oversight in the life sciences sector by prioritizing mes integration, ultimately safeguarding patient safety and enhancing product quality.
Improved Data Accuracy: Ensuring Compliance with MES Integration
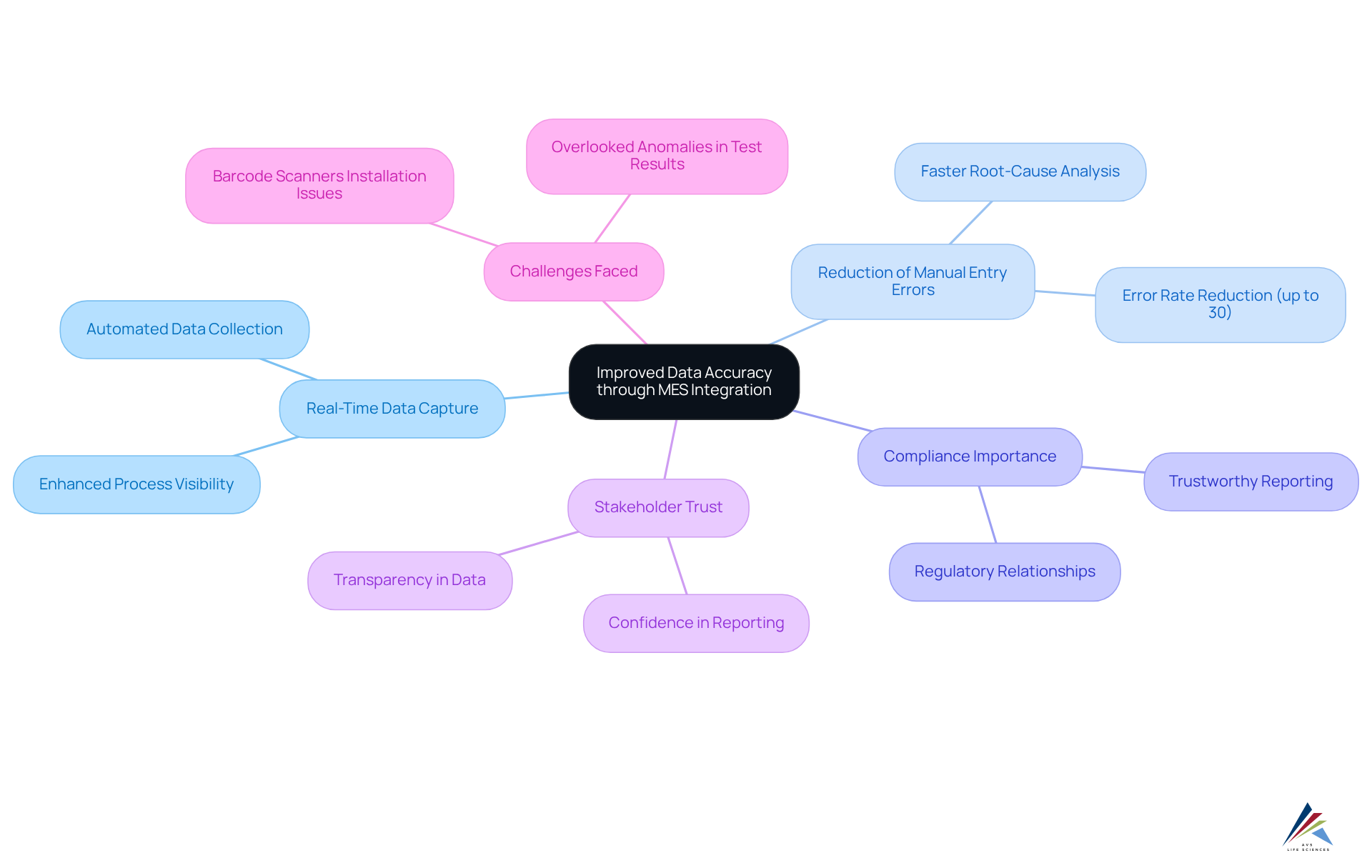
A key advantage of MES integration lies in the substantial enhancement of data accuracy. By capturing real-time data directly from production processes, MES integration significantly reduces the risk of manual entry errors, which can account for up to 30% of inaccuracies in reporting. This enhancement is essential for adherence reporting and evaluations, ensuring that all information provided to oversight organizations is both trustworthy and confirmable.
Compliance officers consistently emphasize that accurate data is foundational for meeting legal requirements and maintaining stakeholder trust. Furthermore, the MES integration fosters a culture of transparency, allowing stakeholders to confidently rely on the integrity of the data presented. This not only supports compliance but also , ultimately leading to smoother audits and fewer compliance-related issues.
During the upgrade phase, challenges arose, such as the installation of barcode scanner cameras upside down, which initially resulted in overlooked anomalies in test results. Addressing these concerns enabled the QC laboratory team to assess their methods and enhance overall data integrity.
Real-Time Data Visibility: Streamlining Compliance Monitoring
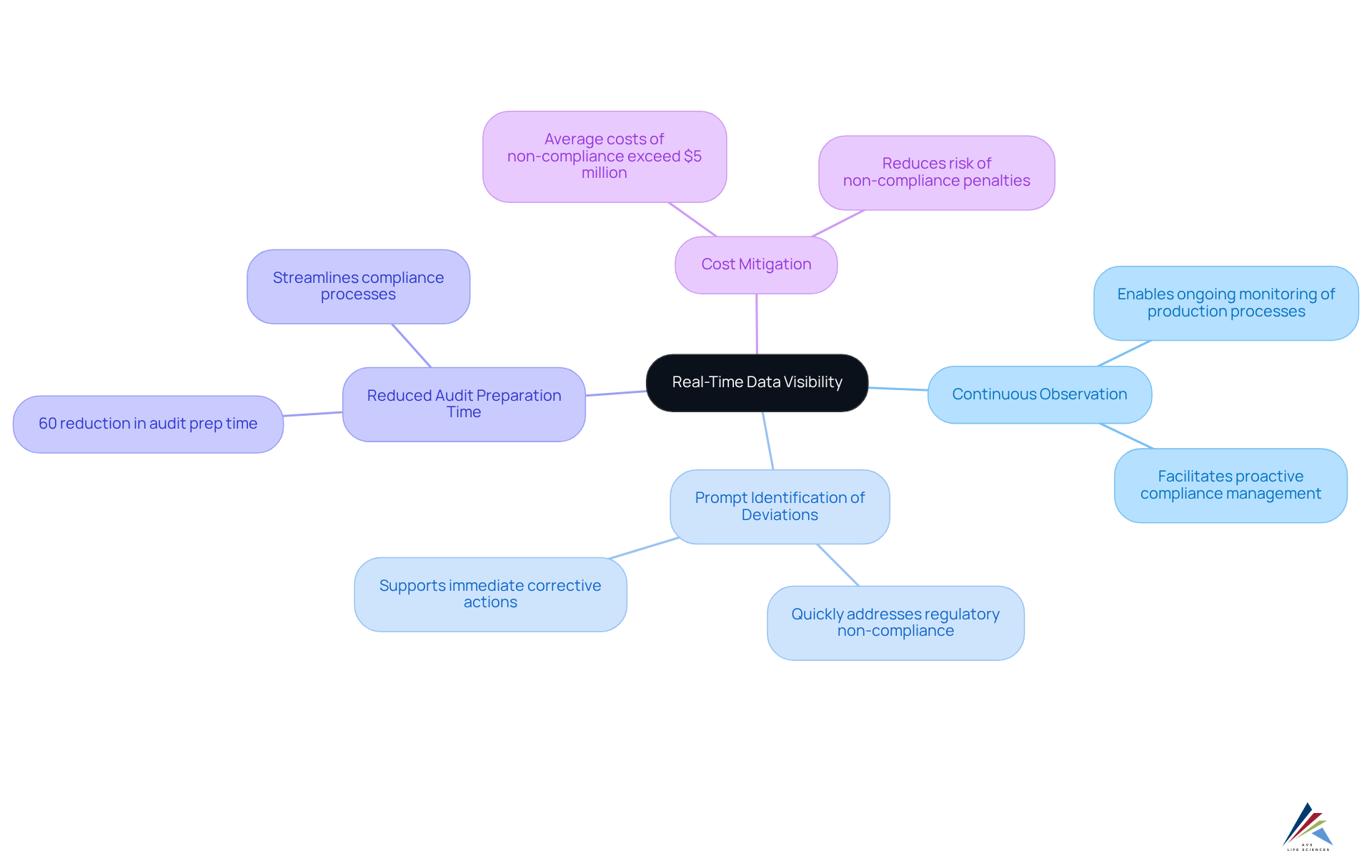
Incorporating MES provides oversight personnel with real-time insight into production processes, enabling continuous observation of operations. This capability facilitates the prompt identification of , allowing for swift corrective actions.
For example, organizations can leverage real-time data to address issues as they arise, significantly mitigating the risk of non-compliance penalties. In fact, companies employing real-time monitoring report a 60% reduction in audit preparation time, highlighting the efficiency gains associated with this approach.
Experts emphasize that access to real-time information not only enhances adherence to regulations but also fosters a proactive culture of compliance, ultimately leading to improved operational standards and reduced financial risks. By ensuring adherence throughout the production lifecycle, organizations can minimize potential expenses linked to non-compliance, which can average over $5 million for breaches.
Therefore, the incorporation of MES integration is not merely a technological enhancement; it is a strategic necessity for ensuring compliance in an increasingly complex regulatory environment.
Reduction of Human Error: Strengthening Compliance Efforts
The mes integration of a Manufacturing Execution System (MES) is essential for minimizing human error in compliance-related tasks. By automating data gathering and reporting, MES significantly reduces reliance on manual entry, which is often susceptible to inaccuracies. This automation not only but also elevates the overall quality of production, resulting in improved outcomes and a decrease in compliance issues.
The comprehensive computer system validation procedure, as outlined in the GAMP 5 Guide, encompasses critical stages such as:
- Planning
- Defining user requirements
- Installation qualification (IQ)
- Operational qualification (OQ)
- Performance qualification (PQ)
Each stage is meticulously documented to ensure alignment with FDA regulations and GXP standards. By leveraging MES integration, organizations can enhance their validation methods, ensuring robustness and compliance, ultimately facilitating efficient vaccine manufacturing through automated batch reporting and data integration.
Enhanced Quality Management: Supporting Compliance Standards
The integration of MES integration significantly enhances quality management systems by equipping organizations with essential tools for real-time monitoring and control of production quality. This capability is crucial for ensuring that products consistently meet regulatory standards throughout the manufacturing process, particularly in adherence to GXP and FDA regulations.
By improving quality management practices, organizations can substantially reduce the risk of non-compliance, thereby enhancing overall product quality—a vital factor for maintaining regulatory approvals. Implementing MES systems can lead to a remarkable 25-40% decrease in defect rates, underscoring their importance in elevating product quality standards.
Furthermore, the MES integration with ERP systems facilitates real-time decision support and optimizes production planning, which is essential for effective adherence management. Organizations must also confront challenges related to user adoption during MES implementation, highlighting the necessity of robust change management practices.
As Nikhil Joshi aptly states, 'Implementing MES systems significantly enhances operational efficiency and production visibility while providing real-time data for better quality management and waste reduction.' This integration not only streamlines regulatory efforts but also fosters a culture of , which is critical for addressing the stringent requirements of the pharmaceutical sector and beyond.

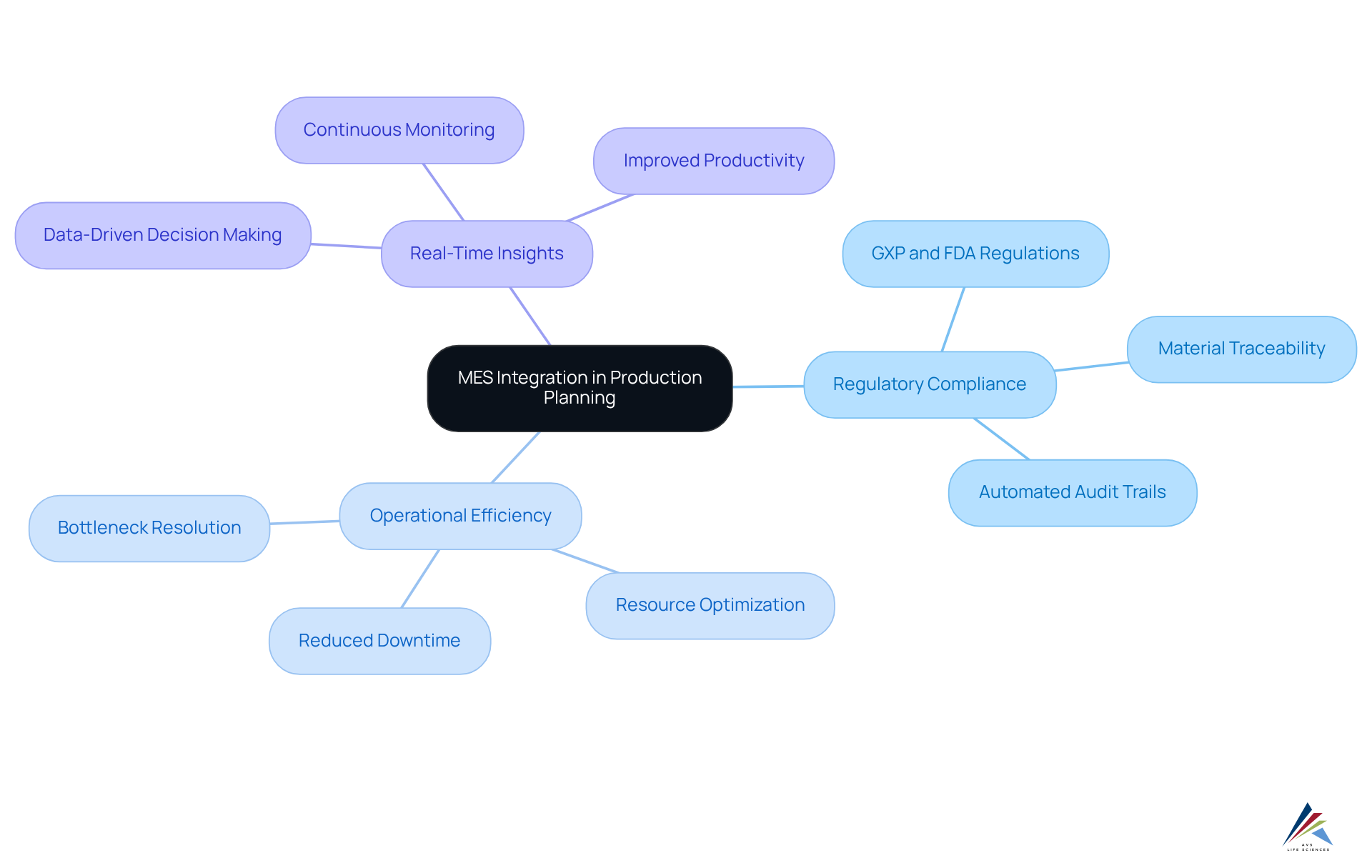
Optimized Production Planning: Aligning with Compliance Requirements
The integration of Manufacturing Execution Systems (MES integration) significantly enhances production planning, ensuring that manufacturing processes strictly align with regulatory requirements. By delivering real-time insights into production schedules, resource allocation, and workflow management, MES integration empowers organizations to strategically plan their operations in compliance with standards, including GXP and FDA regulations. This optimization not only boosts operational efficiency but also mitigates the risk of regulatory violations resulting from inadequate planning.
For example, real-time data from MES allows manufacturers to swiftly identify and resolve bottlenecks, thereby improving productivity and ensuring adherence to Good Manufacturing Practices (GMP), as defined by the WHO's current Good Manufacturing Practice (cGMP) regulations. Furthermore, the thorough traceability provided by MES integration is crucial for regulatory compliance, as it guarantees material traceability and minimizes the risk of nonconformity in controlled sectors.
As Nic Azad, Communications Lead, emphasizes, 'The role of MES integration extends beyond improving production efficiency; it’s a cornerstone of the manufacturing execution strategy, vital for navigating the complexities of modern manufacturing while ensuring consistency and reliability across the production lifecycle.' This underscores MES as an essential tool for in the pharmaceutical industry, particularly regarding AVS Life Sciences' commitment to providing comprehensive quality management and regulatory adherence solutions.
Improved Supply Chain Visibility: Facilitating Compliance Oversight
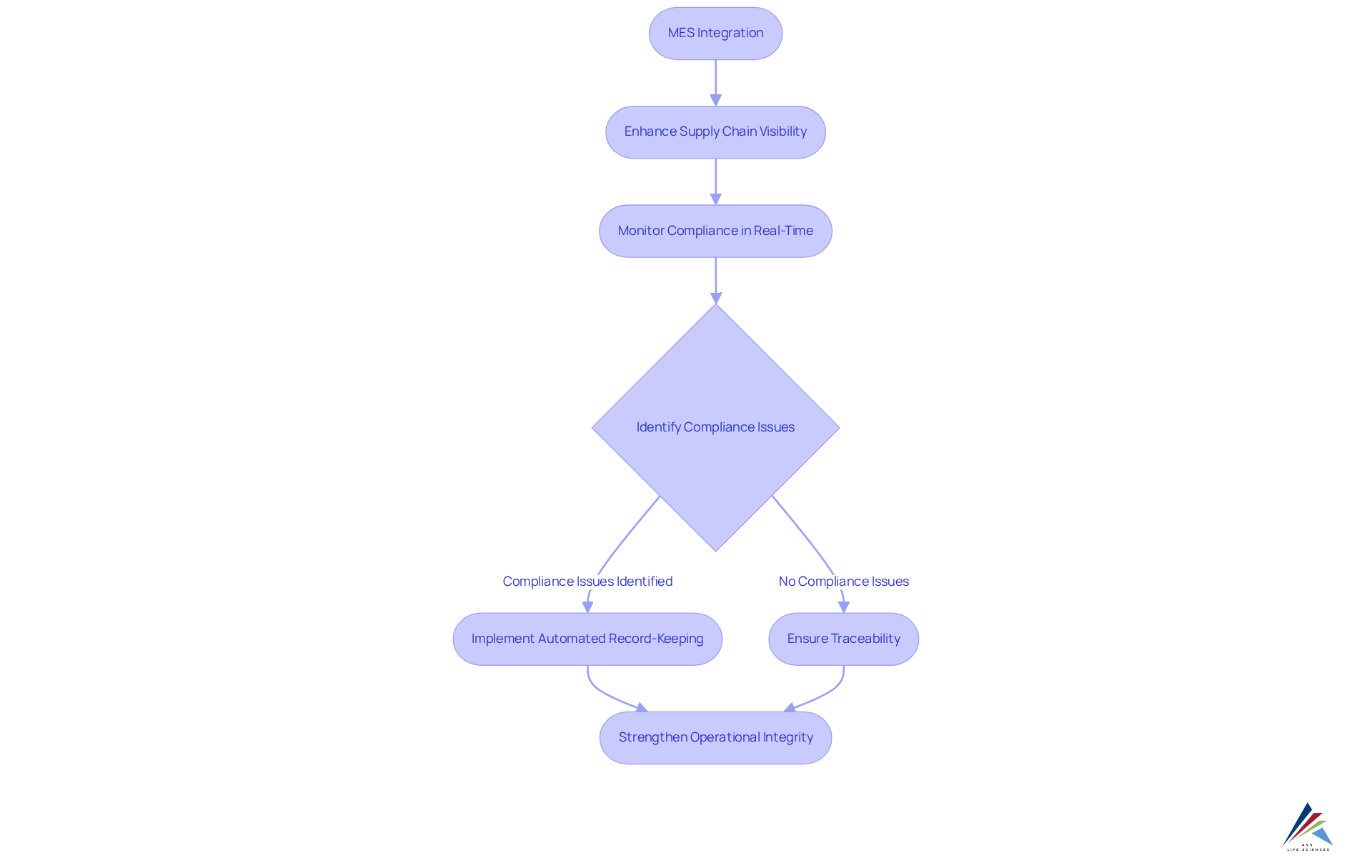
The integration of Manufacturing Execution Systems (MES integration) significantly enhances supply chain visibility, enabling oversight personnel to monitor every aspect of the supply chain in real-time. This heightened visibility is essential for ensuring that all suppliers and partners adhere to regulatory standards, particularly in controlled sectors such as pharmaceuticals and medical devices.
By enhancing oversight capabilities, organizations can quickly identify and address compliance issues, thereby reducing the risk of non-compliance and strengthening overall operational integrity. For example, AVS Life Sciences recently supported a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project adhered to strict timelines and budgets while ensuring for complete traceability, which is vital for compliance audits and inspections.
The MES integration of automated record-keeping guarantees full traceability of materials and processes, conforming to standards specified in Title 21, Part 211 and ISO 9001. Additionally, user-configurable dashboards within MES integration allow oversight officers to efficiently monitor adherence metrics, ensuring that all operations align with established governance frameworks.
As one oversight officer remarked, 'Monitoring our supply chain for regulatory adherence is not just a necessity; it’s a strategic advantage that shields our organization from potential risks.' This proactive approach to regulatory oversight not only enhances operational efficiency but also fosters a culture of accountability and transparency throughout the supply chain.
Integration of Disparate Systems: Overcoming Compliance Challenges
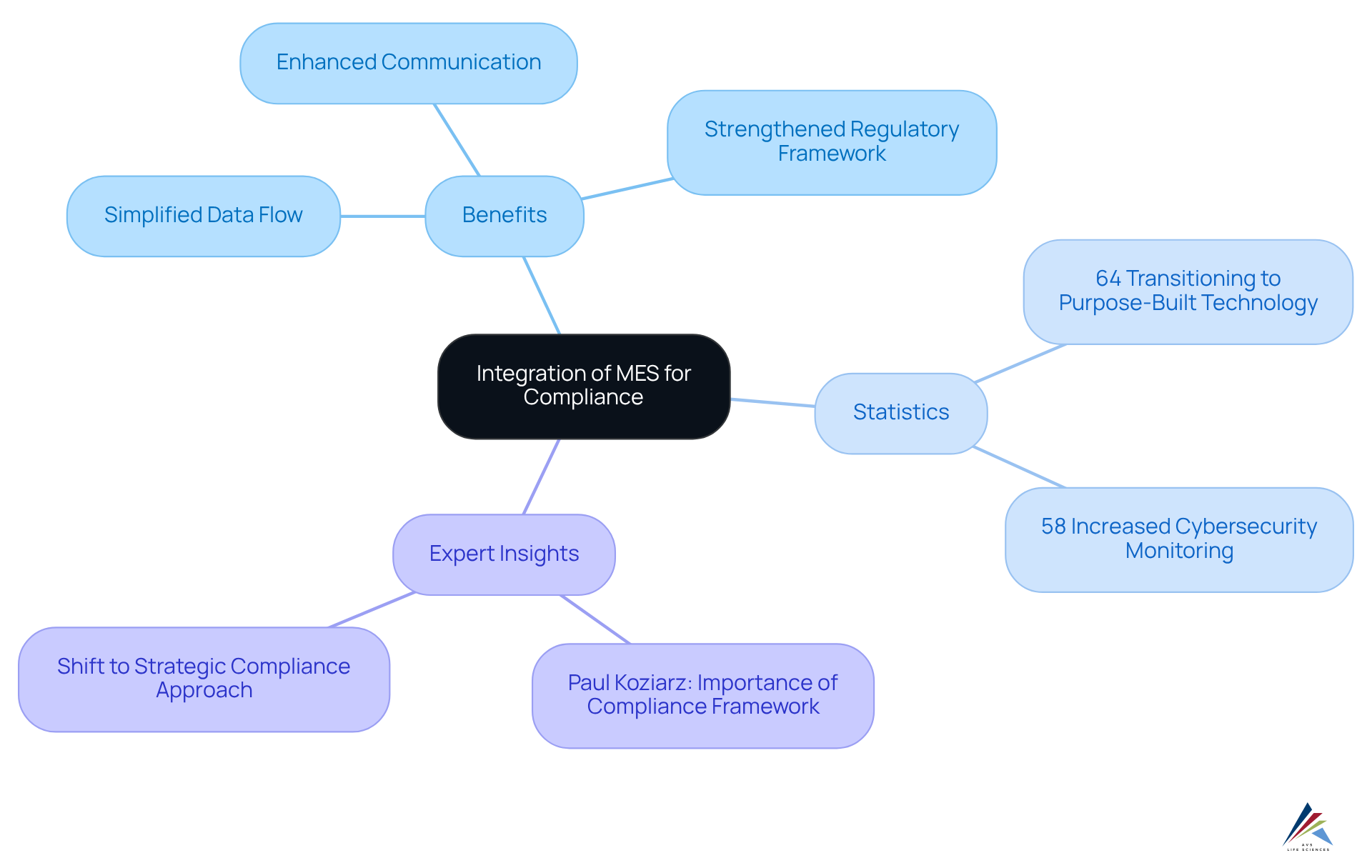
Organizations frequently encounter challenges with adherence due to the complexities of disparate systems. The mes integration of Manufacturing Execution Systems (MES) serves as a strategic solution, unifying various platforms into a cohesive framework. This integration not only simplifies data flow but also enhances interdepartmental communication, leading to a more . By addressing these challenges, organizations can significantly strengthen their regulatory frameworks.
The advantages of MES integration go beyond basic data management. For instance, 64% of businesses are transitioning to purpose-built technology for regulatory management, recognizing that integrated systems can yield improved oversight and reduced complexity. Industry leaders emphasize the necessity of a unified regulatory strategy; as Paul Koziarz notes, "Without adherence to regulations, many organizations wouldn’t have security controls in place, and there would be no consistency of standards among the protocols being used."
Moreover, organizations that have successfully implemented MES integration report improved adherence efforts. A notable increase in cybersecurity monitoring—58% of regulatory teams have intensified their efforts in response to new rules—demonstrates the proactive approach that integrated systems can foster. By leveraging MES, companies can not only meet legal obligations but also cultivate a culture of adherence that permeates all levels of the organization.
Thorough System Analysis: Identifying Compliance Gaps
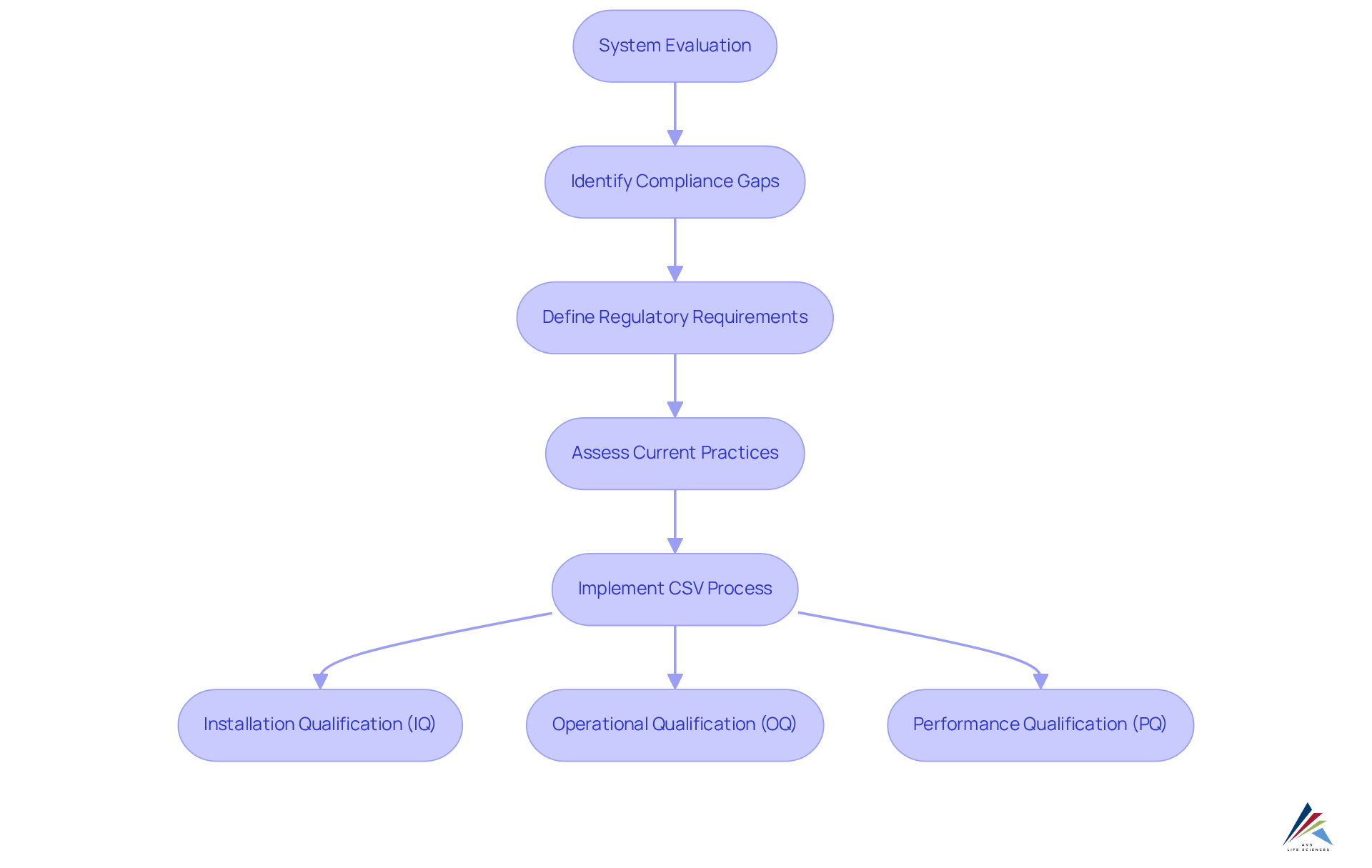
Performing a comprehensive system evaluation is essential for identifying gaps that may lead to oversight issues. The mes integration significantly enhances this analysis by providing extensive data and insights into production workflows. By consistently assessing systems and procedures, organizations can proactively identify and address potential adherence challenges, ensuring compliance with legal standards.
A systematic evaluation of adherence gaps, which includes defining regulatory requirements and assessing current practices, can reveal weaknesses in regulatory compliance. This not only mitigates risks but also fosters operational resilience.
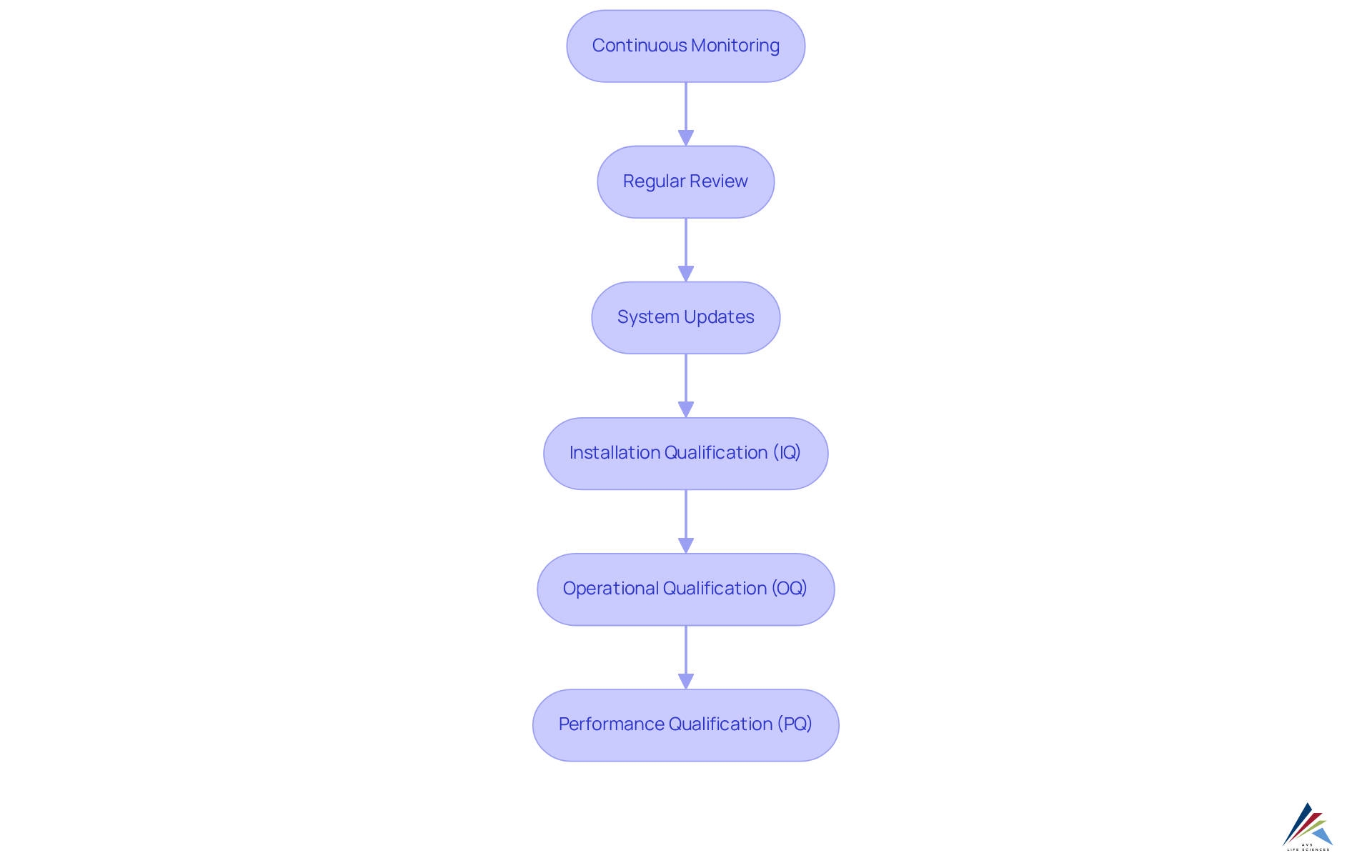
Furthermore, implementing a robust Computer System Validation (CSV) process, which encompasses stages such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), guarantees that systems function as intended and meet regulatory demands.
As emphasized by industry specialists, mes integration not only boosts operational efficiency but also allows organizations to swiftly adapt to evolving regulatory requirements. By leveraging mes integration alongside a comprehensive CSV framework, companies can transform regulatory management from a reactive obligation into a strategic advantage, ultimately enhancing their reputation and operational agility.
Continuous Monitoring and Maintenance: Sustaining Compliance
Continuous monitoring and maintenance of MES integration systems are paramount for sustaining compliance over time. Organizations must regularly review and update their systems to adapt to evolving regulatory requirements and industry standards. The comprehensive computer system validation (CSV) process, encompassing stages such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), is critical in this endeavor.
For instance, White Raven achieved GMP certification in just 18 months by implementing regular updates to their MES systems, demonstrating the effectiveness of proactive adherence strategies. This approach not only aids in but also fosters a culture of quality and responsibility within the organization.
As Peter F. Drucker aptly noted, efficiency in adherence activities is vital; there is nothing so futile as executing efficiently that which should not be done at all. By prioritizing system updates and adhering to the MES integration process, organizations can enhance their compliance sustainability, thereby demonstrating their commitment to quality and regulatory adherence.
Conclusion
The integration of Manufacturing Execution Systems (MES) presents a transformative strategy for compliance officers in the life sciences sector, significantly enhancing regulatory adherence and operational efficiency. By automating compliance processes and offering real-time data visibility, MES integration streamlines regulatory management, substantially reduces human error, and improves data accuracy—critical elements for meeting stringent compliance standards.
Key insights from the article underscore the multiple advantages of MES integration, such as:
- Enhanced quality management
- Optimized production planning
- Improved supply chain visibility
Organizations that embrace MES can anticipate notable reductions in defect rates and downtime, fostering a proactive culture of compliance that facilitates swift corrective actions in response to regulatory deviations. The phased implementation approach guarantees minimal disruption while maximizing the long-term benefits of the system.
Ultimately, the significance of MES integration cannot be overstated. It stands as a strategic necessity for compliance officers, empowering organizations to adeptly navigate the complexities of regulatory requirements. By prioritizing continuous monitoring and maintenance of these systems, companies not only protect their compliance status but also cultivate a culture of quality and accountability that resonates throughout their operations. Embracing MES integration transcends merely meeting current regulations; it positions organizations for future success in an ever-evolving regulatory landscape.
Frequently Asked Questions
What is the primary benefit of integrating Manufacturing Execution Systems (MES) in life sciences?
The primary benefit of MES integration in life sciences is enhanced adherence to regulatory compliance, including Good Manufacturing Practices (GMP), GXP, and FDA standards, while also improving operational efficiency.
How does MES integration affect operational efficiency and compliance?
MES integration automates compliance-related tasks, streamlines regulatory management, and allows organizations to focus on core activities while maintaining high standards of quality and compliance.
What impact does MES have on GMP adherence specifically?
MES facilitates real-time monitoring and documentation, leading to a 15-30% reduction in downtime and a 25-40% decrease in defect rates, which significantly improves production quality and reliability.
What is the role of real-time data provided by MES?
Real-time data from MES enables swift corrective actions, ensuring compliance and operational excellence by allowing organizations to address issues as they arise.
What is a key advantage of MES integration regarding data accuracy?
MES integration enhances data accuracy by capturing real-time data directly from production processes, significantly reducing manual entry errors, which can account for up to 30% of inaccuracies in reporting.
How does accurate data contribute to compliance?
Accurate data is foundational for meeting legal requirements and maintaining stakeholder trust, fostering a culture of transparency that supports compliance and strengthens relationships with regulatory agencies.
What are the challenges faced during the MES upgrade phase?
Challenges included issues like the installation of barcode scanner cameras upside down, which initially led to overlooked anomalies in test results. Addressing these concerns improved overall data integrity.
What benefits do organizations experience from real-time data visibility through MES?
Organizations gain real-time insight into production processes, enabling the prompt identification of deviations from regulatory standards and reducing audit preparation time by 60%.
How does MES integration contribute to reducing financial risks associated with non-compliance?
By ensuring adherence throughout the production lifecycle, MES integration minimizes potential expenses linked to non-compliance, which can average over $5 million for breaches.
What is the typical timeframe for successful MES implementation?
Successful MES implementation typically requires a phased approach spanning 8-12 months, which includes assessment, design, development, testing, and rollout to minimize disruption.
