7 Key Benefits of Continuous Validation for Compliance Officers

Overview
The article delineates seven key benefits of continuous validation for compliance officers, asserting its pivotal role in bolstering regulatory adherence, operational efficiency, and product quality within the pharmaceutical sector. It articulates how ongoing validation processes effectively mitigate compliance risks, reduce costs, and cultivate a culture of accountability. Ultimately, these benefits contribute to enhanced patient outcomes and competitive advantages in an industry characterized by rapid evolution.
Introduction
Continuous validation has emerged as a pivotal strategy for compliance officers in the pharmaceutical industry, driven by the imperative for rigorous adherence to ever-evolving regulatory standards. This approach not only enhances operational efficiency but also significantly improves product quality, thereby safeguarding both organizational integrity and patient safety.
However, as the landscape of regulatory compliance becomes increasingly complex, the question arises: how can continuous validation be effectively integrated into existing frameworks to mitigate risks and ensure sustained adherence?
Exploring the multifaceted benefits of this proactive methodology reveals its critical role in shaping a resilient compliance strategy for the future.
AVS Life Sciences: Comprehensive Continuous Validation Solutions for Pharmaceutical Compliance
AVS Life Sciences provides a comprehensive array of ongoing verification solutions specifically designed for the pharmaceutical sector, ensuring that organizations comply with stringent regulatory requirements and quality assurance benchmarks. By focusing on Good Manufacturing Practices (GMP), GXP, and CAPA, AVS empowers clients throughout the product lifecycle with tailored services that include:
- Assessment and commissioning
- Quality compliance consulting
- Engineering support
All underpinned by robust documentation practices.
Current trends highlight an increasing emphasis on continuous validation as a strategy to enhance operational efficiency and product quality. The integration of advanced analytics and real-time data monitoring is revolutionizing verification processes, facilitating proactive adjustments and improved decision-making. This transition not only streamlines compliance efforts but also fosters a culture of within organizations, aligning with best practices in documentation and standard operating procedures (SOPs).
The benefits of ongoing verification are extensive. It allows pharmaceutical companies to ensure continuous validation throughout the product lifecycle, mitigating the risk of non-compliance and safeguarding product integrity. Furthermore, as the demand for ongoing assessment services is expected to rise significantly in 2025, organizations that adopt these strategies are likely to gain a competitive edge in the rapidly evolving life sciences industry.
Expert opinions underscore the importance of ongoing verification in pharmaceutical compliance, emphasizing its role in minimizing risks associated with batch-to-batch variability and process uncertainties. By leveraging AVS Life Sciences' expertise in GXP, CAPA, and meticulous documentation practices, oversight officers can refine their assessment processes, ensuring that quality and regulatory standards are not only achieved but exceeded, ultimately leading to improved patient outcomes.

Regulatory Compliance: Ensuring Adherence Through Continuous Validation
Ongoing assessment is crucial for ensuring compliance with regulatory requirements. By implementing continuous validation procedures, entities can proactively identify and address regulatory issues before they escalate. This strategy significantly and fosters a culture of accountability and transparency.
Compliance officers can utilize ongoing assessments to stay informed about regulatory changes, ensuring their entities align with evolving standards. Notably, 67% of entities employing ongoing adherence report larger teams compared to those relying on momentary adherence methods, underscoring the importance of this approach.
Furthermore, organizations that utilize continuous validation strategies often experience enhanced operational efficiency and reduced costs related to regulatory failures. For instance, companies leveraging AI and automation in their regulatory processes have reported average savings of approximately $1.45 million.
A transformative case study involving AVS Life Sciences exemplifies this: a leading biotechnology firm upgraded its GMP facility with AVS's assistance, improving their quality assurance processes and ensuring regulatory compliance. This collaboration not only bolstered their operational capabilities but also highlighted the importance of ongoing assessment in identifying process gaps, leading to valuable lessons learned.
However, implementing ongoing assessment can present challenges, such as the need for adequate training and resources. Organizations may need to invest in training programs to equip staff for managing new verification technologies. Addressing these challenges is vital for maximizing the benefits of ongoing assessment.
As the landscape of regulatory requirements continues to evolve, the importance of continuous validation in ensuring regulatory adherence cannot be overstated.
Product Quality Improvement: The Impact of Continuous Validation
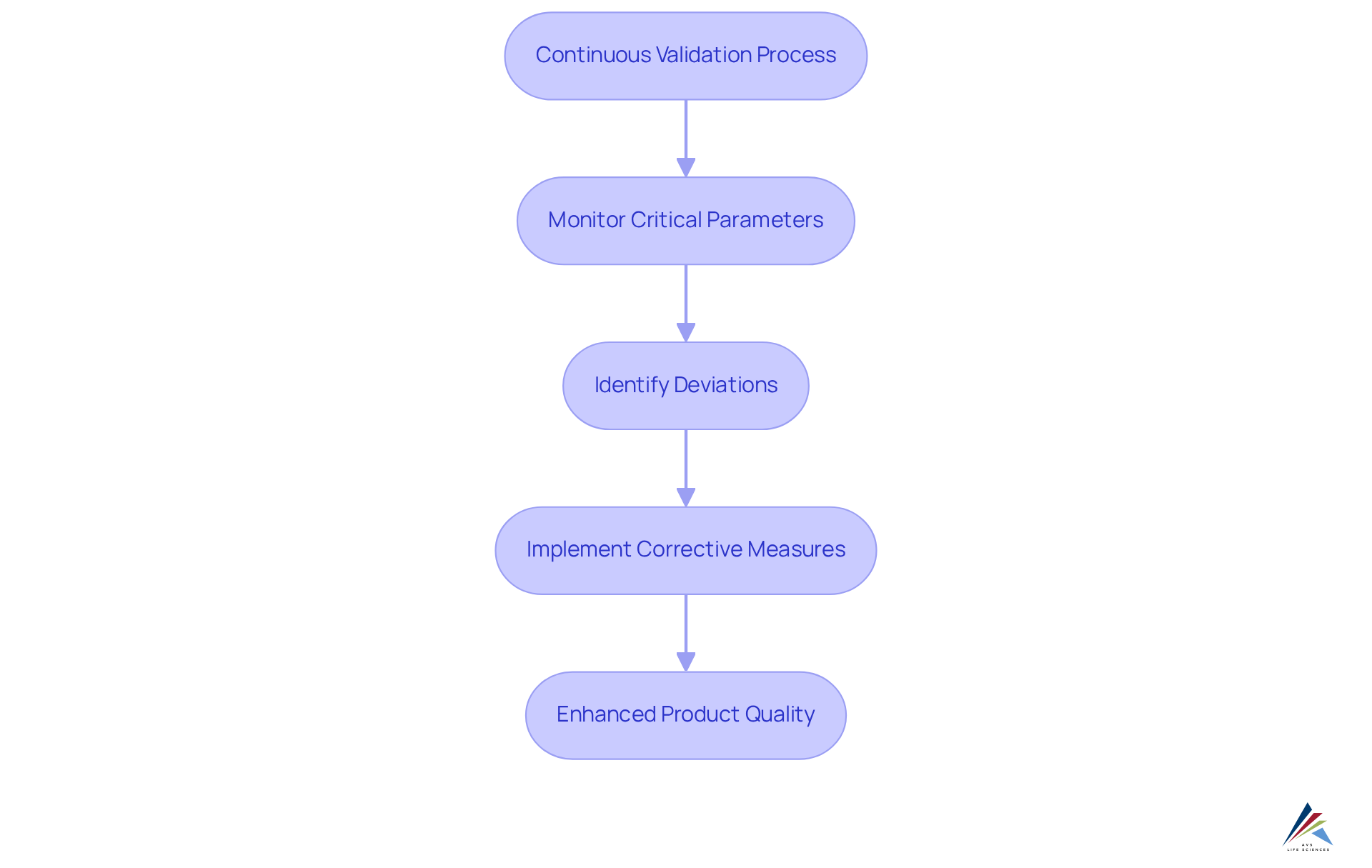
Ongoing assessment stands as a cornerstone for enhancing product quality, ensuring that processes remain within a controlled state. By utilizing continuous validation to monitor critical parameters and processes in real-time, organizations can swiftly identify deviations and implement corrective measures. This proactive strategy not only strengthens product consistency but also significantly , leading to substantial operational cost savings and heightened customer satisfaction.
Notably, achieving a Six Sigma level of performance corresponds to a Defects Per Unit (DPU) of merely 0.00034, translating to just 3.4 defects per million opportunities. Such rigorous standards cultivate trust in the brand and elevate overall market reputation.
Industry leaders emphasize that effective continuous validation goes beyond regulatory requirements; it is a fundamental element of quality assurance that drives operational excellence and enhances customer loyalty. By integrating real-time monitoring systems, organizations can ensure that product quality remains uncompromised, thereby aiding compliance with GXP and FDA regulations while enhancing profitability.
AVS Life Sciences positions itself as a leading provider of quality management and regulatory oversight solutions, offering expert guidance in GMP adherence and verification tailored specifically for the pharmaceutical and biotechnology sectors.
Risk Management: Mitigating Compliance Risks with Continuous Validation
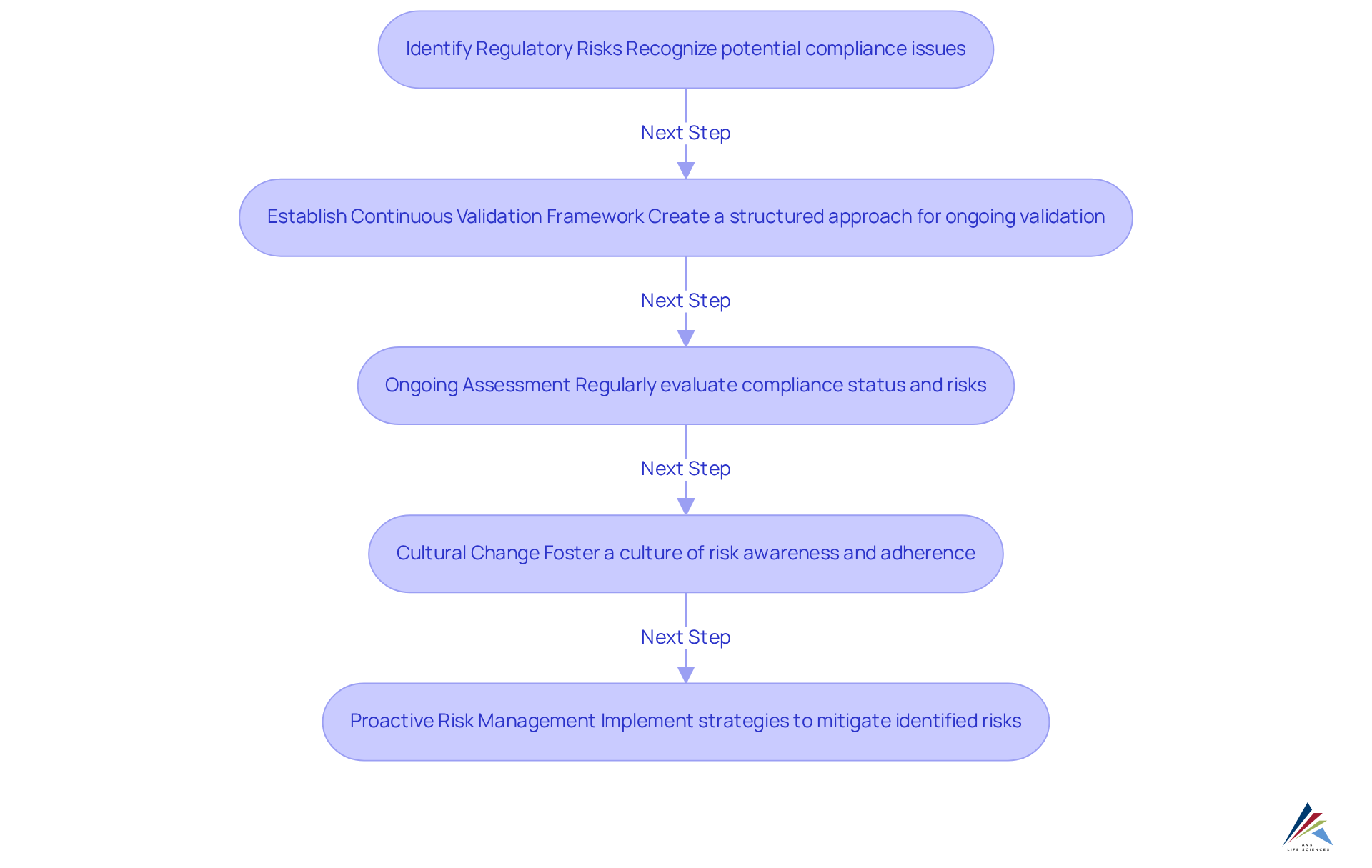
Ongoing validation is a crucial approach for reducing regulatory risks in today's intricate legal environment. By establishing a framework for continuous validation and evaluation, entities can proactively recognize potential risks before they escalate into violations. This approach not only minimizes the likelihood of incurring costly penalties but also protects the organization's reputation.
For instance, AVS Life Sciences successfully assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on schedule and within budget. Our documentation efforts exhibited complete traceability, enabling the client to produce medication with lentivirus vector material, highlighting the effectiveness of ongoing verification in improving quality assurance and regulatory adherence. This partnership enabled the client to concentrate on creating medicines while guaranteeing adherence, ultimately resulting in a substantial decrease in adherence-related expenses and audit results.
Furthermore, ongoing assessment fosters a culture of risk awareness within teams, motivating them to prioritize adherence in their daily activities. This cultural change is essential, as 73% of regulatory breaches involve human error, emphasizing the need for a vigilant workforce. By incorporating continuous validation into their regulatory strategies, organizations empower their employees to identify and tackle regulatory issues proactively.
In 2025, the focus on ongoing verification will only increase, as regulatory bodies more frequently embrace real-world evidence to support conventional adherence strategies. This evolution presents both opportunities and challenges, making it essential for oversight officers to utilize ongoing verification as a key resource in their risk management toolkit. Ultimately, the implementation of not only enhances adherence efficiency but also equips organizations to navigate the changing regulatory environment with confidence.
As Sarah Lee aptly stated, "Data-driven adherence represents a paradigm shift from reactive to proactive regulatory management.
Operational Efficiency: Streamlining Processes with Continuous Validation
Incorporating continuous validation into regulatory workflows significantly enhances operational efficiency by automating standard checks and substantially decreasing the need for manual interventions. This strategic approach not only accelerates processes but also , which is critical for maintaining regulatory standards.
Entities that have embraced ongoing assessment report time savings of up to 60% in regulatory tasks, enabling oversight officers to redirect their focus toward strategic initiatives rather than merely reacting to regulatory demands. By ensuring that adherence is seamlessly integrated into daily operations, organizations can enhance their agility and responsiveness, ultimately leading to improved outcomes and reduced operational risks.
Continuous validation fosters a culture of proactive adherence, enabling teams to stay ahead of regulatory demands while maintaining high standards of quality and efficiency.
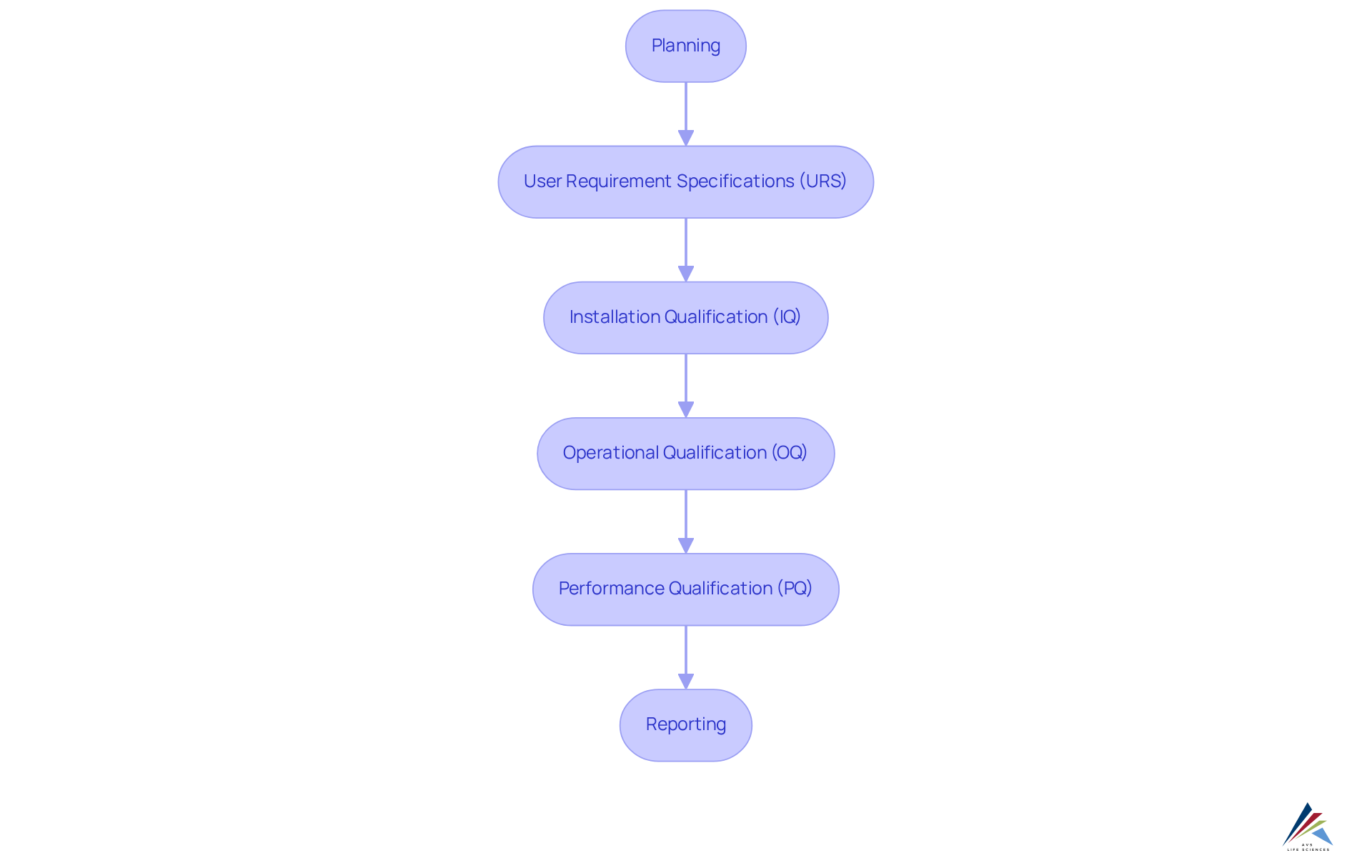
The computer system verification process, as detailed in the GAMP 5 Guide, emphasizes the significance of organized phases such as:
- Planning
- User Requirement Specifications (URS)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Reporting
These stages guarantee that systems are thoroughly validated, providing a dependable framework for adherence to essential regulatory standards such as GXP and FDA regulations.
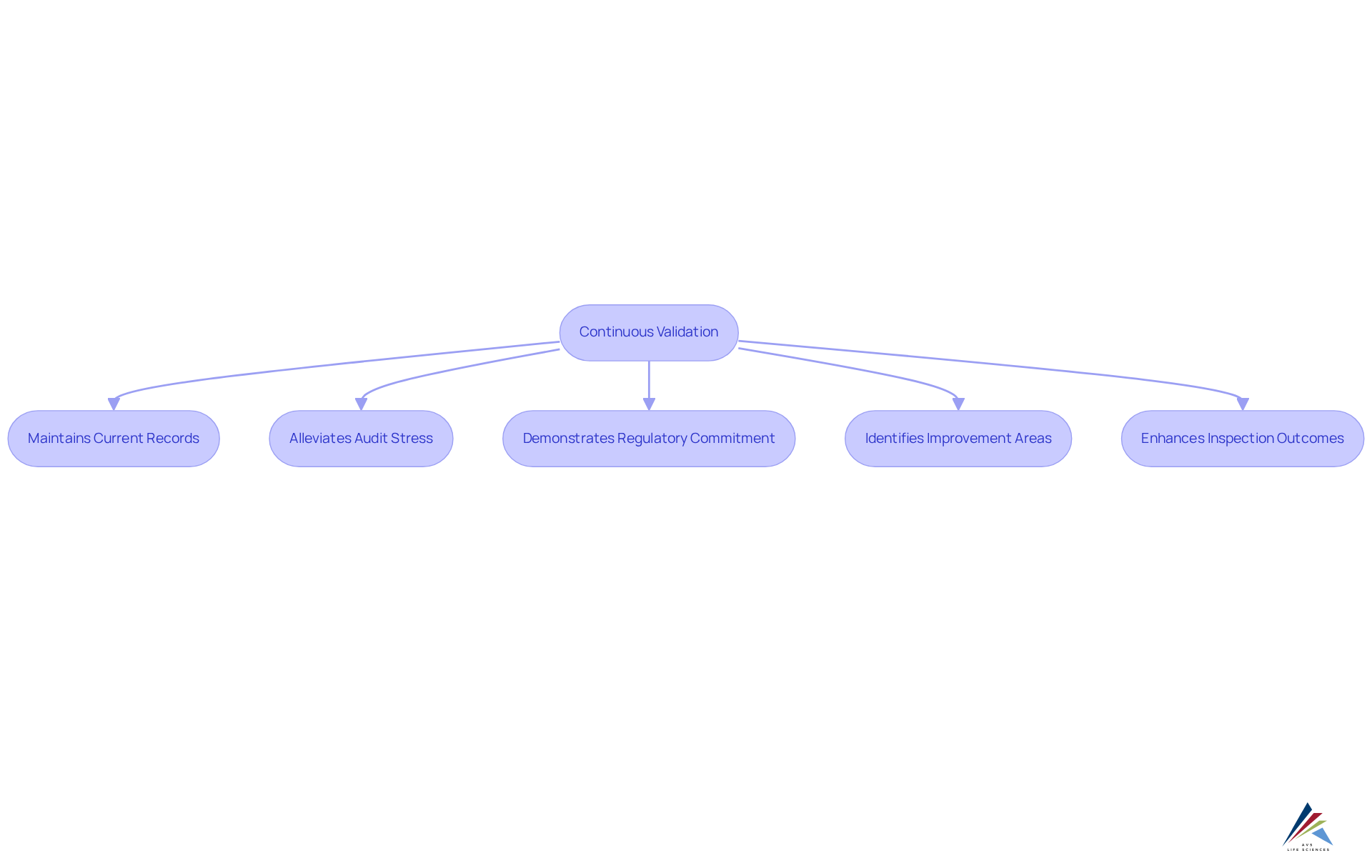
Audit Readiness: Preparing for Inspections with Continuous Validation
Ongoing verification through continuous validation significantly enhances audit preparedness by ensuring that companies maintain current records of adherence actions and verification procedures. This continuous validation not only equips firms for inspections but also alleviates the stress commonly associated with audits. Compliance officers can easily access up-to-date information, demonstrating their commitment to regulatory adherence.
For instance, AVS Life Sciences exemplifies the effectiveness of ongoing assessment through its successful upgrade of a biotechnology GMP facility. Here, meticulous documentation efforts ensured complete traceability and compliance with regulatory standards. This not only bolstered patient confidence but also strengthened data security.
Moreover, continuous validation helps in identifying areas for improvement, enabling entities to proactively address potential issues before they arise during an audit. This method has been linked to enhanced inspection outcomes, as companies employing continuous validation strategies often experience fewer audit interruptions and a higher rate of compliance success.
Cost-Effectiveness: Financial Benefits of Continuous Validation
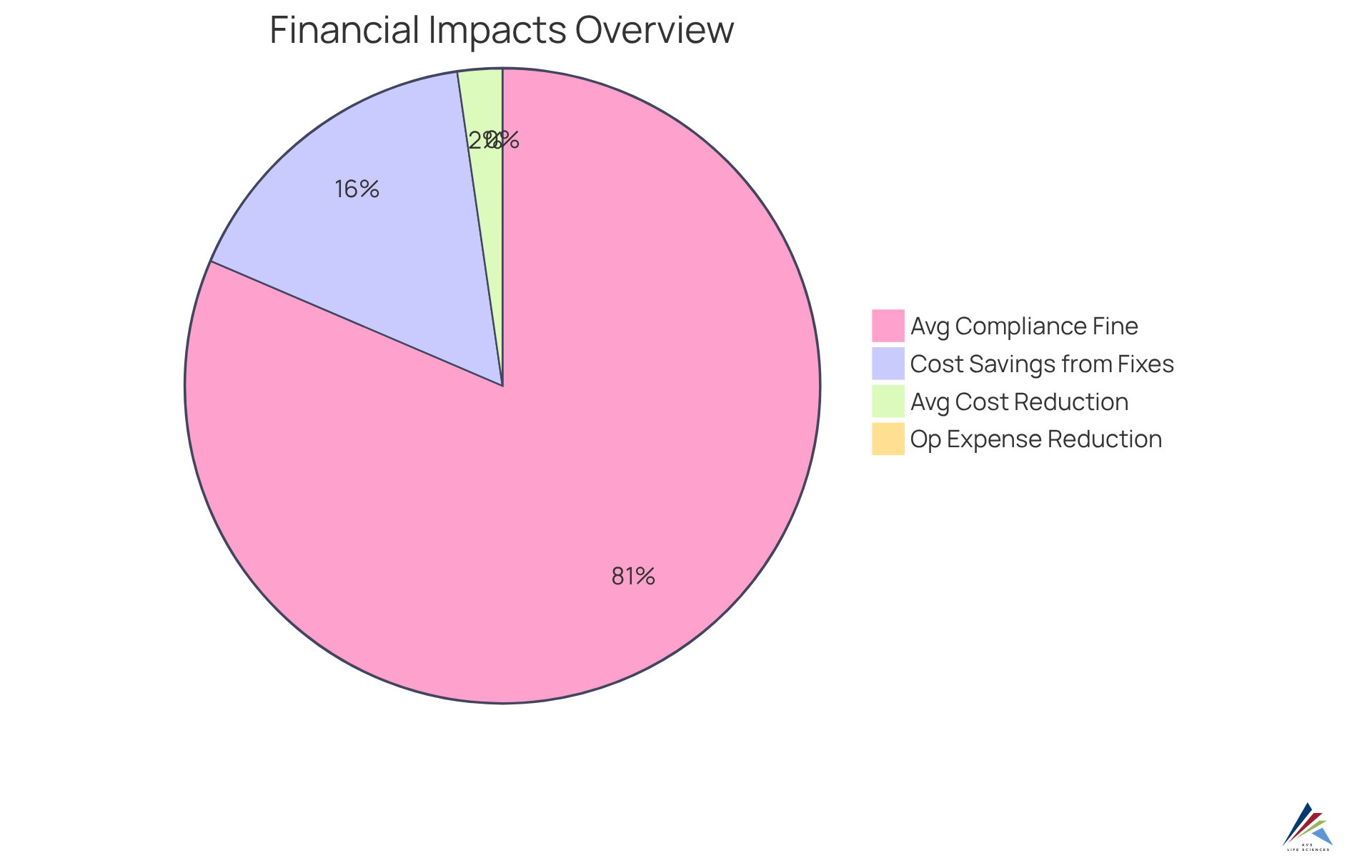
Investing in continuous validation offers significant for organizations in the pharmaceutical sector. By proactively addressing regulatory challenges, companies can greatly diminish the necessity for costly emergency fixes and reduce the risk of compliance breaches, which may result in substantial fines and penalties. In fact, compliance violations can impose millions in costs on pharmaceutical firms, with fines averaging approximately $2.5 million per incident.
Furthermore, the efficiencies gained through automation and optimized processes can lead to reduced operational expenses, with companies reporting reductions of up to 50% in overhead costs following the implementation of integrated verification systems. Compliance officers can leverage these financial benefits to persuade stakeholders that continuous validation goes beyond mere regulatory obligation; it represents a strategic investment that enhances the organization's long-term sustainability and profitability.
By presenting data indicating an average first-year cost reduction of $70,000 stemming from ongoing improvement initiatives, oversight officers can effectively demonstrate the tangible returns on investment associated with continuous validation efforts.
Integration with Quality Management Systems: Enhancing Compliance through Continuous Validation
Incorporating continuous validation within quality management systems (QMS) is essential for enhancing adherence efforts. By embedding continuous validation procedures into QMS, organizations can achieve a cohesive alignment of quality and regulatory initiatives. This integration facilitates seamless data flow and real-time oversight, enabling regulatory officers to make informed decisions based on accurate, up-to-date information. Moreover, a well-integrated system cultivates a culture of continuous improvement, positioning continuous validation as an ongoing commitment rather than a one-time task.
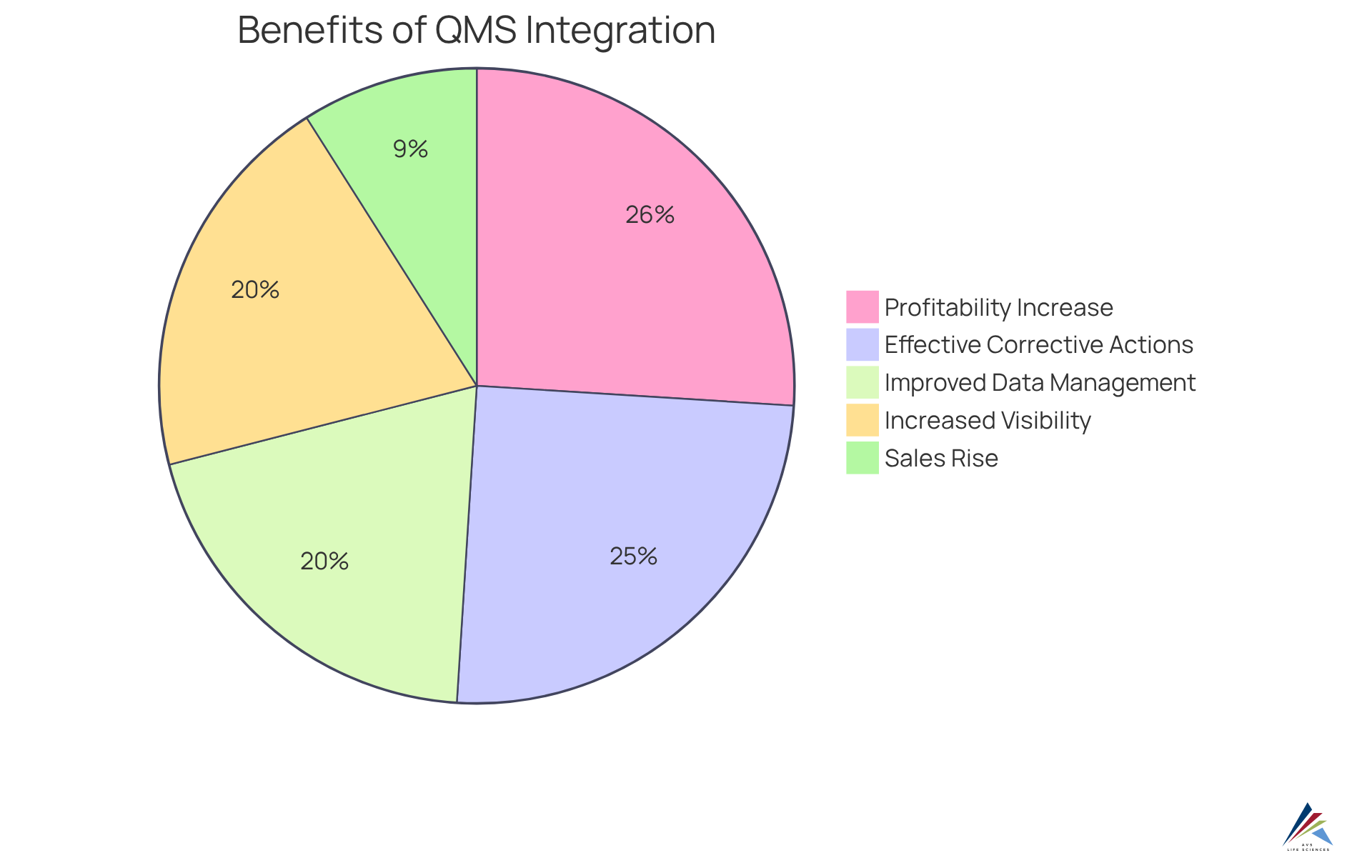
Organizations that have successfully enhanced their adherence through QMS integration report substantial benefits. For example, companies employing automated QMS solutions have seen a 26% increase in profitability and a 9% rise in sales, underscoring the financial gains of effective quality management. Additionally, 83% of organizations believe that a QMS solution has significantly aided their recovery from product recalls, emphasizing the importance of having a responsive system in place.
The benefits of integrating ongoing verification within QMS extend beyond adherence; they include , increased visibility, and more effective corrective actions. These improvements not only reduce risks associated with regulatory non-compliance but also promote a proactive approach to quality management. As organizations increasingly recognize the strategic importance of quality management, the integration of continuous validation becomes a vital component in achieving sustained compliance and operational excellence.
Training and Support: Empowering Teams for Continuous Validation Success
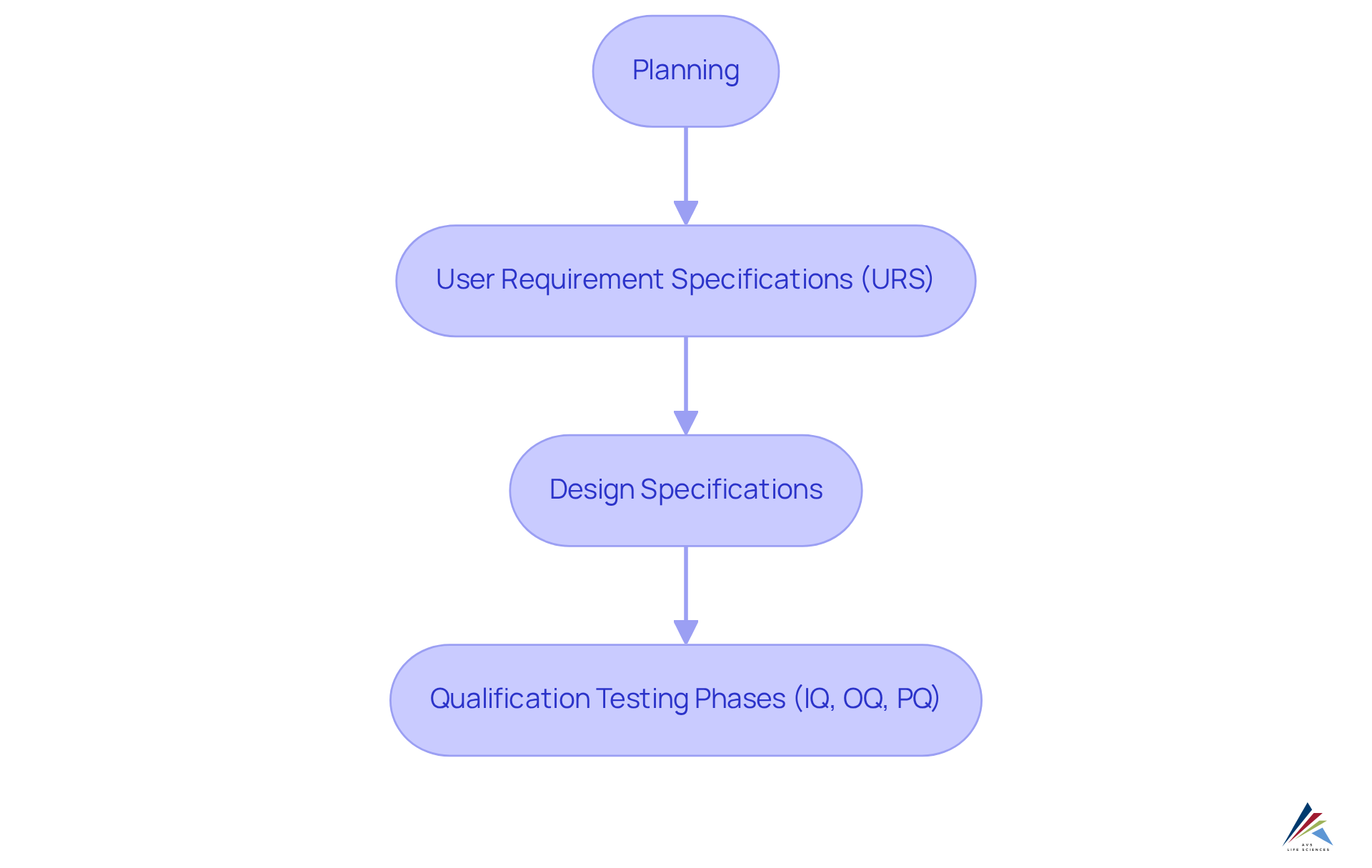
Efficient training and assistance are essential for the successful execution of continuous validation in the life sciences field. Organizations must invest in comprehensive training programs that equip employees with the knowledge and skills necessary to navigate the complexities of the computer system verification process. This process encompasses critical stages such as:
- Planning
- User Requirement Specifications (URS)
- Design Specifications
- Various qualification testing phases (IQ, OQ, PQ)
By fostering an environment of ongoing education, regulatory officers can ensure that their teams are well-prepared to adapt to evolving regulations and industry benchmarks. Furthermore, continuous validation from management empowers teams to take ownership of their responsibilities, thereby promoting a proactive approach to ongoing verification. Aligning with established standards, such as those outlined in the GAMP 5 Guide, underscores the significance of thorough training in achieving comprehensive quality management and regulatory compliance.
Strategic Advantages: Leveraging Continuous Validation for Pharmaceutical Compliance
Ongoing assessment provides companies with substantial strategic advantages in the competitive pharmaceutical landscape. By embracing a proactive regulatory approach, businesses can distinguish themselves from competitors who still rely on traditional verification methods. This innovative framework not only enhances regulatory compliance but also cultivates agility, enabling organizations to swiftly respond to market fluctuations.
For example, AVS Life Sciences played a pivotal role in assisting a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade resulted in a remarkable 30% increase in and a 25% reduction in verification time, underscoring the effectiveness of ongoing assessment in enhancing quality assurance.
Companies that implement Continuous Process Verification (CPV) have reported greater operational flexibility and reduced downtime, with assessments completed at least seven times faster using GO!FIVE® software. Moreover, the integration of CPV with Quality by Design (QbD) principles facilitates real-time monitoring of Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs), ensuring that product quality remains high while complying with regulatory standards.
The Annual Product Quality Review (APQR) is a vital component of CPV, systematically evaluating product quality data throughout its lifecycle. Regulatory officers should advocate for continuous validation as a core element of their strategic initiatives, as it not only promotes adherence to standards but also drives business success and innovation in a continually evolving industry.
The insights gained from the case study highlight the significance of meticulous documentation and proactive quality assessments, which can markedly enhance compliance efforts.

Conclusion
Continuous validation emerges as a pivotal strategy for compliance officers in the pharmaceutical sector, ensuring not only adherence to regulatory demands but also enhancing operational efficiency and product quality. By integrating ongoing verification into their processes, organizations can foster a culture of proactive compliance that mitigates risks and drives continuous improvement throughout the product lifecycle.
The article underscores several key benefits of continuous validation, including its role in regulatory compliance, risk management, and operational efficiency. Ongoing assessment allows companies to identify and address potential issues before they escalate, ultimately leading to improved audit readiness and significant cost savings. Furthermore, the integration of continuous validation with quality management systems amplifies its impact, ensuring that organizations remain agile and responsive to evolving regulatory landscapes.
As the pharmaceutical industry continues to evolve, embracing continuous validation is not merely a best practice; it is a strategic imperative. Compliance officers are encouraged to advocate for these solutions within their organizations, recognizing that a proactive approach to validation will not only enhance compliance but also position their companies for long-term success in a competitive market. Investing in continuous validation is an investment in quality, efficiency, and ultimately, patient safety.
Frequently Asked Questions
What services does AVS Life Sciences provide for pharmaceutical compliance?
AVS Life Sciences offers a comprehensive array of ongoing verification solutions, including assessment and commissioning, quality compliance consulting, and engineering support, all focused on ensuring compliance with Good Manufacturing Practices (GMP), GXP, and CAPA.
Why is continuous validation important in the pharmaceutical sector?
Continuous validation is crucial as it enhances operational efficiency and product quality, mitigates the risk of non-compliance, and supports a culture of continuous improvement within organizations.
How does AVS Life Sciences support ongoing verification processes?
AVS Life Sciences supports ongoing verification through advanced analytics and real-time data monitoring, facilitating proactive adjustments and improved decision-making while ensuring robust documentation practices.
What are the benefits of ongoing verification in the pharmaceutical industry?
Ongoing verification helps ensure continuous validation throughout the product lifecycle, mitigates non-compliance risks, safeguards product integrity, and positions organizations to gain a competitive edge as demand for these services rises.
What impact does continuous validation have on operational efficiency and costs?
Organizations that implement continuous validation strategies often experience enhanced operational efficiency and reduced costs related to regulatory failures, with some companies reporting average savings of approximately $1.45 million.
What challenges might organizations face when implementing ongoing assessments?
Organizations may encounter challenges such as the need for adequate training and resources, requiring investment in training programs to equip staff for managing new verification technologies.
How does continuous validation contribute to product quality improvement?
Continuous validation helps monitor critical parameters and processes in real-time, allowing organizations to swiftly identify deviations, implement corrective measures, and ultimately enhance product consistency and reduce defect rates.
What is the significance of achieving a Six Sigma level of performance in product quality?
Achieving a Six Sigma level corresponds to a Defects Per Unit (DPU) of 0.00034, translating to just 3.4 defects per million opportunities, which cultivates trust in the brand and enhances overall market reputation.
How does AVS Life Sciences position itself in the market?
AVS Life Sciences positions itself as a leading provider of quality management and regulatory oversight solutions, offering expert guidance in GMP adherence and verification tailored specifically for the pharmaceutical and biotechnology sectors.
