7 EDC Systems Enhancing Compliance in Clinical Trials

Overview
This article examines seven electronic data capture (EDC) systems that significantly enhance compliance in clinical trials. The systems, including Medidata Rave, OpenClinica, and Xybion, are designed with features that not only improve data accuracy but also streamline operational processes and ensure adherence to regulatory standards. These capabilities collectively support the integrity and success of clinical research. By addressing compliance challenges, these EDC solutions emerge as essential tools for organizations aiming to elevate their clinical trial processes.
Introduction
As the clinical research landscape evolves, the integration of Electronic Data Capture (EDC) systems has emerged as a pivotal factor in ensuring compliance and enhancing the efficiency of clinical trials. These advanced systems not only streamline data management but also assist organizations in navigating the complex web of regulatory requirements. This ultimately leads to higher quality research outcomes.
However, with numerous EDC solutions available, how can sponsors determine which systems will best meet their compliance needs and improve data integrity? This article explores seven leading EDC systems that are transforming compliance in clinical trials, shedding light on their unique features and benefits.
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for EDC Implementation
AVS Life Sciences offers a comprehensive suite of services that facilitate the efficient implementation of EDC systems in research studies. Their extensive knowledge of regulatory compliance guarantees that EDC systems adhere to rigorous industry standards, including Good Manufacturing Practices (GMP) and ISO regulations.
By applying robust quality management strategies, AVS Life Sciences empowers clients to enhance their data collection processes while maintaining high standards of data integrity and compliance. This integrated approach not only boosts the effectiveness of research studies but also significantly .
As the research landscape evolves—with an estimated 60% of new studies embracing EDC systems—AVS Life Sciences stands out as a trusted partner, committed to helping clients navigate the complexities of regulatory requirements and achieve successful study outcomes.
Medidata Rave EDC: Leading Efficiency in Electronic Data Capture for Clinical Trials
Medidata Rave is recognized as a premier EDC system, part of the broader category of EDC systems, that significantly enhances efficiency in electronic information collection for studies, all while ensuring strict adherence to GXP and FDA regulations.
With a proven track record of supporting over 36,000 studies and 11 million individuals, Rave's intuitive interface allows for rapid information entry and real-time oversight, which are critical for maintaining data accuracy.
The system adeptly accommodates complex study designs and integrates seamlessly with other research management tools, empowering researchers to access and analyze data swiftly.
Additionally, Medidata Rave's robust compliance features, including adherence to Standard Operating Procedures (SOPs) and effective documentation practices, along with internal and external auditing capabilities, aid organizations in meeting regulatory requirements, establishing it as a preferred choice among research sponsors.
Recently, the platform has introduced AI-driven enhancements to bolster protocol compliance, further reinforcing its position as a favored option within the industry.
With relying on Medidata's platform, its efficacy in overseeing research studies has been demonstrated through numerous successful applications, solidifying its reputation as a leader in EDC systems and an indispensable tool for quality management in the life sciences sector.
OpenClinica: User-Friendly EDC System for Enhanced Data Management
OpenClinica distinguishes itself as one of the leading EDC systems that prioritizes user experience, making it an exemplary choice for clinical research teams. Its intuitive interface empowers users to navigate the system effortlessly, significantly minimizing the average learning curve for new staff.
With , OpenClinica enables researchers to tailor the data collection process to meet diverse study requirements. Automated validation checks enhance information accuracy, thereby reducing errors that could compromise study integrity.
Moreover, OpenClinica's robust reporting features facilitate rapid insights generation, supporting timely decision-making throughout the study process. This combination of attributes has resulted in high user satisfaction scores for OpenClinica, which effectively utilizes EDC systems, boasting an overall rating of 4.5 from over 200 reviews and underscoring its efficacy in enhancing information management within research studies.
SimpleTrials: Integration of EDC with Clinical Trial Management for Streamlined Processes
SimpleTrials serves as a robust clinical study management system that seamlessly integrates Electronic Information Capture (EDC) features, significantly enhancing information collection and management processes. This integration promotes a centralized data flow, ensuring that all stakeholders have access to real-time information, which is vital for informed decision-making.
By connecting EDC with study management, SimpleTrials effectively minimizes redundant manual processes, thereby fostering collaboration among research teams. This streamlined approach not only boosts but also reinforces adherence to regulatory standards, positioning SimpleTrials as an indispensable resource for study sponsors.
Industry leaders emphasize that such integration is essential for improving data precision and integrity, ultimately leading to more successful testing outcomes.
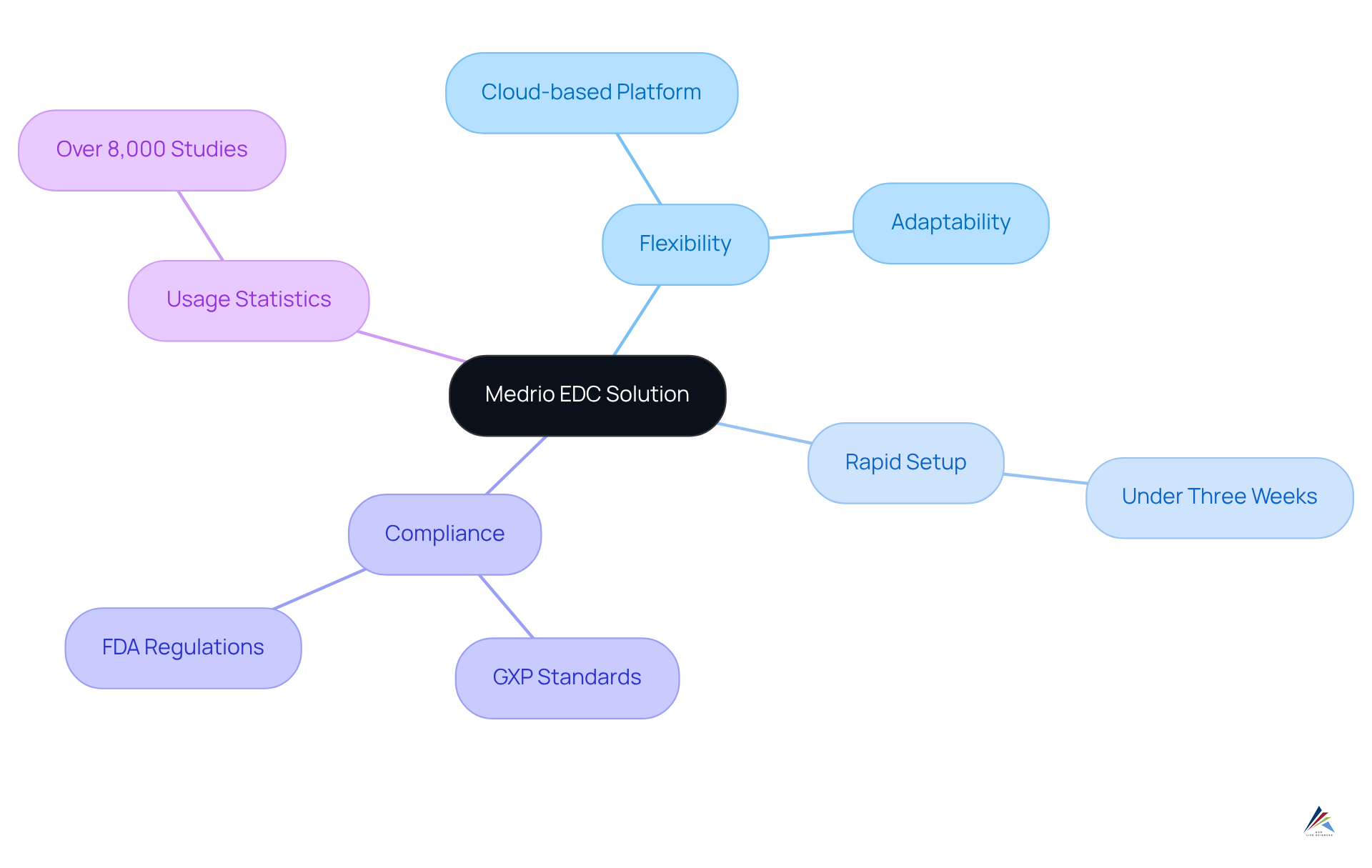
Medrio: Flexible EDC Solution for Accelerated Clinical Trials
Medrio presents a highly versatile edc systems solution, expertly tailored to accelerate research studies by aligning with the unique requirements of each project while ensuring compliance with GXP standards and FDA regulations. Its cloud-based platform facilitates rapid setup and seamless modifications, empowering researchers to swiftly adapt to changing study demands without incurring significant delays. Typically, setup is completed in under three weeks, establishing Medrio as a , particularly in research where time is of the essence.
The intuitive interface, combined with robust information management features, simplifies the processes of information collection and oversight, ensuring that experiments progress smoothly and effectively. This flexibility is particularly advantageous for sponsors of research studies aiming to optimize timelines while adhering to stringent regulatory compliance standards, including exemplary documentation practices and the implementation of edc systems and standard operating procedures (SOPs).
Notably, Medrio has been utilized in over 8,000 studies, underscoring its effectiveness in enhancing study efficiency and preserving information integrity.
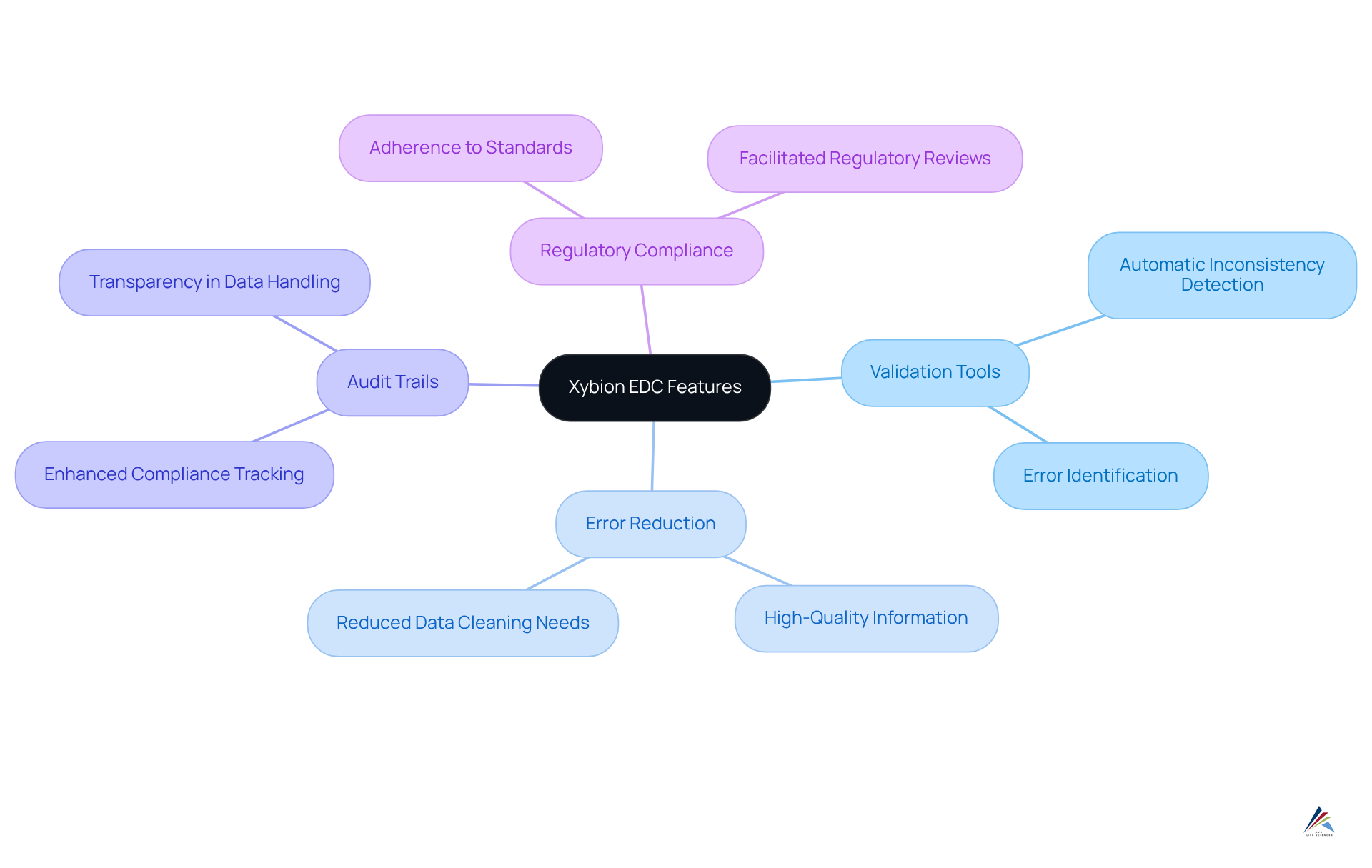
Xybion: EDC Features for Enhanced Data Accuracy and Compliance
Xybion's edc systems tackle critical inherent in clinical trials by prioritizing information accuracy and regulatory adherence. This platform features advanced validation tools that automatically identify inconsistencies and errors during data entry. Such a proactive strategy significantly reduces the necessity for extensive data cleaning later in the process, ensuring that only high-quality information is recorded.
Organizations utilizing Xybion's validation tools have reported a notable reduction in errors, thereby enhancing overall information integrity. Furthermore, Xybion's robust audit trails and compliance features facilitate adherence to stringent regulatory requirements, positioning it as a trusted solution for organizations committed to maintaining the highest standards of information integrity in their research.
By implementing Xybion's edc systems, organizations can not only streamline their processes but also ensure compliance with rigorous demands, ultimately driving the success of their clinical trials.
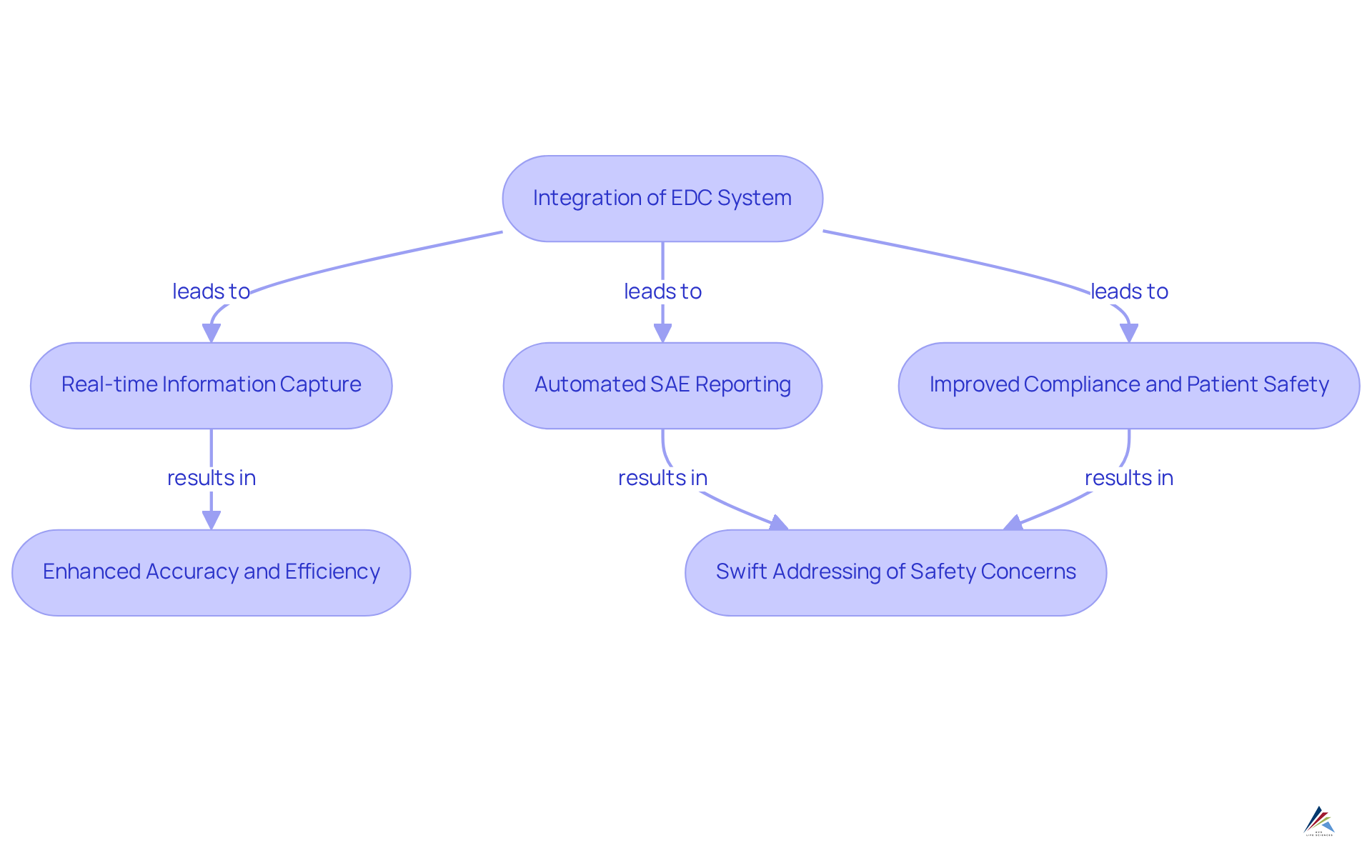
DataReconciliation.com: EDC Systems for Effective SAE Reporting
DataReconciliation.com excels in delivering EDC systems that enhance the reporting of Serious Adverse Events (SAEs) in clinical trials. Their platform is designed to integrate seamlessly with existing EDC systems, facilitating real-time information capture and SAE reporting. This capability is crucial for maintaining and ensuring compliance with regulatory standards.
By automating the SAE reporting process, DataReconciliation.com significantly enhances the accuracy and efficiency of data management. Consequently, clinical trial sponsors can swiftly address any safety concerns that may arise during the study, thereby improving compliance and fostering a safer clinical environment.
Conclusion
The evolution of Electronic Data Capture (EDC) systems in clinical trials has become pivotal in ensuring compliance and enhancing data integrity. These systems streamline the data collection process and align with stringent regulatory requirements, making them indispensable tools for researchers. This article highlights various EDC solutions, each uniquely contributing to the efficiency and accuracy of clinical trials while addressing the complexities of compliance.
Key insights from the discussion include:
- The robust features of platforms like Medidata Rave, which enhance efficiency and protocol compliance.
- OpenClinica's user-friendly design that reduces the learning curve for new users.
- SimpleTrials showcases the importance of integrating EDC with clinical trial management systems to foster collaboration and operational efficiency.
- Solutions like Xybion and DataReconciliation.com emphasize the need for accurate data management and effective reporting of Serious Adverse Events, critical for maintaining patient safety and regulatory adherence.
In light of these advancements, it is clear that embracing EDC systems is essential for modern clinical research. Organizations are encouraged to explore these innovative solutions to improve their compliance frameworks and data management processes. As the landscape of clinical trials continues to evolve, the integration of EDC systems will play a significant role in driving successful study outcomes and enhancing the overall quality of research.
Frequently Asked Questions
What services does AVS Life Sciences provide for EDC implementation?
AVS Life Sciences offers a comprehensive suite of services that facilitate the efficient implementation of Electronic Data Capture (EDC) systems in research studies, ensuring adherence to regulatory compliance and industry standards.
How does AVS Life Sciences ensure compliance with regulatory standards?
AVS Life Sciences guarantees that EDC systems adhere to rigorous industry standards, including Good Manufacturing Practices (GMP) and ISO regulations, by applying robust quality management strategies.
What are the benefits of using AVS Life Sciences for data collection processes?
By working with AVS Life Sciences, clients can enhance their data collection processes while maintaining high standards of data integrity and compliance, which boosts the effectiveness of research studies and reduces risks associated with regulatory non-compliance.
What is Medidata Rave, and what role does it play in clinical trials?
Medidata Rave is a leading Electronic Data Capture (EDC) system that enhances efficiency in electronic information collection for clinical trials while ensuring strict adherence to GXP and FDA regulations.
How does Medidata Rave support research studies?
Medidata Rave has a proven track record of supporting over 36,000 studies and 11 million individuals, featuring an intuitive interface for rapid information entry and real-time oversight, which is critical for maintaining data accuracy.
What features make Medidata Rave a preferred choice among research sponsors?
Medidata Rave accommodates complex study designs, integrates seamlessly with other research management tools, and includes robust compliance features such as adherence to Standard Operating Procedures (SOPs) and effective documentation practices.
What recent enhancements have been made to Medidata Rave?
Medidata Rave has introduced AI-driven enhancements to bolster protocol compliance, further reinforcing its position as a favored option within the industry.
How many users rely on Medidata Rave for their research studies?
Over 1 million registered users rely on Medidata Rave's platform, which has demonstrated efficacy in overseeing research studies through numerous successful applications.
