7 Corporate Compliance Services for Pharmaceutical Success

Overview
The article primarily emphasizes seven essential corporate compliance services that are vital for the success of pharmaceutical companies. These services—validation, regulatory guidance, ongoing support, and training programs—are indispensable for navigating the complexities of regulatory frameworks. By ensuring adherence to industry standards, these services ultimately enhance operational efficiency and product quality in a highly regulated environment. Understanding these compliance challenges is crucial for any organization aiming to thrive in this sector. Engaging with these services not only mitigates risks but also positions companies for sustained success.
Introduction
The pharmaceutical industry operates within a complex web of regulations that demand unwavering compliance to ensure safety and efficacy in drug development. As organizations strive to navigate these intricate requirements, understanding essential corporate compliance services becomes crucial for achieving success. This article delves into seven key services that not only streamline compliance but also empower pharmaceutical companies to thrive amidst evolving regulatory landscapes. How can these services transform challenges into opportunities for growth and innovation in an increasingly scrutinized environment?
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for Pharmaceutical Companies
AVS Life Sciences distinguishes itself in the pharmaceutical industry by delivering a comprehensive suite of services designed to satisfy rigorous oversight and assurance requirements. Their primary offerings encompass:
- Engineering support
With a dedicated team of over 300 seasoned professionals, AVS Life Sciences empowers clients to adeptly , ensuring compliance with and regulations at every phase.
As we look towards 2025, the drug industry is poised to place . Data indicates that 68% more swiftly. Furthermore, the global is projected to grow at a rate of 7-9%, driven by increased scrutiny and . AVS Life Sciences is strategically positioned to lead in this dynamic environment, offering vital support that aligns with contemporary trends in medication quality management.
ComplianceWeek: Essential Updates on Regulatory Changes for Pharma Compliance
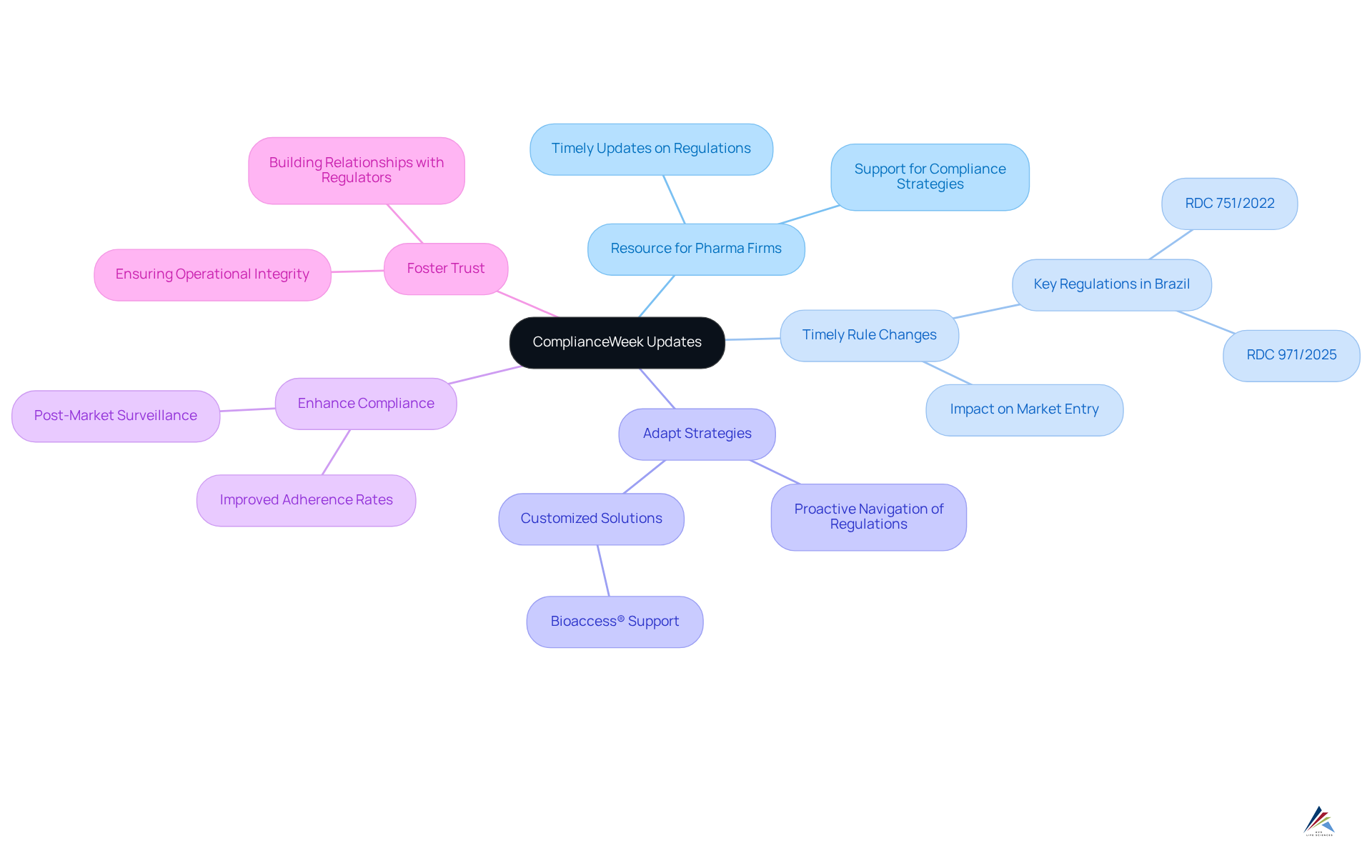
ComplianceWeek serves as an indispensable resource for pharmaceutical firms, delivering that critically impact . For organizations striving to adapt their strategies effectively, is paramount. By leveraging insights from ComplianceWeek, companies can proactively , thereby ensuring their remain robust and responsive. This proactive stance not only but also positions organizations to uphold operational integrity and foster trust with .
COMPLY: Modern Compliance Management Solutions for Pharmaceutical Firms
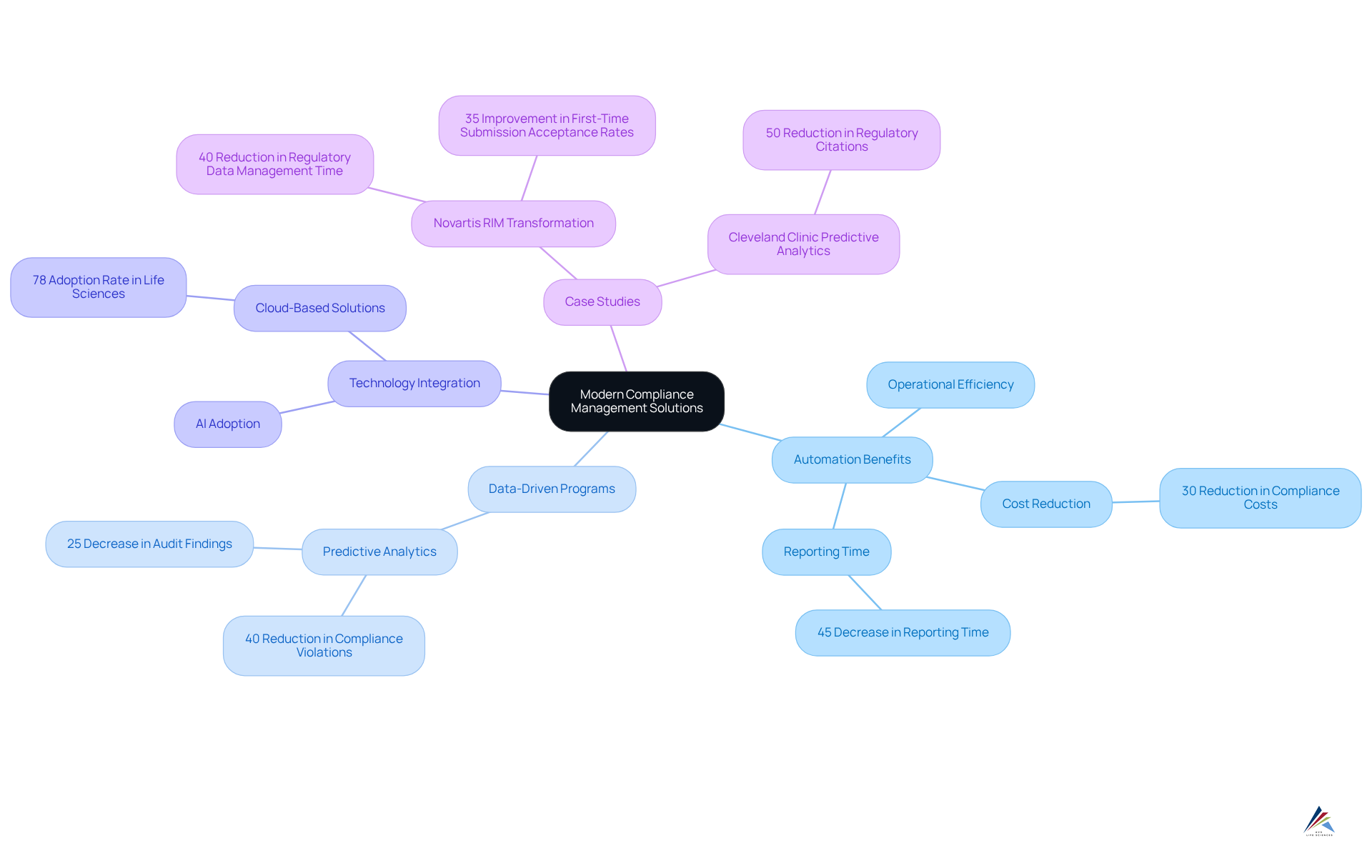
COMPLY provides innovative tailored specifically for the healthcare industry, significantly enhancing the . By leveraging these advanced tools, pharmaceutical companies can substantially boost operational efficiency, mitigate the risk of regulatory breaches, and secure a .
Automation in adherence management accelerates the processing of legal requirements while fostering a proactive approach to compliance, empowering organizations to swiftly adapt to . Industry leaders have noted that companies implementing data-driven adherence programs have realized a 30% reduction in adherence-related costs and a 45% decrease in reporting time, underscoring the importance of these advancements within the broader management landscape.
Moreover, the integration of cutting-edge technologies, including AI and cloud-based solutions, has gained traction, with 78% of embracing these tools for regulatory functions. This shift towards automation not only streamlines regulatory processes but also , positioning pharmaceutical firms for sustained success.
a prominent biotechnology company's GMP facility from a Biosafety Level 1 to a Level 2, completing the project on time and within budget. Our meticulous documentation practices ensured complete traceability, validated by the client’s quality assurance team, ultimately allowing them to concentrate on developing life-saving medicines.
To discover how AVS Life Sciences can , contact us today.
Quality Compliance Consulting: Navigating Regulatory Standards in Pharmaceuticals
Quality adherence consulting is essential for the intricate landscape of compliance standards. At AVS Life Sciences, our expert consultants provide tailored strategies that align with:
- , including:
- GCP
- GLP
- CFR Part 11 adherence
Our comprehensive GXP regulatory services encompass critical areas such as:
- Deviations
- Investigations
This ensures strict compliance with FDA regulations.
By leveraging the expertise of our , organizations can enhance their , mitigate risks, and guarantee that their products meet the highest quality standards throughout the drug development lifecycle. To bolster your compliance initiatives and achieve optimal results, consider partnering with AVS Life Sciences for their , which are tailored to your unique requirements. Engage with us today to elevate your compliance efforts and ensure your organization thrives in a competitive landscape.
Validation and Commissioning Services: Ensuring Regulatory Compliance in Pharma
services are indispensable for ensuring that medical facilities and equipment adhere to stringent industry standards. These services utilize systematic methodologies to verify that systems are designed, installed, and operate according to defined requirements. By implementing comprehensive validation and commissioning protocols, , elevate product quality, and protect patient safety.
Organizations that adopt effective , for example, report and a significant decrease in . A meticulously organized can lead to a 30% reduction in testing cycle durations, expediting time-to-market for critical products while maintaining industry standards.
Industry leaders underscore the importance of these services, asserting that mastering CQV is vital for achieving operational excellence within the healthcare sector. As oversight environments evolve, the integration of is becoming increasingly essential, enhancing accuracy and efficiency in compliance efforts.
Ultimately, the successful implementation of , including validation and commissioning, not only ensures adherence to legal requirements but also .
Ongoing Compliance Support: Sustaining Regulatory Adherence in Pharmaceuticals
Continuous assistance through is crucial for pharmaceutical firms striving to maintain adherence in a constantly changing environment. This support includes corporate compliance services, which encompass:
- Prompt updates on compliance changes
By fostering a culture of , organizations can proactively identify and address potential issues, and industry best practices. Routine evaluations not only aid in recognizing regulatory gaps but also promote a , significantly reducing the likelihood of costly infractions.
For instance, organizations that implement robust can experience a notable reduction in errors; research indicates that role-specific training can lead to a decrease in mistakes by as much as 40%. Adherence specialists emphasize that organizations should view corporate compliance services .
As one expert noted, 'The companies that succeed won’t be those that do the minimum necessary for adherence, but those that embrace .' This perspective underscores the importance of integrating compliance into the core operational strategy, ensuring that all employees are equipped with the knowledge and tools necessary to uphold legal standards effectively.
Regulatory Submissions Guidance: Streamlining Approval Processes for Pharma Products
is crucial for drug companies aiming to optimize their approval processes. This guidance encompasses to oversight bodies, ensuring that all essential information is included and presented with clarity.
By leveraging , , and exemplary documentation practices, companies can significantly enhance their prospects for . Our and adherence to industry regulations not only accelerates the time-to-market for products but also ensures alignment with sector standards, ultimately .
Training Programs: Empowering Pharmaceutical Teams for Compliance Excellence
Training programs serve as a cornerstone of regulatory excellence within the pharmaceutical sector. These initiatives equip employees with essential knowledge and skills necessary to adeptly navigate intricate legal landscapes. By cultivating a culture of compliance through ongoing education, organizations can ensure their teams are well-prepared to uphold the and regulatory adherence.
Statistics indicate that organizations are projected to provide training across an average of 12 subject areas over a two to three-year period. Notably, those that deliver —such as the code of conduct and conflict of interest—experience significant improvements in employee confidence and compliance with regulations.
Moreover, continuous learning transcends being merely a best practice; it has become an imperative. Industry leaders underscore that , ultimately leading to reduced risks and enhanced operational efficiency.
, such as those implemented by , which offers comprehensive regulatory and quality solutions, demonstrate that tailored initiatives can yield , underscoring the advantages of empowering teams through structured training. With an impressive , AVS Life Sciences exemplifies client satisfaction and effectiveness in training, particularly within the biopharmaceutical and medical device sectors.
As the healthcare landscape evolves, organizations must assess their training programs against the average of 12 topical areas to ensure compliance with the necessary standards.
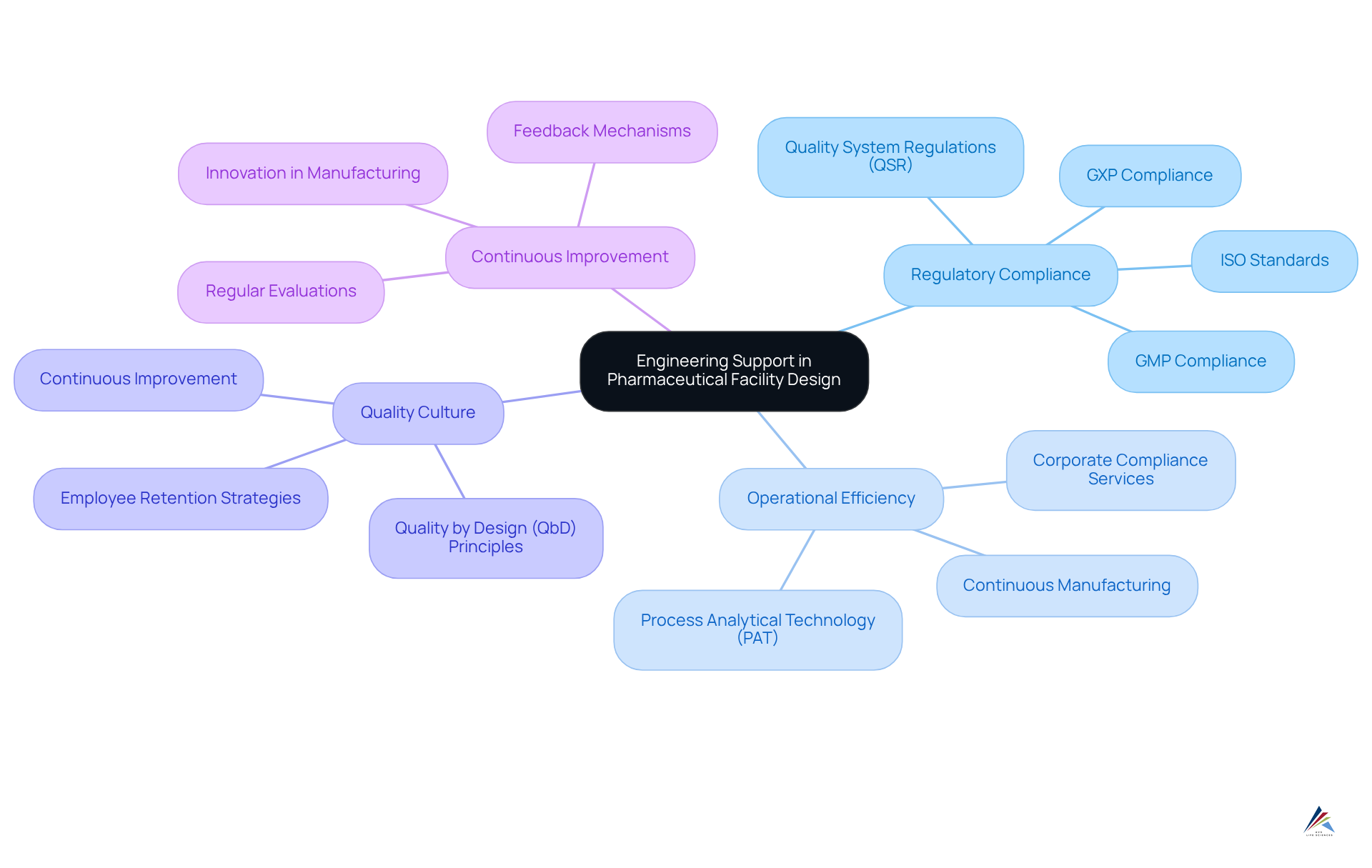
Engineering Support: Designing Compliant Facilities for Pharmaceutical Operations
Engineering support is essential in designing drug manufacturing facilities that adhere to stringent regulatory standards, particularly concerning and . This support leverages advanced engineering principles to create environments conducive to safe and efficient manufacturing processes, ensuring facilities meet and optimal management practices.
By prioritizing in facility design, drug manufacturers can significantly enhance operational efficiency, mitigate risks, and ensure their products consistently meet the highest standards. Industry leaders underscore that effective facility design not only satisfies legal requirements but also fosters a culture of quality and continuous improvement.
For example, Tim Cook highlights the significance of strategic manufacturing, asserting, "The way we look at manufacturing is this: the U.S.'s strategy should be to skate where the puck is going, not where it is." This interconnected approach to [corporate compliance services](https://teamguru.com/blog/25-inspirational-business-process-improvement-quotes/1632) and is crucial for navigating the complexities of the healthcare landscape in 2025 and beyond.
Moreover, adherence to GMP and Quality by Design (QbD) principles is vital in ensuring not only comply with regulations but also boost overall productivity. To implement these concepts effectively, companies must conduct regular evaluations of their facility designs to align with evolving legal standards and operational demands, utilizing corporate compliance services alongside expert solutions in from AVS Life Sciences.
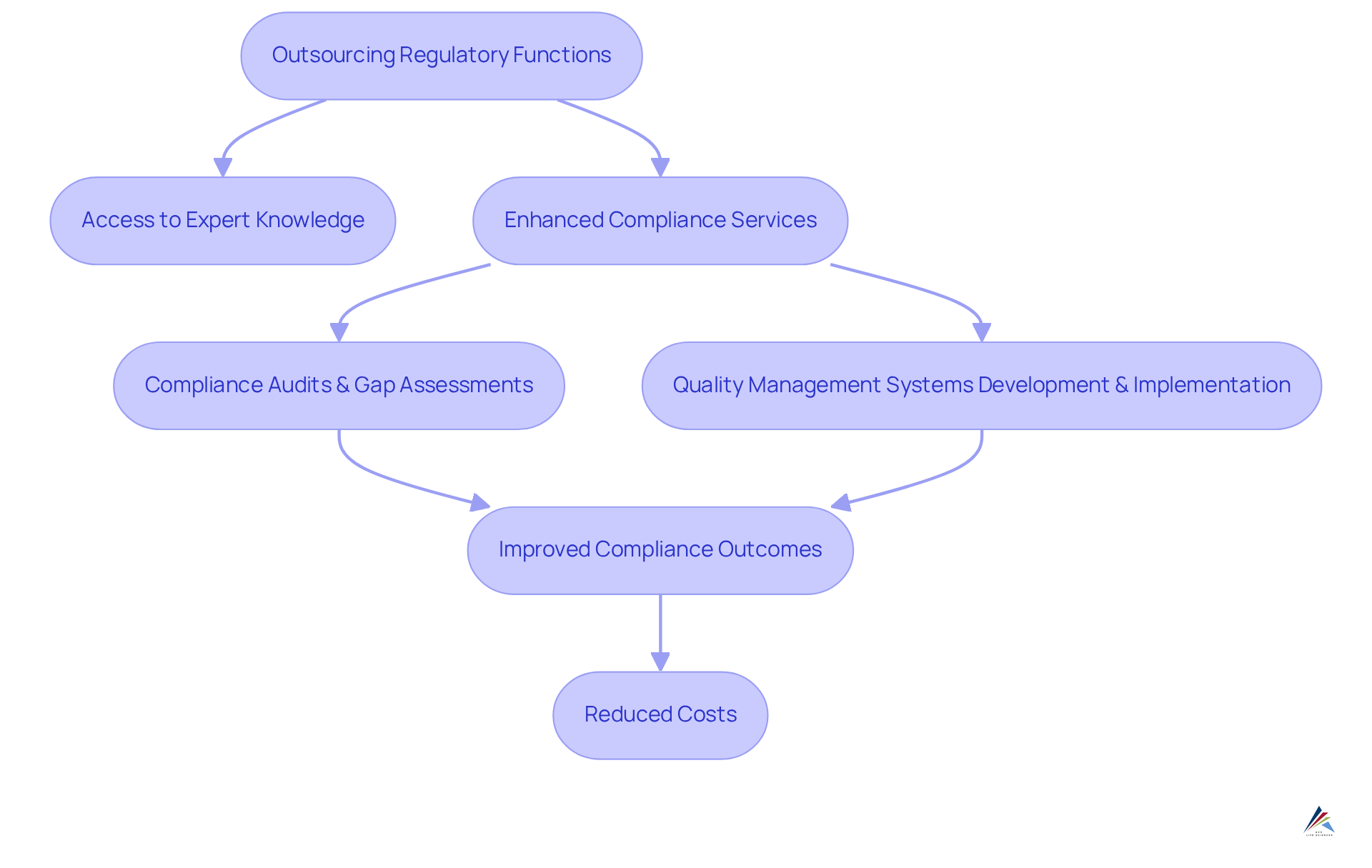
Managed Services: Efficient Compliance Management for Pharmaceutical Companies
through managed services provides pharmaceutical firms with a strategic advantage in . By partnering with specialized providers such as AVS Life Sciences, organizations gain access to that significantly enhance their to meet complex compliance requirements. AVS Life Sciences offers comprehensive GXP regulatory services, including:
- Compliance Audits & Gap Assessments
- Development & Implementation
This ensures quality and conformity throughout the drug development lifecycle. This approach , allowing firms to concentrate on their core operations, which ultimately boosts efficiency and effectiveness in regulatory efforts.
As we look ahead to 2025, the benefits of outsourcing regulatory functions are expected to become even more pronounced, as organizations increasingly recognize the value of leveraging external expertise to navigate evolving regulations. Industry leaders emphasize that outsourcing not only alleviates operational burdens but also fosters a culture of corporate compliance services, which is essential for long-term success.
The impact of outsourcing on efficiency is substantial; firms that adopt this strategy often report improved compliance outcomes and reduced costs. For instance, appointing a C-level regulatory leader can result in an average savings of $1.25 million, showcasing the financial advantages of strategic management. Furthermore, 59% of security and IT leaders indicate that their organizations manage multiple systems that must comply with regulatory standards, highlighting the complexity of oversight and the necessity for outsourcing. As the pharmaceutical sector continues to evolve, the trend toward outsourcing corporate compliance services is poised to expand, equipping organizations with greater agility and resilience in addressing regulatory challenges. By selecting AVS Life Sciences as a trusted partner, companies can ensure adherence to the highest industry standards.
Conclusion
The landscape of pharmaceutical compliance presents intricate challenges that are continuously evolving, highlighting the necessity for robust corporate compliance services. Leveraging specialized offerings like those from AVS Life Sciences can significantly enhance a pharmaceutical company's ability to navigate these regulatory complexities, ensuring they not only meet but exceed industry standards.
Key insights reveal the critical role of various compliance services, including:
- Validation
- Commissioning
- Ongoing support
- Training programs
These services empower organizations to streamline operations, mitigate risks, and foster a culture of quality essential for success in the competitive pharmaceutical market. The integration of advanced technologies and automation further enhances compliance efficiency, enabling firms to adapt swiftly to regulatory changes.
In conclusion, embracing a proactive approach to compliance is not merely a legal obligation but a strategic advantage that drives operational excellence and builds trust within the industry. As the pharmaceutical sector prepares for the challenges of 2025, organizations must prioritize their compliance frameworks to remain agile and resilient. By investing in comprehensive compliance solutions, pharmaceutical companies position themselves for sustained success and contribute to the overall integrity of healthcare.
Frequently Asked Questions
What services does AVS Life Sciences provide to pharmaceutical companies?
AVS Life Sciences offers a comprehensive suite of services including validation and commissioning, consulting on standards adherence, engineering support, and guidance on regulatory submissions.
How does AVS Life Sciences help clients in the pharmaceutical industry?
With a dedicated team of over 300 seasoned professionals, AVS Life Sciences assists clients in navigating the complexities of the life sciences product lifecycle, ensuring compliance with high standards and regulations throughout all phases.
What is the projected growth rate for the global life sciences oversight and compliance market?
The global life sciences oversight and compliance market is projected to grow at a rate of 7-9%, driven by increased scrutiny and technological innovations.
What role does ComplianceWeek play for pharmaceutical firms?
ComplianceWeek serves as a vital resource by providing timely updates on regulatory changes that impact adherence practices, enabling organizations to adapt their strategies and ensure robust corporate compliance services.
How does COMPLY enhance regulatory management for pharmaceutical firms?
COMPLY provides innovative regulatory management solutions that automate regulatory procedures, significantly boosting operational efficiency, mitigating the risk of regulatory breaches, and securing a competitive advantage.
What benefits have companies experienced by implementing data-driven adherence programs?
Companies that have implemented data-driven adherence programs have reported a 30% reduction in adherence-related costs and a 45% decrease in reporting time.
What technologies are being embraced by life sciences organizations for regulatory functions?
78% of life sciences organizations are adopting advanced technologies, including AI and cloud-based solutions, to streamline regulatory processes and enhance overall productivity.
Can you provide an example of a project completed by AVS Life Sciences?
AVS Life Sciences successfully upgraded a biotechnology company's GMP facility from a Biosafety Level 1 to a Level 2, completing the project on time and within budget, with meticulous documentation practices ensuring complete traceability.
