5 Steps to Navigate Breakthrough Device Designation Successfully

Overview
The article delineates the critical steps required to adeptly navigate the breakthrough device designation process as established by the FDA. It articulates the essential criteria for eligibility, the necessary documentation, the FDA review process, and post-designation compliance. A thorough understanding and meticulous preparation are emphasized as pivotal factors that can significantly streamline the pathway to market for innovative medical devices.
Introduction
Navigating the intricate landscape of medical device approval presents significant challenges, particularly regarding the FDA's Breakthrough Device Designation. This program aims to expedite access to innovative therapies that address critical health needs. However, many companies encounter difficulties in grasping the eligibility criteria and documentation essential for success. As the demand for faster, more effective medical solutions escalates, developers must consider how to meet the necessary standards and streamline their pathway to market. This guide delineates five essential steps to effectively navigate the breakthrough device designation process, empowering innovators to deliver life-changing technologies to patients with increased efficiency.
Understand Breakthrough Device Designation
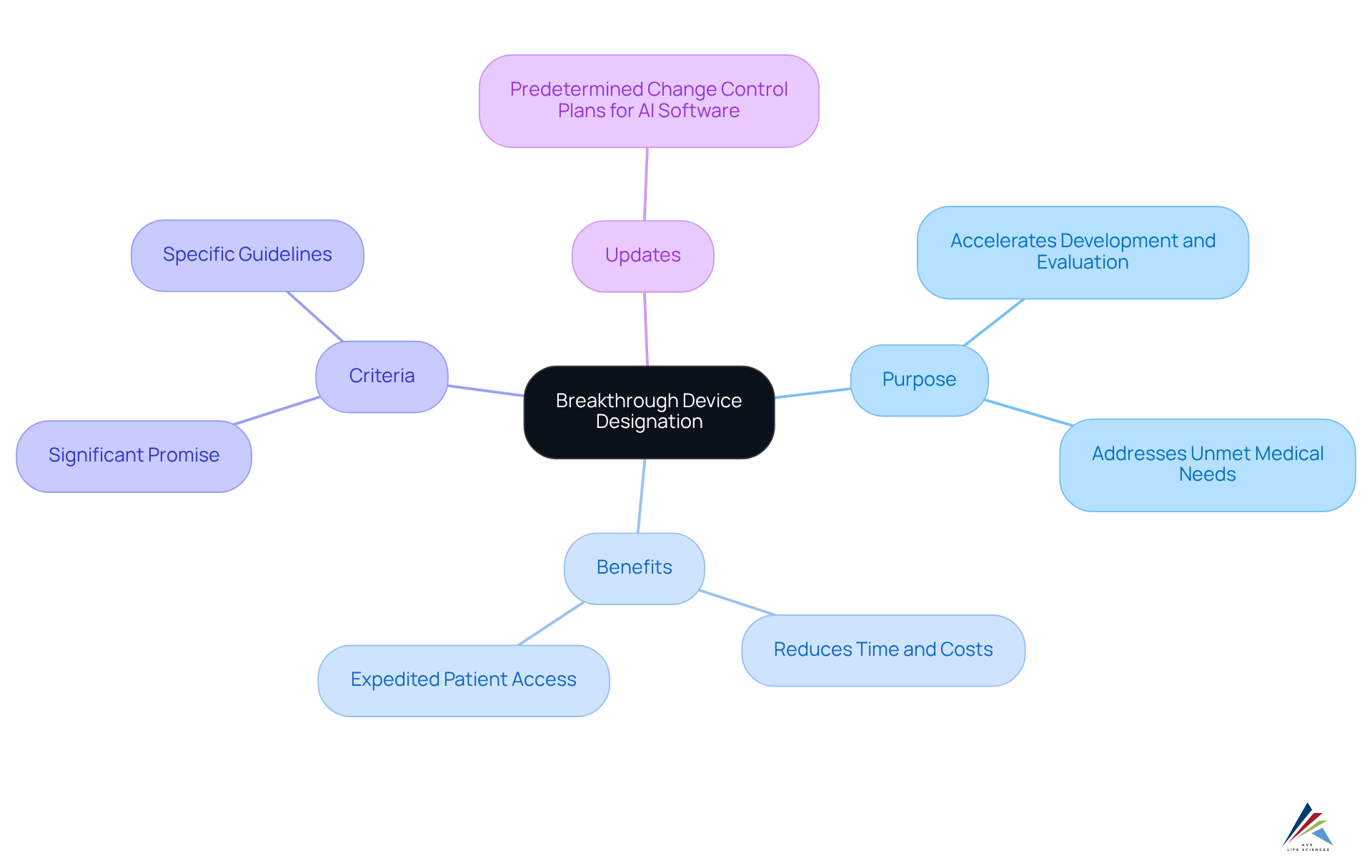
The breakthrough device designation represents a pivotal program established by the FDA, aimed at accelerating the development and evaluation of medical instruments that deliver more effective treatment or diagnosis for life-threatening or irreversibly debilitating conditions.
The breakthrough device designation is exclusively reserved for tools demonstrating significant promise in addressing unmet medical needs, thus facilitating expedited patient access to innovative therapies.
Understanding the nuances of the breakthrough device designation is imperative, as it can substantially reduce both the time and costs associated with product launches.
Familiarity with the FDA's guidelines and the specific criteria that qualify an instrument for breakthrough device designation is essential for ensuring preparedness for the subsequent stages of the oversight process.
AVS Life Sciences, your committed partner, provides comprehensive regulatory compliance and quality management solutions, including validation services and regulatory training, specifically tailored to the life sciences sector.
Recent updates from the FDA, such as , further underscore the dynamic nature of medical regulation and the necessity of remaining informed.
Identify Eligibility Criteria for Designation
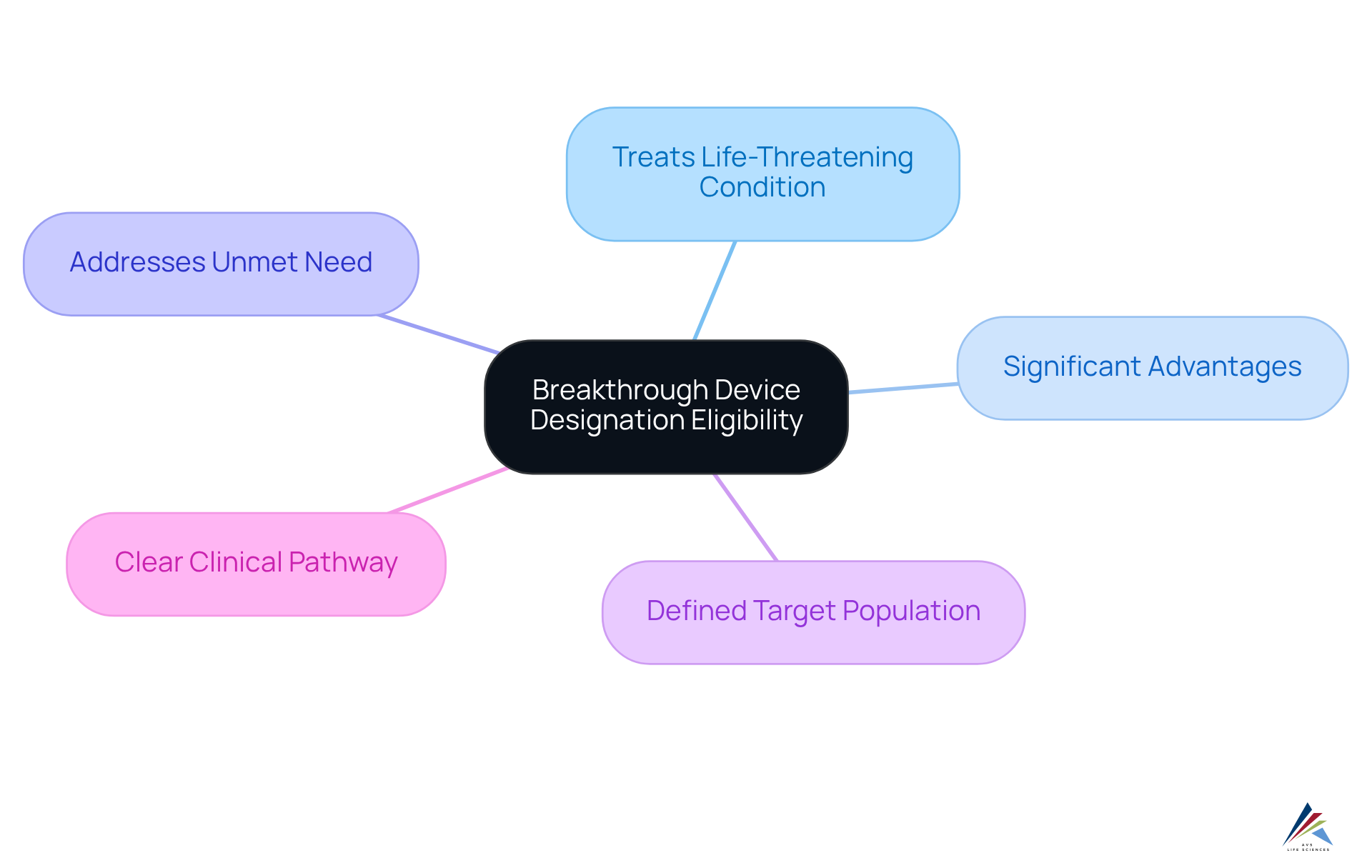
To qualify for the breakthrough device designation, a product must meet several key criteria:
- It must be intended to treat or diagnose a life-threatening or irreversibly debilitating condition.
- It must provide significant advantages over existing approved alternatives.
- It must demonstrate the potential to address an unmet medical need.
- The instrument should have a well-defined target population.
- There should be a clear clinical pathway.
It is essential to review the to ensure your product aligns with these criteria before proceeding with your application.
Prepare Required Documentation and Submissions
When preparing your application for the breakthrough device designation, it is imperative to compile several . Start with:
- A detailed description of the product, its intended use, and the clinical evidence that supports its effectiveness.
- Comprehensive information on the manufacturing process, quality control measures, and any prior regulatory submissions.
- A summary of the device's potential benefits in comparison to existing treatments.
Ensure that all documentation is clear, concise, and well-organized, as this will facilitate the FDA's evaluation process and enhance your chances of success.

Navigate the FDA Review Process
Upon submission of your application, the FDA conducts a thorough examination of the provided documentation. This initial assessment determines whether the application meets the established eligibility criteria. If accepted, the FDA may request further information or clarification, making it essential to maintain open lines of communication throughout this phase. Timely responses can significantly , as recent statistics indicate that effective communication can reduce review timelines by up to 30%. Engaging proactively with the FDA not only fosters a collaborative relationship but also enhances the likelihood of a favorable outcome.
Successful communication strategies include:
- Scheduling pre-submission meetings to clarify expectations
- Utilizing clear, concise documentation to address potential concerns
As Brandon Arbiter from Tidepool emphasizes, 'When I buy an interoperable insulin pump, I want to be able to connect it to the CGM and apps that work best for me.' This highlights the importance of understanding the FDA's perspective and aligning your application with their expectations.

Manage Post-Designation Compliance and Monitoring
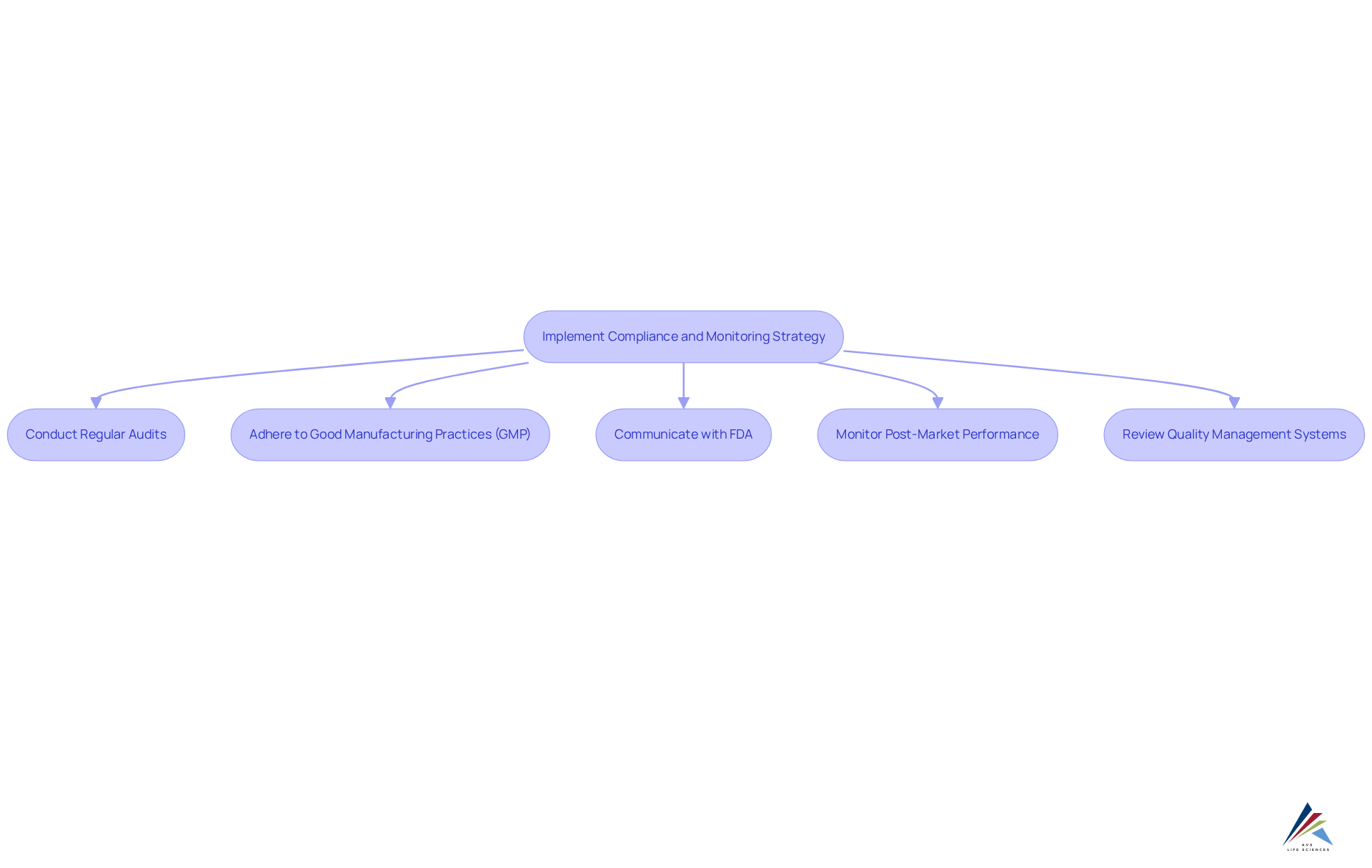
Following the acquisition of the breakthrough device designation, it is essential to implement a robust compliance and monitoring strategy, leveraging AVS Life Sciences' . This strategy must encompass:
- Regular audits of manufacturing processes
- Strict adherence to Good Manufacturing Practices (GMP)
- Continuous communication with the FDA regarding any modifications to the device or its intended use
Establishing a system for monitoring post-market performance and adverse events is crucial, as this data will be vital for future compliance submissions and maintaining high standards. Additionally, consistently reviewing and updating your quality management systems to align with new regulatory requirements or industry standards is key, ensuring your organization remains at the forefront of best practices in the life sciences sector.
Conclusion
Successfully navigating the breakthrough device designation process is crucial for innovators aiming to bring transformative medical devices to market. This designation accelerates the development and evaluation of devices intended for life-threatening or debilitating conditions, enhancing patient access to groundbreaking therapies. Understanding the intricacies of this process is essential for any organization looking to leverage its advantages.
Key steps in this journey include:
- Identifying eligibility criteria
- Preparing comprehensive documentation
- Effectively navigating the FDA review process
- Managing post-designation compliance
Each of these steps requires meticulous attention to detail and proactive communication with the FDA to ensure that applications are complete and aligned with regulatory expectations. Insights shared throughout the article highlight how strategic preparation and ongoing compliance can significantly impact the success of breakthrough devices.
In conclusion, the breakthrough device designation represents a valuable opportunity for medical innovators. Embracing this process can lead to expedited access to the market, ultimately benefiting patients in need of advanced treatment options. Stakeholders in the life sciences sector are encouraged to remain informed and engaged, utilizing available resources and expertise to navigate this complex landscape effectively. By doing so, they can contribute to the evolution of healthcare and the development of solutions that save lives.
Frequently Asked Questions
What is the breakthrough device designation?
The breakthrough device designation is a program established by the FDA to accelerate the development and evaluation of medical instruments that provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating conditions.
Who can apply for the breakthrough device designation?
Any medical instrument that demonstrates significant promise in addressing unmet medical needs can apply for the breakthrough device designation.
What are the benefits of obtaining breakthrough device designation?
Obtaining breakthrough device designation can substantially reduce both the time and costs associated with product launches, facilitating expedited patient access to innovative therapies.
What are the eligibility criteria for breakthrough device designation?
To qualify, a product must: 1. Be intended to treat or diagnose a life-threatening or irreversibly debilitating condition. 2. Provide significant advantages over existing approved alternatives. 3. Demonstrate the potential to address an unmet medical need. 4. Have a well-defined target population. 5. Present a clear clinical pathway.
Where can I find more information about the eligibility criteria?
It is essential to review the FDA's official guidance documents to ensure your product aligns with the criteria for breakthrough device designation before proceeding with your application.
What support does AVS Life Sciences provide regarding breakthrough device designation?
AVS Life Sciences offers comprehensive regulatory compliance and quality management solutions, including validation services and regulatory training, specifically tailored to the life sciences sector.
Why is it important to stay informed about FDA updates?
Staying informed about FDA updates, such as guidance on Predetermined Change Control Plans for AI-enabled software functions, is crucial due to the dynamic nature of medical regulation.
