4 Strategies for Ensuring Data Integrity in Digital Validation

Overview
The article articulates four key strategies for ensuring data integrity in digital validation, underscoring the critical need for accurate, consistent, and reliable information to meet regulatory standards. It begins by addressing the compliance challenges faced by organizations in this domain. To combat these challenges, it details best practices such as:
- Implementing robust validation protocols
- Enforcing stringent access control measures
- Conducting routine audits
- Providing comprehensive staff training
- Leveraging advanced technology, including Electronic Lab Notebooks and Laboratory Information Management Systems
These strategies not only enhance data integrity but also significantly improve operational efficiency. Ultimately, the article calls for proactive engagement with these compliance solutions, empowering organizations to uphold the highest standards of data integrity.
Introduction
Ensuring data integrity in digital validation is not merely a regulatory requirement; it stands as a cornerstone of trust within the life sciences sector. As organizations navigate the complexities of compliance, the accuracy and reliability of their data become paramount, influencing everything from clinical trials to regulatory approvals. Yet, the challenge persists: how can companies effectively safeguard their data against errors and breaches while adhering to stringent regulations? This article delves into four essential strategies that organizations can implement to uphold data integrity, mitigate risks, and enhance operational efficiency in an increasingly digital landscape.
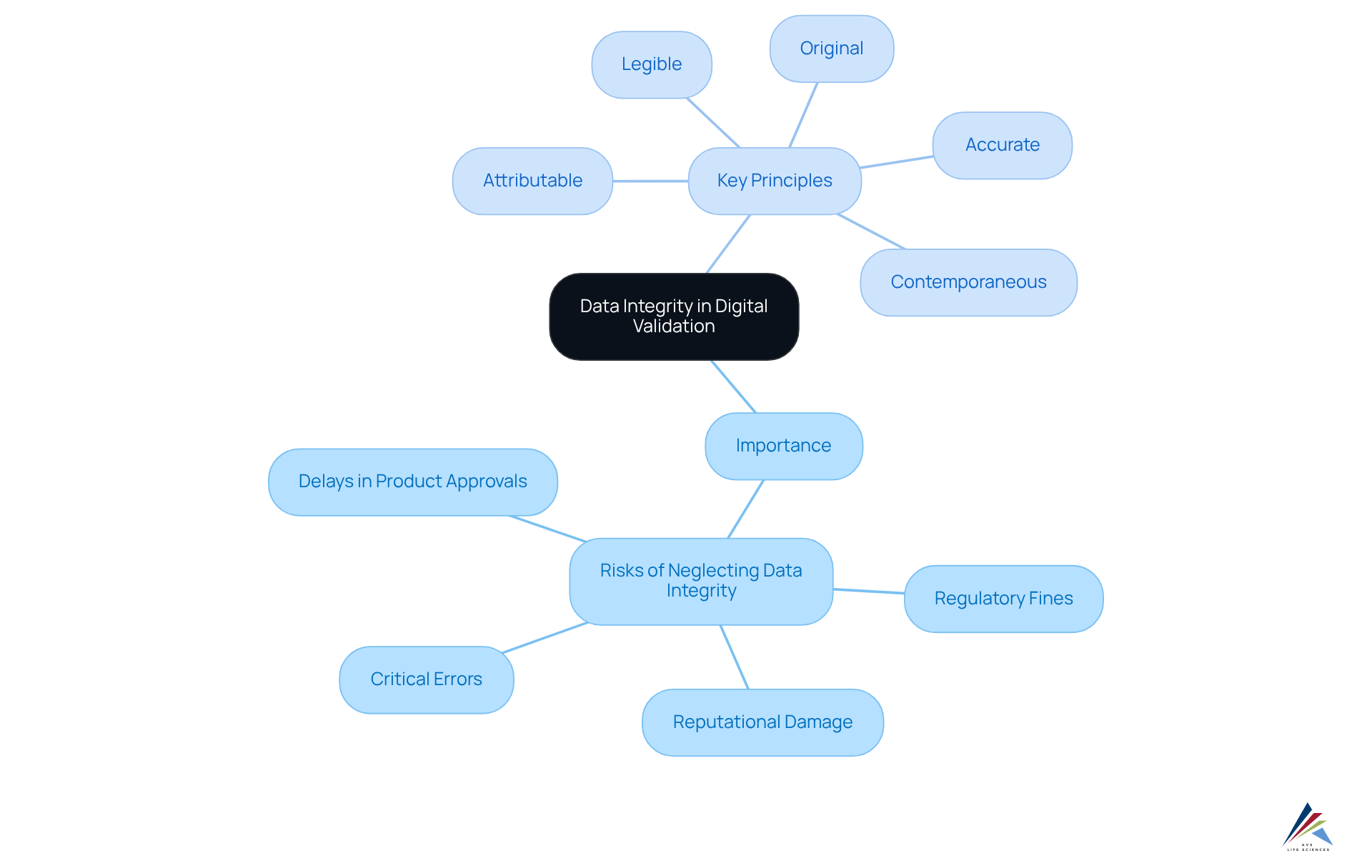
Define Data Integrity and Its Importance in Digital Validation
Data integrity in digital validation encompasses the accuracy, consistency, and dependability of information throughout its lifecycle, playing a pivotal role. This integrity is essential for ensuring that the data generated and utilized in clinical trials, manufacturing, and regulatory submissions is trustworthy. Organizations that neglect information accuracy expose themselves to significant risks, including critical errors, regulatory fines, and reputational damage. For example, a pharmaceutical company that fails to uphold information accuracy may encounter substantial challenges during FDA inspections, resulting in delays in product approvals and potential market withdrawals. Therefore, prioritizing information reliability, alongside implementing robust practices such as Information Reliability Deviations, Investigations, and CAPA, is vital for any organization in the life sciences sector.
Key principles of data integrity include:
- Attributable: Data must be traceable to the individual responsible for its creation or modification.
- Legible: Data should be clear and comprehensible.
- Contemporaneous: Data must be recorded at the time of the activity.
- Original: Data should represent the first record of the information.
- Accurate: Data must be correct and devoid of errors.
These principles underpin effective digital validation strategies, which are essential for maintaining data integrity in digital validation and ensuring that all information remains reliable and compliant with industry standards. By adhering to exemplary documentation practices and recognizing the ramifications of poor information management, organizations can mitigate risks and enhance their operational efficiency.
Understand Regulatory Frameworks Impacting Data Integrity
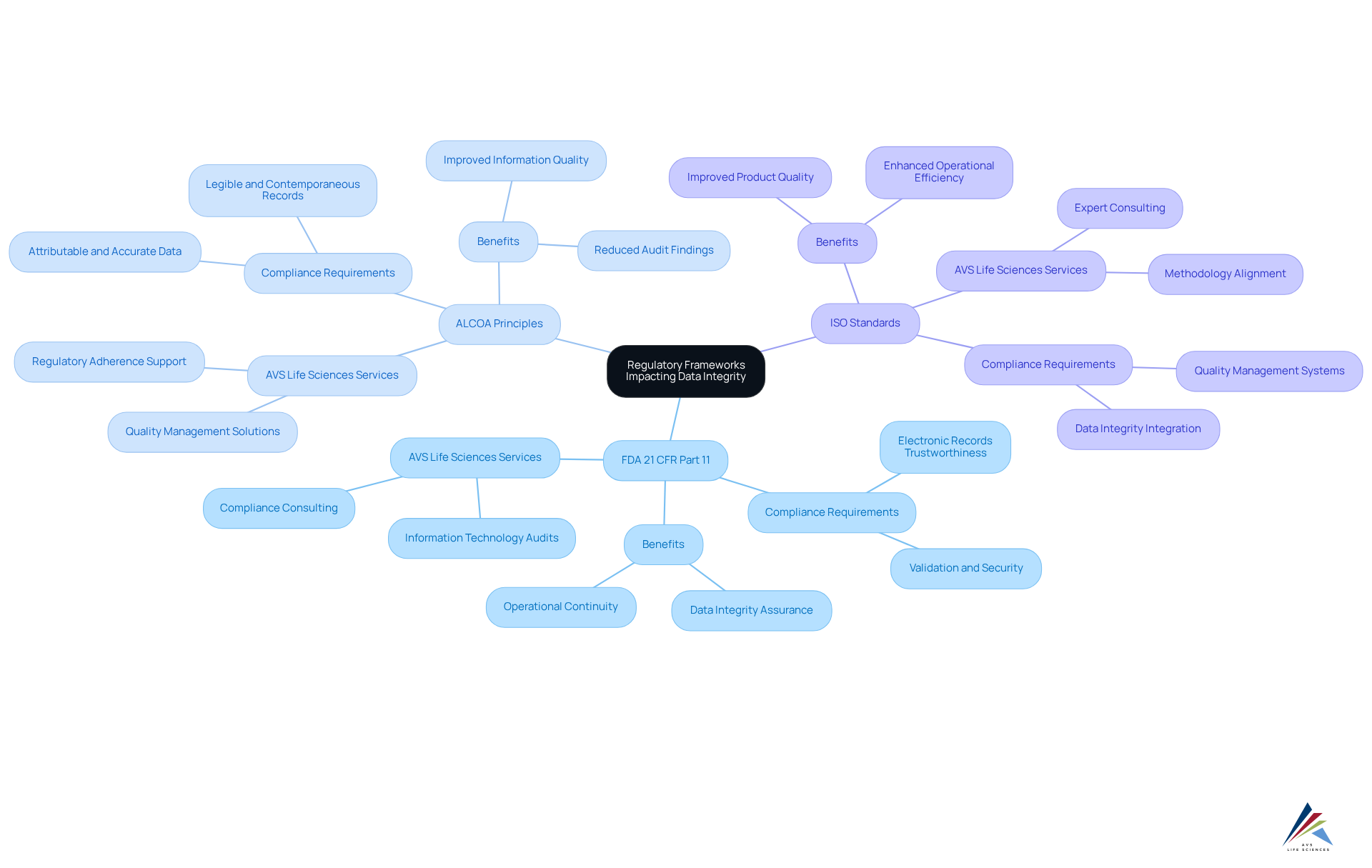
Regulatory frameworks play a pivotal role in shaping information reliability practices within the pharmaceutical and life sciences sectors. Key regulations that organizations must navigate include:
-
FDA 21 CFR Part 11: This regulation delineates the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records. Compliance with this regulation is not merely a best practice; it is essential for ensuring data integrity in digital validation procedures. Non-compliance can lead to substantial penalties and operational disruptions. AVS Life Sciences offers comprehensive services, including Information Technology Audits, to assist organizations in meeting the stringent requirements of this regulation.
-
ALCOA Principles: The ALCOA (Attributable, Legible, Contemporaneous, Original, Accurate) principles are essential for ensuring data integrity in digital validation. Organizations must align their information gathering and management practices with these principles to uphold compliance and mitigate regulatory scrutiny. Companies that effectively implement ALCOA principles have reported significant improvements in information quality and a reduction in audit findings. AVS Life Sciences emphasizes these principles in its quality management and regulatory adherence solutions.
-
ISO Standards: Various ISO standards, such as ISO 9001 and ISO 13485, highlight the necessity of quality management systems that integrate data integrity as a core component. Adherence to these standards not only drives compliance but also enhances operational efficiency and product quality, thereby ensuring data integrity in digital validation. AVS Life Sciences aids organizations in aligning their methodologies with these standards through expert consulting services.
Organizations must remain vigilant regarding these regulations and seamlessly incorporate them into their information management practices. Consistent training and assessments, as provided by AVS Life Sciences, are critical to ensuring adherence and fostering a culture of information reliability within the organization. By prioritizing these frameworks, organizations will be strategically positioned to navigate the complexities of compliance and uphold trust with stakeholders.
Implement Best Practices for Maintaining Data Integrity in Validation
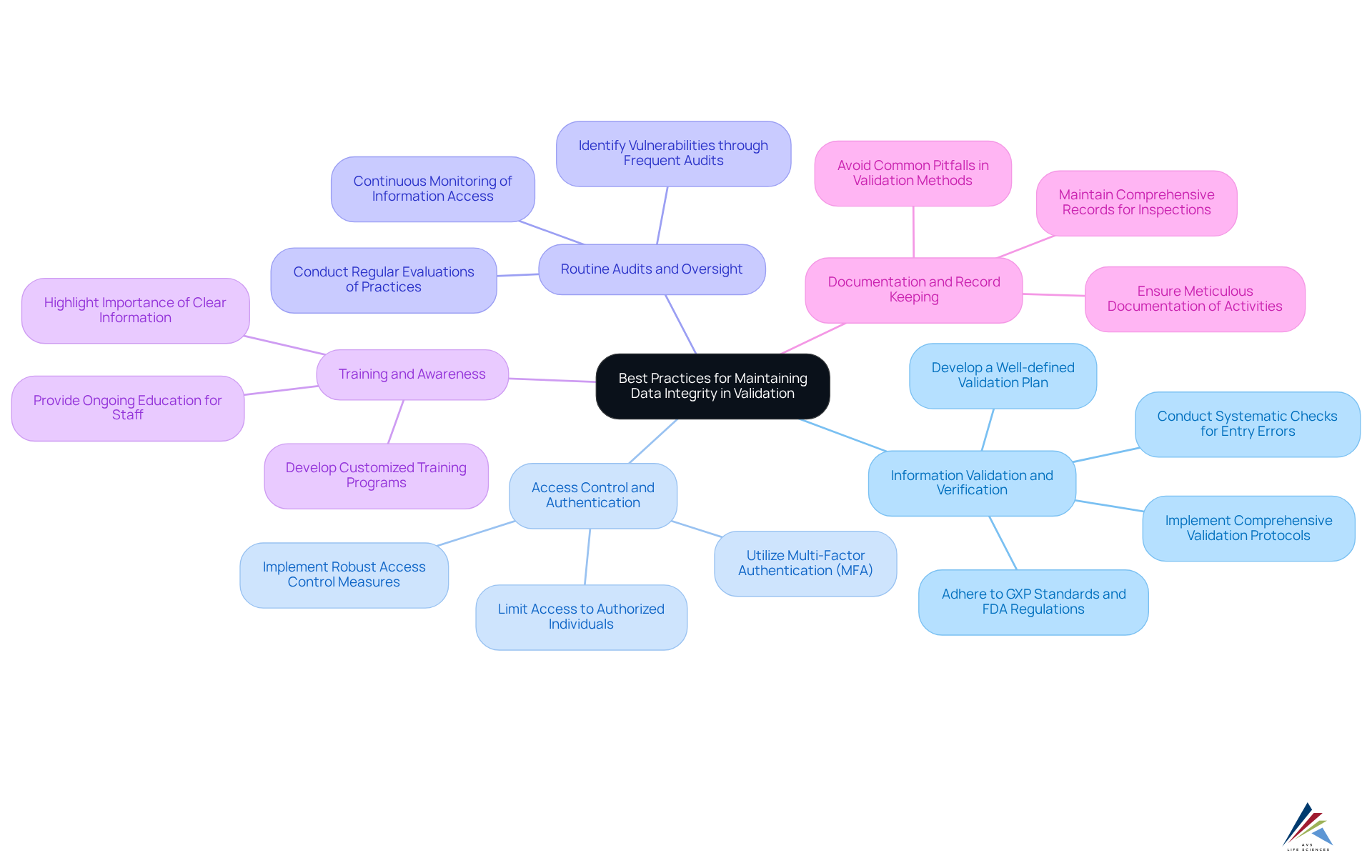
To uphold data integrity in validation processes, organizations must adopt best practices that address compliance challenges effectively:
-
Information Validation and Verification: Implement comprehensive information validation protocols to ensure that the information is both accurate and complete. This involves conducting systematic checks for entry errors and inconsistencies, which are essential for maintaining high-quality information. A well-defined Validation Plan should outline standardization requirements, validation checks, and procedures to guarantee consistency across all information management activities. AVS Life Sciences emphasizes the importance of adhering to GXP standards and FDA regulations to ensure data integrity in digital validation.
-
Access Control and Authentication: Limit access to confidential information strictly to authorized individuals. Utilizing multi-factor authentication (MFA) significantly enhances security, effectively preventing unauthorized access and potential breaches. With cyberattacks using stolen or compromised credentials rising by 71% year-over-year, robust access control measures are more critical than ever. AVS Life Sciences offers expert guidance on implementing these controls in compliance with regulatory requirements.
-
Routine Audits and Oversight: Conduct regular evaluations of information management practices to uncover potential integrity concerns. Continuous monitoring of information access and changes is vital for maintaining data integrity in digital validation, which facilitates prompt corrective actions. Frequent audits can help identify vulnerabilities that may lead to costly breaches, which reached an all-time high average cost of $4.88 million in 2024. AVS Life Sciences' case studies illustrate the effectiveness of thorough auditing techniques in maintaining compliance and quality assurance, as demonstrated in our collaboration with a biotechnology client that successfully upgraded their GMP facility.
-
Training and Awareness: Provide ongoing education for staff regarding information accuracy concepts and optimal practices. A knowledgeable workforce is essential for maintaining high standards of data integrity in digital validation, as employees play a critical role in the information management process. Highlighting the importance of clear information, as emphasized by authorities like Michael Stonebraker, can further enhance the effectiveness of training programs. AVS Life Sciences assists organizations in developing customized training programs that align with their specific regulatory requirements.
-
Documentation and Record Keeping: Ensure meticulous documentation of all information management activities, including entries, modifications, and audits. This comprehensive record-keeping serves as a crucial reference during regulatory inspections, demonstrating adherence and accountability. Maintaining thorough documentation can also help organizations avoid common pitfalls in information validation methods. AVS Life Sciences emphasizes the significance of strong documentation practices in achieving successful outcomes in regulatory compliance.
By implementing these optimal strategies, organizations can significantly enhance their information accuracy and ensure compliance with regulatory standards, ultimately safeguarding their operations against breaches affecting data integrity in digital validation.
Leverage Technology and Tools to Enhance Data Integrity
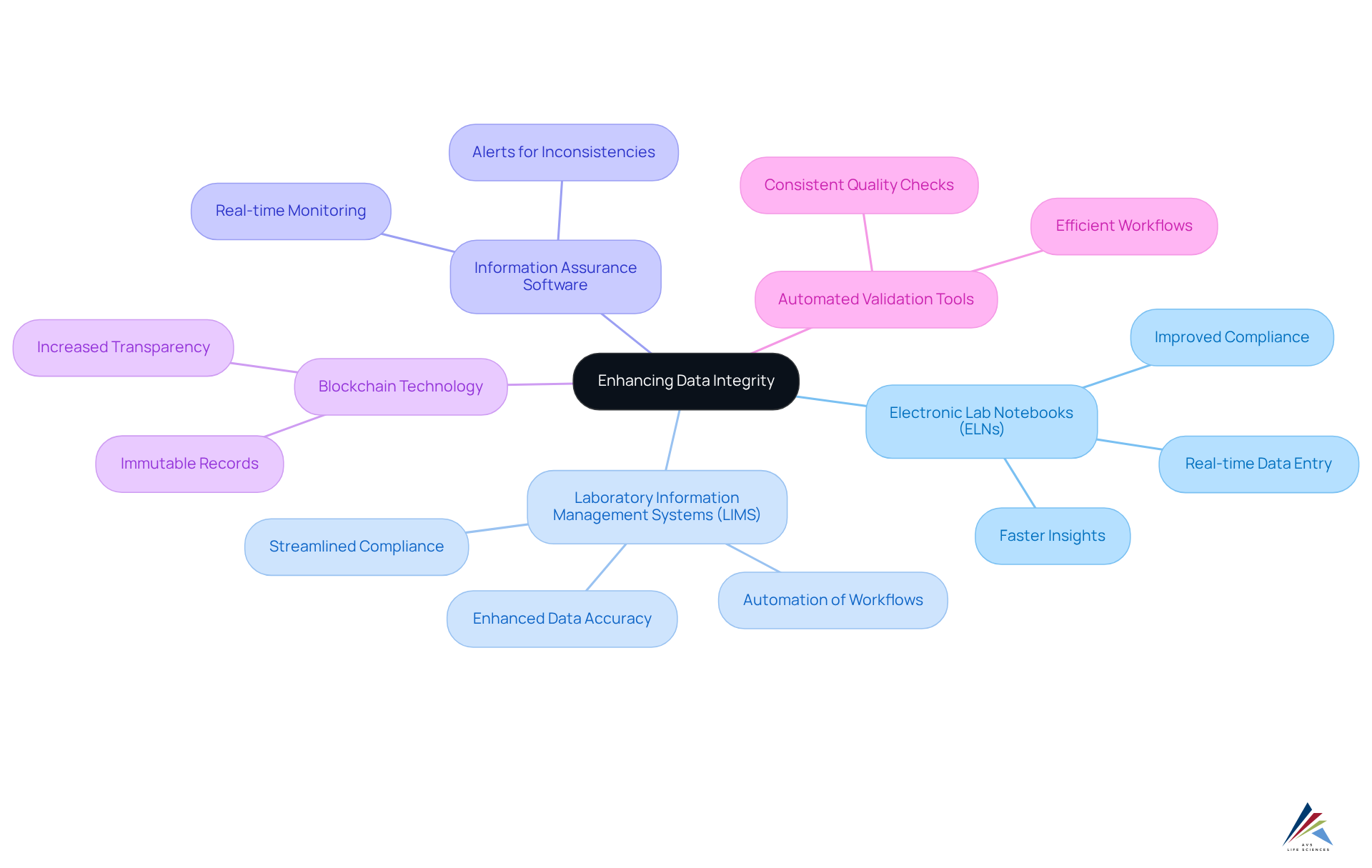
Technology is essential for ensuring data integrity in digital validation processes. Organizations face compliance challenges that can be effectively addressed through a variety of tools and technologies, including:
- Electronic Lab Notebooks (ELNs): ELNs enable real-time data entry, ensuring that data is recorded contemporaneously. This significantly decreases the risk of mistakes linked to manual information entry, thereby improving accuracy.
- Laboratory Information Management Systems (LIMS): LIMS automate information collection, storage, and retrieval processes, streamlining management. This automation guarantees that information stays precise and easily obtainable, which is vital for adherence and operational effectiveness. A transformative case study involving AVS Life Sciences illustrates how effective LIMS implementation can enhance quality assurance and regulatory compliance in a biotechnology GMP facility.
- Information Assurance Software: Specialized software solutions oversee information accuracy in real-time, offering alerts for inconsistencies or unauthorized modifications. This proactive strategy aids in preserving the accuracy of information throughout its lifecycle.
- Blockchain Technology: By providing an unchangeable record of information transactions, blockchain improves transparency and trust in information reliability. This technology is especially advantageous in clinical trials and supply chain management, where information accuracy is crucial.
- Automated Validation Tools: Automation decreases the time and effort needed for validation processes, ensuring that information quality checks are consistently applied. This leads to more efficient workflows and reliable outcomes.
Integrating these technologies into validation processes enables organizations to strengthen data integrity in digital validation, streamline compliance, and enhance overall operational efficiency. It is essential to reference the guidelines for Computer System Validation (CSV) to ensure that these technologies are applied effectively and in alignment with regulatory requirements.
Conclusion
Prioritizing data integrity in digital validation is not merely a regulatory requirement; it is a cornerstone of trust and reliability in the life sciences sector. The accuracy and consistency of data throughout its lifecycle are paramount, as they directly influence the quality of clinical trials, manufacturing processes, and regulatory submissions. Organizations must recognize that neglecting data integrity can lead to severe repercussions, including regulatory fines and reputational harm. Thus, adopting best practices and robust strategies is essential.
Key insights from the discussion underscore the necessity of adhering to regulatory frameworks such as FDA 21 CFR Part 11 and the ALCOA principles, which provide a solid foundation for maintaining data integrity. Implementing best practices—including information validation, access control, routine audits, and effective training—ensures that data remains accurate and secure. Moreover, leveraging advanced technologies like Electronic Lab Notebooks and Laboratory Information Management Systems enhances operational efficiency and compliance, ultimately safeguarding data integrity.
In light of these considerations, organizations are urged to take proactive steps in fostering a culture of data integrity. By investing in training, adhering to regulatory standards, and utilizing cutting-edge technology, companies can not only mitigate risks but also enhance their overall operational effectiveness. Ensuring data integrity is a continuous process that demands attention and commitment; however, the rewards—trust, compliance, and improved quality—are invaluable in today’s data-driven landscape.
Frequently Asked Questions
What is data integrity in digital validation?
Data integrity in digital validation refers to the accuracy, consistency, and dependability of information throughout its lifecycle, ensuring that data generated and utilized in clinical trials, manufacturing, and regulatory submissions is trustworthy.
Why is data integrity important?
Data integrity is crucial because neglecting information accuracy can lead to significant risks, including critical errors, regulatory fines, and reputational damage. For instance, pharmaceutical companies may face challenges during FDA inspections if they fail to maintain data accuracy, resulting in delays in product approvals and potential market withdrawals.
What are the key principles of data integrity?
The key principles of data integrity include: - Attributable: Data must be traceable to the individual responsible for its creation or modification. - Legible: Data should be clear and comprehensible. - Contemporaneous: Data must be recorded at the time of the activity. - Original: Data should represent the first record of the information. - Accurate: Data must be correct and devoid of errors.
How can organizations ensure data integrity?
Organizations can ensure data integrity by prioritizing information reliability, implementing robust practices such as Information Reliability Deviations, Investigations, and Corrective and Preventive Actions (CAPA), and adhering to exemplary documentation practices.
What are the consequences of poor information management?
Poor information management can lead to significant risks, including regulatory fines, reputational damage, operational inefficiencies, and challenges during regulatory inspections, which can ultimately affect product approvals and market presence.
