4 Strategies for Effective MLR Review in Compliance

Overview
This article examines four pivotal strategies for enhancing Medical, Legal, and Regulatory (MLR) review processes within compliance management. It underscores the necessity of:
- Establishing clear guidelines
- Promoting cross-department collaboration
- Employing standardized checklists
- Conducting regular training sessions
These strategies not only improve efficiency and adherence in MLR evaluations but also significantly mitigate legal risks and bolster marketing initiatives. By implementing these measures, organizations can navigate the complex landscape of compliance more effectively, ensuring that they remain both competitive and compliant.
Introduction
In the fast-paced pharmaceutical industry, the stakes are higher than ever regarding the need for promotional materials to comply with stringent legal and regulatory standards. Medical, Legal, and Regulatory (MLR) reviews are critical in safeguarding public health and maintaining corporate integrity.
However, organizations frequently encounter significant challenges in executing these evaluations efficiently. By exploring effective strategies for MLR review compliance, companies can streamline their processes and mitigate risks associated with regulatory scrutiny and content overload.
How can organizations overcome these obstacles and optimize their MLR review systems to enhance compliance and accelerate time to market? The answer lies in implementing robust compliance solutions that not only address these challenges but also position companies for success in a highly regulated environment.
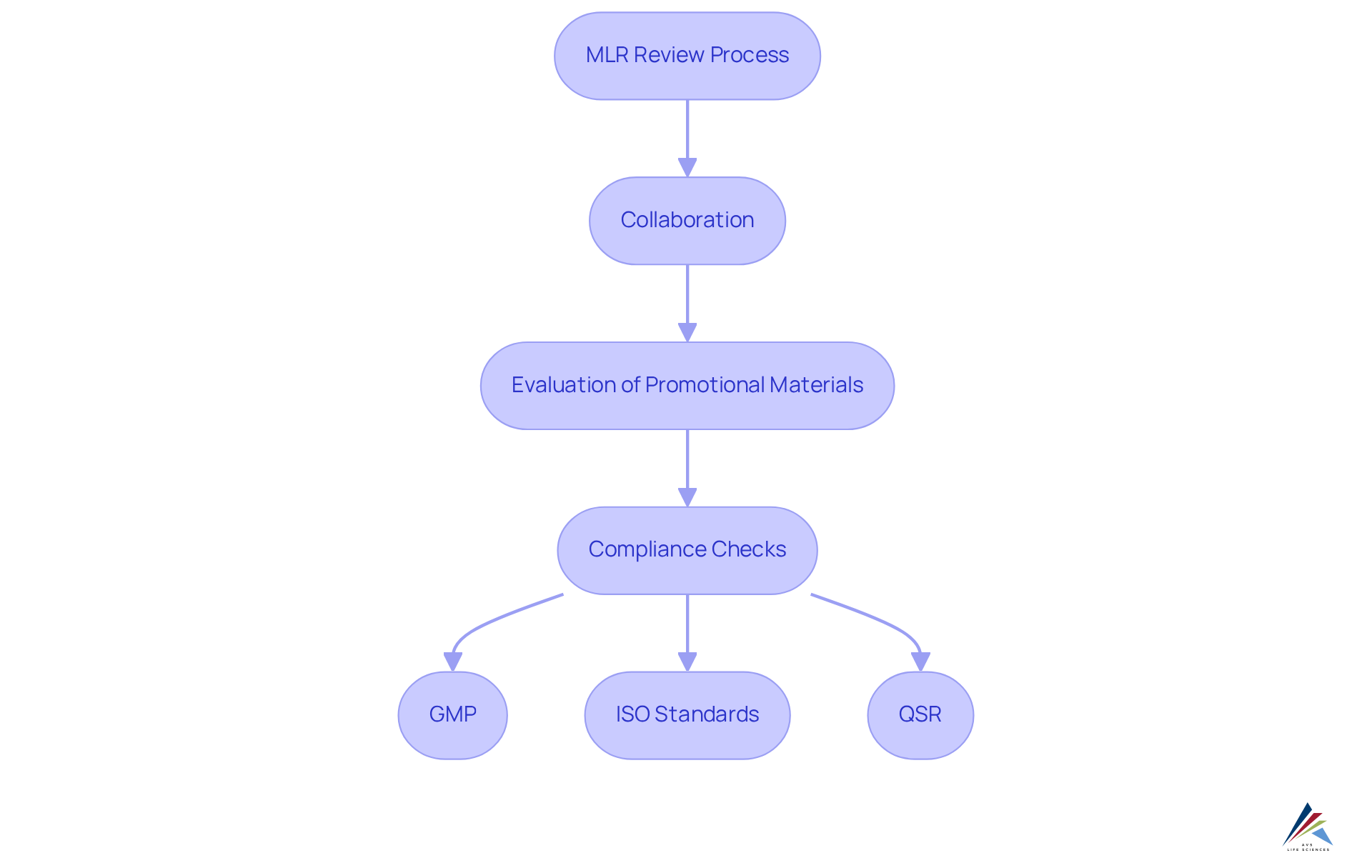
Define MLR Review and Its Importance in Compliance
MLR, or Medical, Legal, and Regulatory assessment, represents a systematic approach that evaluates promotional materials to ensure compliance with applicable laws and regulations. This assessment is crucial in the pharmaceutical sector, where the can significantly impact public health and corporate reputation.
In 2024, the U.S. market for medical, legal, and regulatory assessment software was valued at over USD 5.9 billion, underscoring the growing importance of these activities. The MLR evaluation process involves collaboration among healthcare professionals, legal consultants, and regulatory specialists, who scrutinize content for adherence to:
Key elements such as Data Integrity Deviations, Investigations, and CAPA are essential for ensuring compliance throughout this process. Organizations that have optimized their workflows for MLR review have reported a 57% reduction in cycle times and a 55% decrease in time spent in evaluation meetings, illustrating the efficiency gains achievable through effective MLR review systems.
By establishing a robust MLR review framework, organizations can mitigate legal risks, enhance product credibility, and maintain consumer trust, ultimately supporting their marketing initiatives and regulatory objectives. The case study on 'Optimizing MLR Workflows' exemplifies these findings, highlighting the tangible benefits of integrating Standard Operating Procedures (SOPs) and Technical Writing into MLR activities.
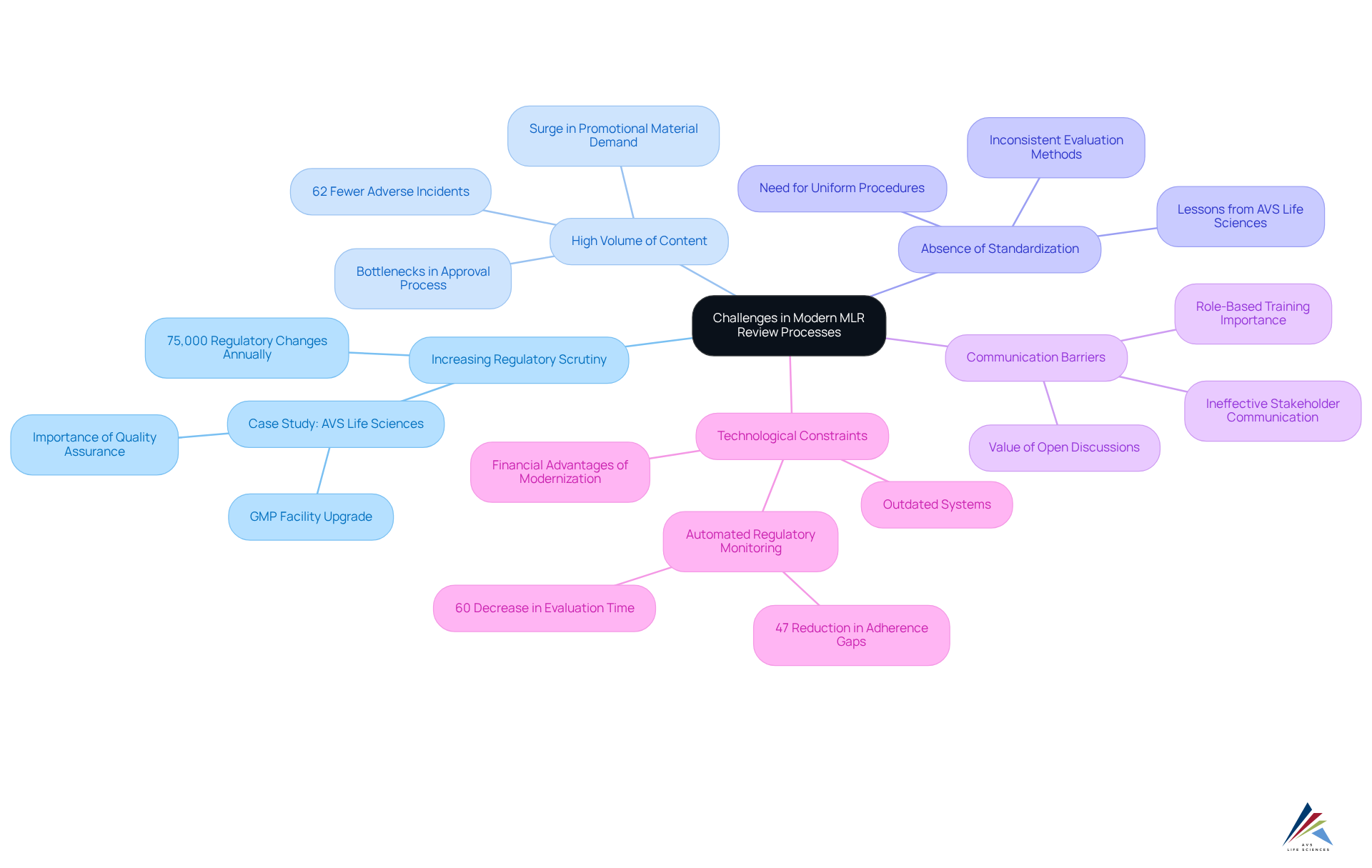
Identify Key Challenges in Modern MLR Review Processes
Contemporary MLR evaluation procedures encounter numerous significant obstacles that can hinder both efficiency and adherence. These challenges include:
- Increasing Regulatory Scrutiny: The pharmaceutical industry is facing heightened regulatory oversight, with companies tracking over 75,000 regulatory changes annually. This evolving landscape necessitates that organizations remain vigilant and adaptable to , which can be daunting. A recent case study from AVS Life Sciences illustrates how a leading biotechnology company successfully upgraded its GMP facility to meet these stringent regulatory demands, showcasing the importance of robust quality assurance practices and operational excellence.
- High Volume of Content: The demand for promotional materials is surging, resulting in increased workloads for evaluation teams. This influx often leads to bottlenecks, delaying the approval of essential marketing materials and potentially impacting product launch timelines. Organizations with developed regulatory capabilities experience 62% fewer significant adverse incidents in clinical trials, underscoring the significance of effective MLR systems in mitigating risks linked to high content volumes. AVS Life Sciences' expertise in enhancing quality control during their facility upgrade demonstrates how effective management can alleviate these pressures.
- Absence of Standardization: Inconsistent evaluation methods across different departments can create confusion and mistakes, complicating adherence efforts. Establishing uniform procedures is essential to ensure that all teams are coordinated and adherence is upheld throughout the evaluation process. The lessons learned from AVS Life Sciences' collaboration with their client emphasize the need for thorough evaluations of existing processes to identify and rectify gaps that may lead to unreliable outcomes.
- Communication Barriers: Ineffective communication among stakeholders can result in misunderstandings and delays. Open lines of communication are vital to facilitate prompt evaluations and approvals, ensuring that all parties are informed and engaged. Role-based training for compliance is crucial to ensure employees understand their responsibilities and the importance of compliance to the organization. The case study further emphasizes the value of open discussions among teams to foster a culture of accountability and shared responsibility.
- Technological Constraints: Many organizations continue to rely on outdated systems that hinder effective collaboration and progress monitoring. Embracing contemporary technological solutions can streamline workflows and enhance the overall efficiency of the MLR evaluation process. For instance, automated regulatory monitoring can minimize adherence gaps by 47% and reduce the time required for regulatory evaluations by over 60%. A major pharmaceutical firm has credited substantial yearly savings to their integrated regulatory and oversight intelligence platform, illustrating the financial advantages of effectively addressing MLR assessment challenges.
Addressing these challenges is essential for organizations striving to enhance their evaluation methods through MLR review and boost compliance, ultimately fostering a more effective and agile marketing strategy.
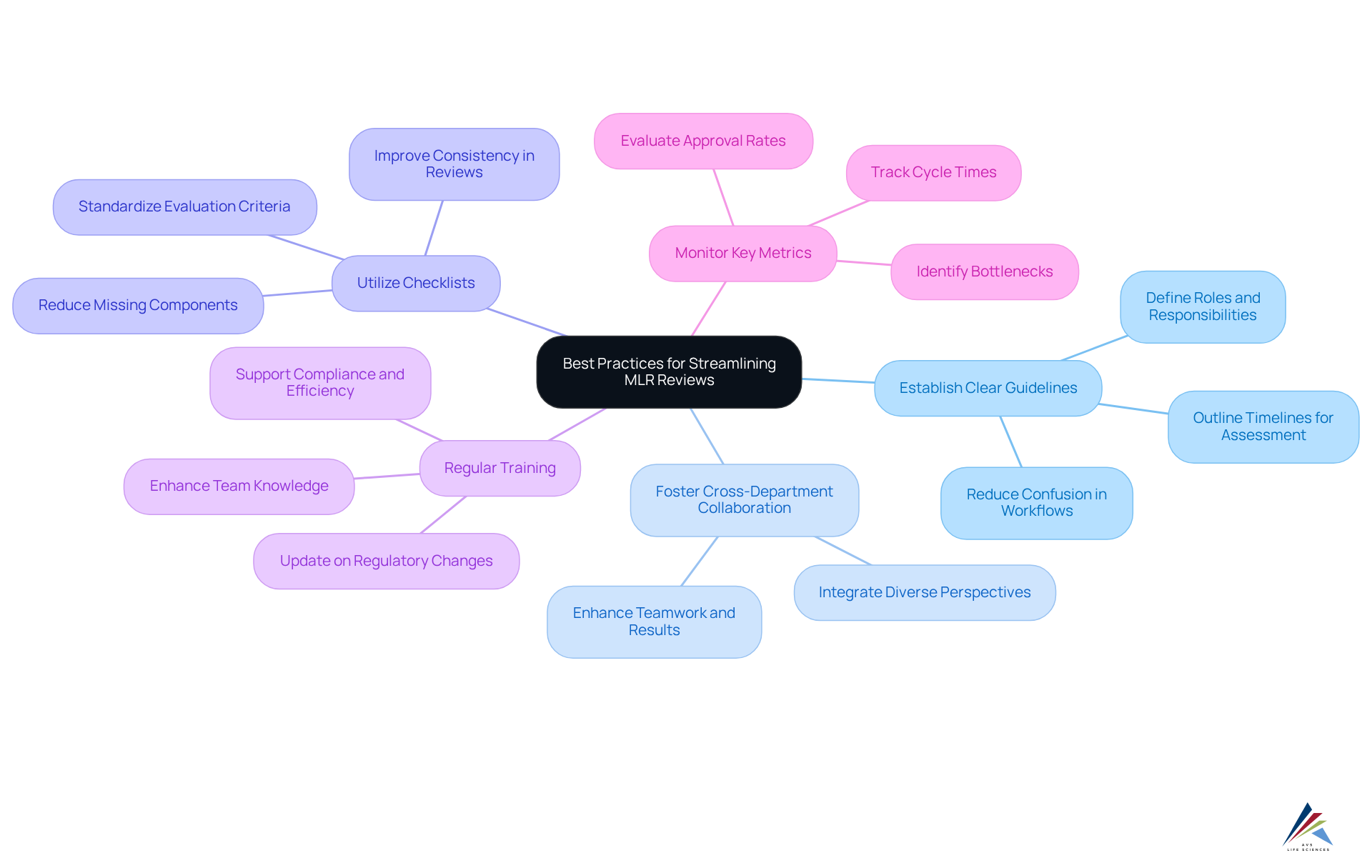
Implement Best Practices for Streamlining MLR Reviews
To enhance the efficiency of MLR reviews, organizations must adopt best practices that leverage the expertise of AVS Life Sciences:
- Establish Clear Guidelines: Organizations should create detailed MLR evaluation guidelines that distinctly outline roles, responsibilities, and timelines for each phase of the assessment. This clarity significantly reduces confusion and streamlines workflows, a principle emphasized in AVS Life Sciences' quality management solutions.
- Foster Cross-Department Collaboration: It is essential to promote cooperation among medical, legal, and regulatory teams to ensure that diverse perspectives are integrated into the evaluation process. Successful teamwork has demonstrated the ability to enhance assessment results and adherence, aligning with AVS Life Sciences' strategy for holistic consulting services.
- Utilize Checklists: Implementing is crucial to ensure that all required criteria are met during the evaluation. This practice not only improves consistency but also reduces the chance of missing vital components, a key focus for AVS Life Sciences in their regulatory solutions.
- Regular Training: Conducting ongoing training sessions for evaluation teams is necessary to keep them updated on regulatory changes and best practices. Investing in team knowledge is essential for upholding compliance and enhancing assessment efficiency, which AVS Life Sciences supports through its expert quality solutions.
- Monitor Key Metrics: Tracking essential performance indicators (KPIs) related to the MLR evaluation, such as cycle times and approval rates, enables organizations to pinpoint bottlenecks and opportunities for enhancement. Ultimately, this results in more effective operations, a goal that AVS Life Sciences strives to accomplish for its clients.
By applying these optimal methods, organizations can significantly improve the efficiency and effectiveness of their MLR review processes. This leads to better adherence outcomes and shortened approval timelines, backed by the knowledge and experience of AVS Life Sciences.
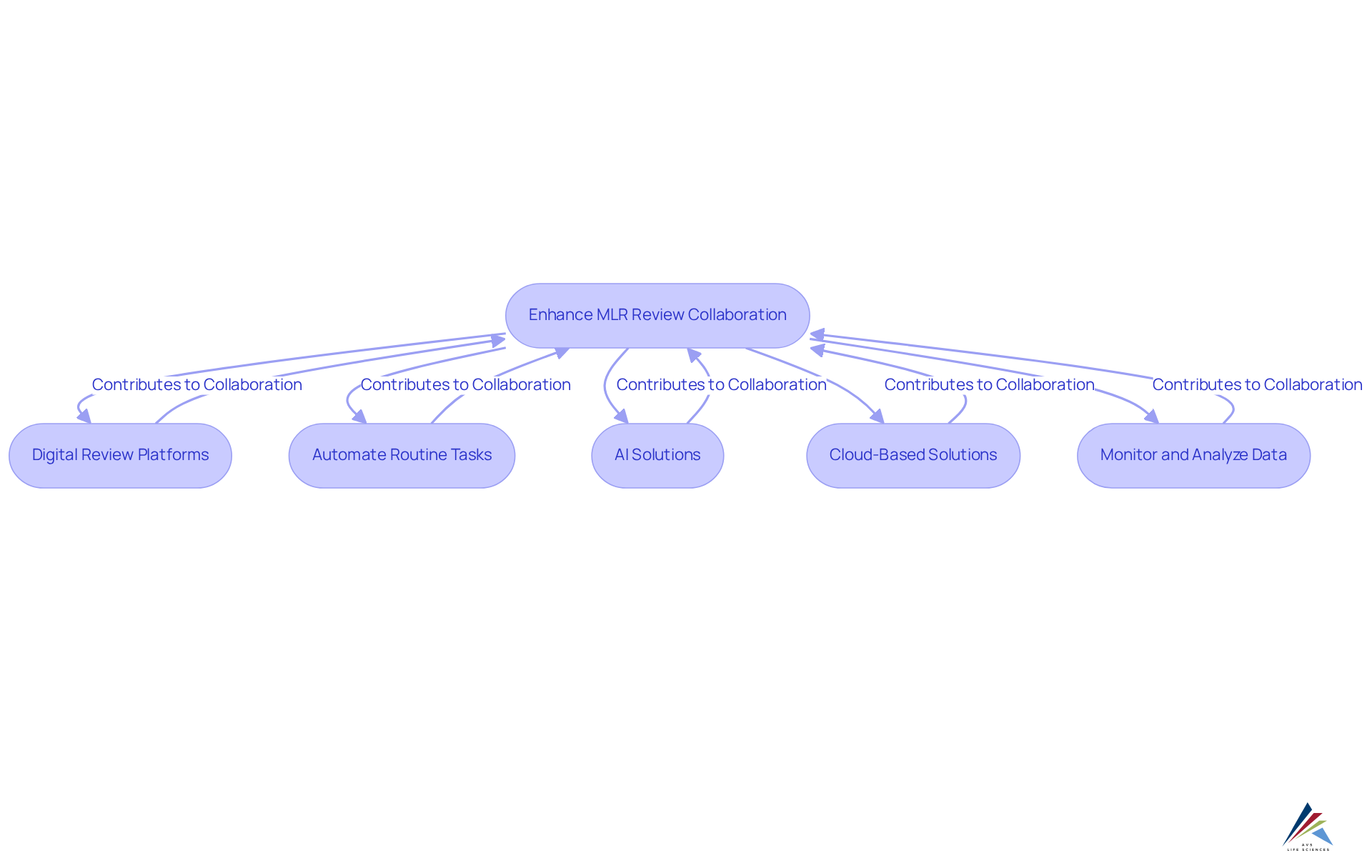
Leverage Technology to Enhance MLR Review Collaboration
Utilizing technology can significantly enhance cooperation and effectiveness in MLR review evaluations, particularly when it is aligned with comprehensive . Consider the following strategies:
- Implement Digital Review Platforms: Leverage digital platforms that enable real-time collaboration among stakeholders, facilitating seamless document sharing and feedback, in accordance with robust documentation practices and standard operating procedures (SOPs).
- Automate Routine Tasks: Employ automation tools to manage repetitive tasks, such as version control and document tracking, allowing reviewers to focus on more complex assessments while ensuring compliance with FDA regulations and GXP standards.
- Embrace AI Solutions: Integrate AI-powered tools capable of assessing content for regulatory concerns, thereby reducing manual workload and expediting evaluations, which enhances data integrity and adherence to regulatory standards.
- Employ Cloud-Based Solutions: Cloud technology provides remote access to documents and fosters collaboration among geographically dispersed teams, ensuring that all stakeholders can participate in the evaluation while complying with CFR Part 11.
- Monitor and Analyze Data: Utilize analytics tools to track MLR evaluations, identifying trends and areas for improvement based on data-driven insights, which are crucial for effective CAPA and investigations.
By adopting these technological advancements, organizations can foster a more efficient and collaborative MLR review, ultimately enhancing compliance and reducing time to market, while leveraging AVS Life Sciences' expertise in GMP compliance and validation.
Conclusion
Establishing an effective MLR (Medical, Legal, and Regulatory) review process is essential for organizations within the pharmaceutical sector. This systematic evaluation not only ensures compliance with various regulatory requirements but also safeguards public health and enhances corporate credibility. By prioritizing a robust MLR framework, companies can navigate the complexities of regulatory landscapes while improving the efficiency of their marketing strategies.
The article outlines several key challenges that modern MLR review processes face:
- Increasing regulatory scrutiny
- High content volumes
- Lack of standardization
- Communication barriers
- Outdated technological systems
Each of these challenges can significantly hinder the effectiveness of MLR evaluations, leading to delays and potential compliance issues. However, by implementing best practices—such as establishing clear guidelines, fostering cross-department collaboration, utilizing standardized checklists, and leveraging technology—organizations can mitigate these obstacles and streamline their review processes.
Ultimately, the significance of optimizing MLR reviews cannot be overstated. As regulatory environments evolve and the demand for promotional materials increases, adopting innovative solutions and best practices is crucial for maintaining compliance and operational efficiency. Organizations are encouraged to embrace these strategies to enhance their MLR review processes, ensuring not only legal adherence but also fostering a culture of accountability and excellence in their marketing initiatives.
Frequently Asked Questions
What is MLR Review?
MLR, or Medical, Legal, and Regulatory assessment, is a systematic approach that evaluates promotional materials to ensure compliance with applicable laws and regulations in the pharmaceutical sector.
Why is MLR Review important?
MLR Review is crucial because it ensures the accuracy and legality of marketing materials, which can significantly impact public health and corporate reputation.
What was the market value of MLR assessment software in 2024?
In 2024, the U.S. market for medical, legal, and regulatory assessment software was valued at over USD 5.9 billion, indicating the growing importance of these activities.
Who is involved in the MLR evaluation process?
The MLR evaluation process involves collaboration among healthcare professionals, legal consultants, and regulatory specialists.
What standards does MLR Review assess for compliance?
MLR Review assesses compliance with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
What are key elements essential for ensuring compliance during MLR Review?
Key elements include Data Integrity Deviations, Investigations, and Corrective and Preventive Actions (CAPA).
What efficiency gains have organizations reported from optimizing MLR workflows?
Organizations that optimized their workflows for MLR review reported a 57% reduction in cycle times and a 55% decrease in time spent in evaluation meetings.
How does a robust MLR review framework benefit organizations?
A robust MLR review framework helps mitigate legal risks, enhance product credibility, and maintain consumer trust, supporting marketing initiatives and regulatory objectives.
What does the case study on 'Optimizing MLR Workflows' demonstrate?
The case study highlights the tangible benefits of integrating Standard Operating Procedures (SOPs) and Technical Writing into MLR activities.
