4 Steps to Implement ISO 22716 Guidelines for Private Label Skincare Manufacturing

Overview
The article presents a structured approach to implementing ISO 22716 guidelines for private label skincare manufacturing, highlighting the critical aspects of quality management, risk mitigation, personnel training, and thorough documentation. It introduces compliance challenges faced by the industry and expands on detailed solutions through a step-by-step framework. This framework includes:
- Conducting a gap analysis
- Defining roles
- Developing standard operating procedures
- Establishing monitoring and auditing processes
Such measures are essential to ensure compliance and drive continuous improvement in manufacturing practices, ultimately prompting engagement with AVS Life Sciences for effective compliance solutions.
Introduction
Navigating the intricate landscape of skincare manufacturing necessitates strict adherence to quality standards, particularly the ISO 22716 guidelines. These guidelines are crucial, as they not only guarantee the safety and efficacy of products but also cultivate consumer trust in private label brands. However, the journey toward compliance can appear formidable, prompting inquiries about the most effective methods for implementing these standards. What essential steps must manufacturers undertake to align their processes with ISO 22716 while ensuring operational efficiency and product integrity?
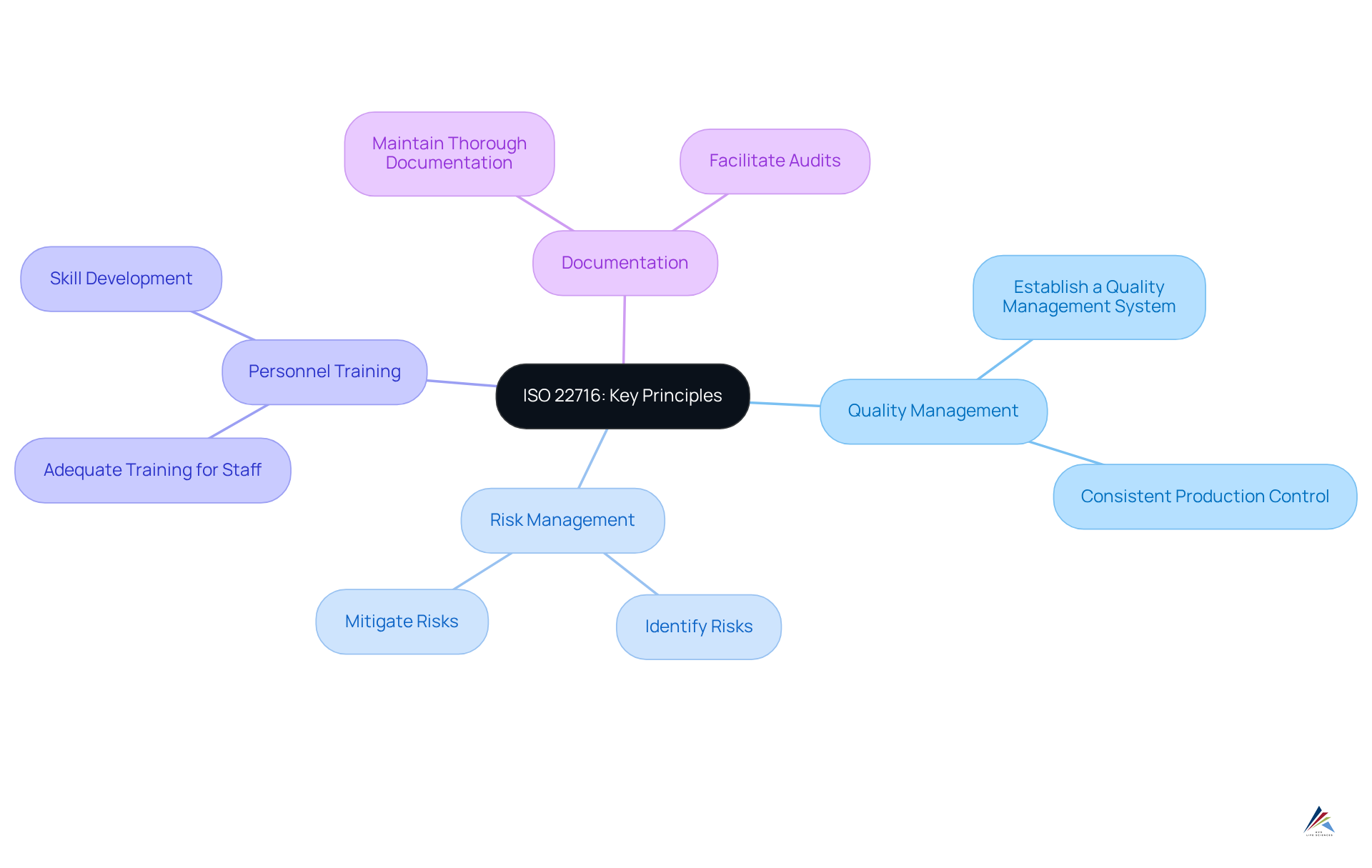
Understand ISO 22716: Key Principles and Objectives
The ISO 22716 guidelines for private label skincare manufacturing outline the Good Manufacturing Practices (GMP) for the cosmetics industry, emphasizing the quality management system. The key principles are as follows:
- Quality Management: Establish a quality management system that guarantees products are consistently produced and controlled in accordance with quality standards.
- Risk Management: Identify and mitigate risks associated with the manufacturing process to ensure product safety.
- Personnel Training: Ensure that all staff are adequately trained and skilled in their roles to uphold the established standards.
- Documentation: Maintain thorough documentation to provide evidence of adherence and facilitate audits.
Understanding these principles is crucial for creating a compliant manufacturing environment that adheres to the ISO 22716 guidelines for private label skincare manufacturing while satisfying both regulatory requirements and consumer expectations.
Establish a Compliance Framework: Step-by-Step Implementation
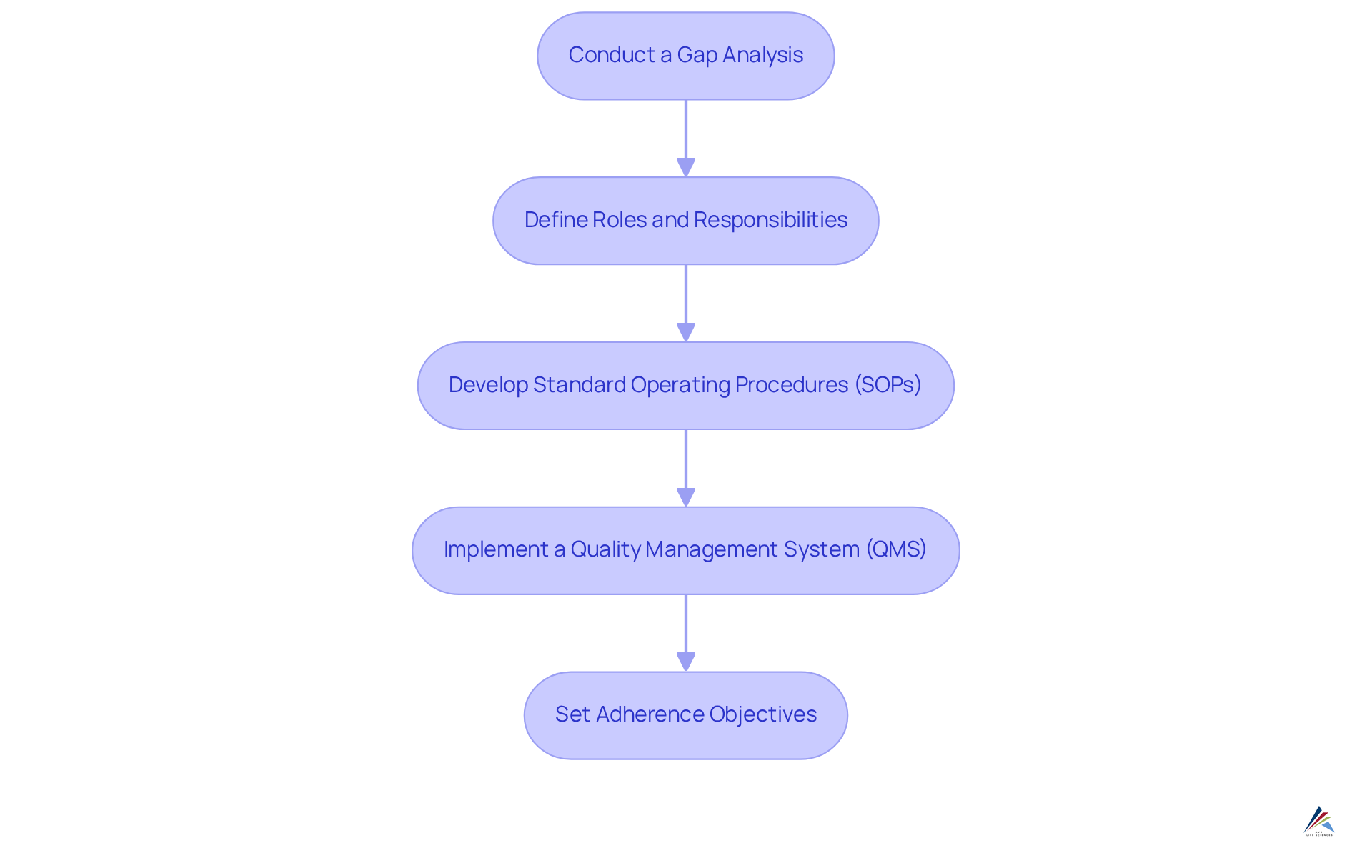
To establish a robust compliance framework for ISO 22716, manufacturers must follow these essential steps:
-
Conduct a Gap Analysis: Begin by assessing current practices against ISO 22716 requirements. This analysis is crucial for identifying deficiencies and areas needing improvement, ensuring that the entity aligns with industry standards. Viewing the gap analysis as a continuous process allows for ongoing enhancements in adherence.
-
Define Roles and Responsibilities: Clearly outline adherence duties within the entity. This step is vital, as effective role definition can significantly improve accountability and simplify adherence to the ISO 22716 guidelines for private label skincare manufacturing. Statistics indicate that organizations with well-defined roles experience 30% fewer compliance issues, underscoring the importance of clarity in compliance tasks.
-
Develop Standard Operating Procedures (SOPs): Create detailed SOPs that encompass processes for manufacturing, quality control, and documentation. These procedures should align with the ISO 22716 guidelines for private label skincare manufacturing, ensuring consistency and quality in operations.
-
Implement a Quality Management System (QMS): Select a QMS that aligns with ISO 22716 and integrates seamlessly with existing processes. A robust QMS that follows the ISO 22716 guidelines for private label skincare manufacturing not only assists in adhering to regulations but also enhances overall operational efficiency.
-
Set Adherence Objectives: Establish measurable adherence goals to track progress and ensure accountability. Consistently examining these objectives aids in sustaining attention on regulatory initiatives and encourages a culture of ongoing enhancement.
By diligently following these steps, manufacturers can establish a strong basis for adherence that supports ongoing quality assurance and reduces risks linked to non-adherence. As Ayush Saxena observes, "Organizations are quickly adopting regulatory programs to establish trust with clients and stakeholders, evade fines and penalties, and develop a robust cybersecurity stance to safeguard sensitive information.
Develop Documentation and Training Programs for Staff
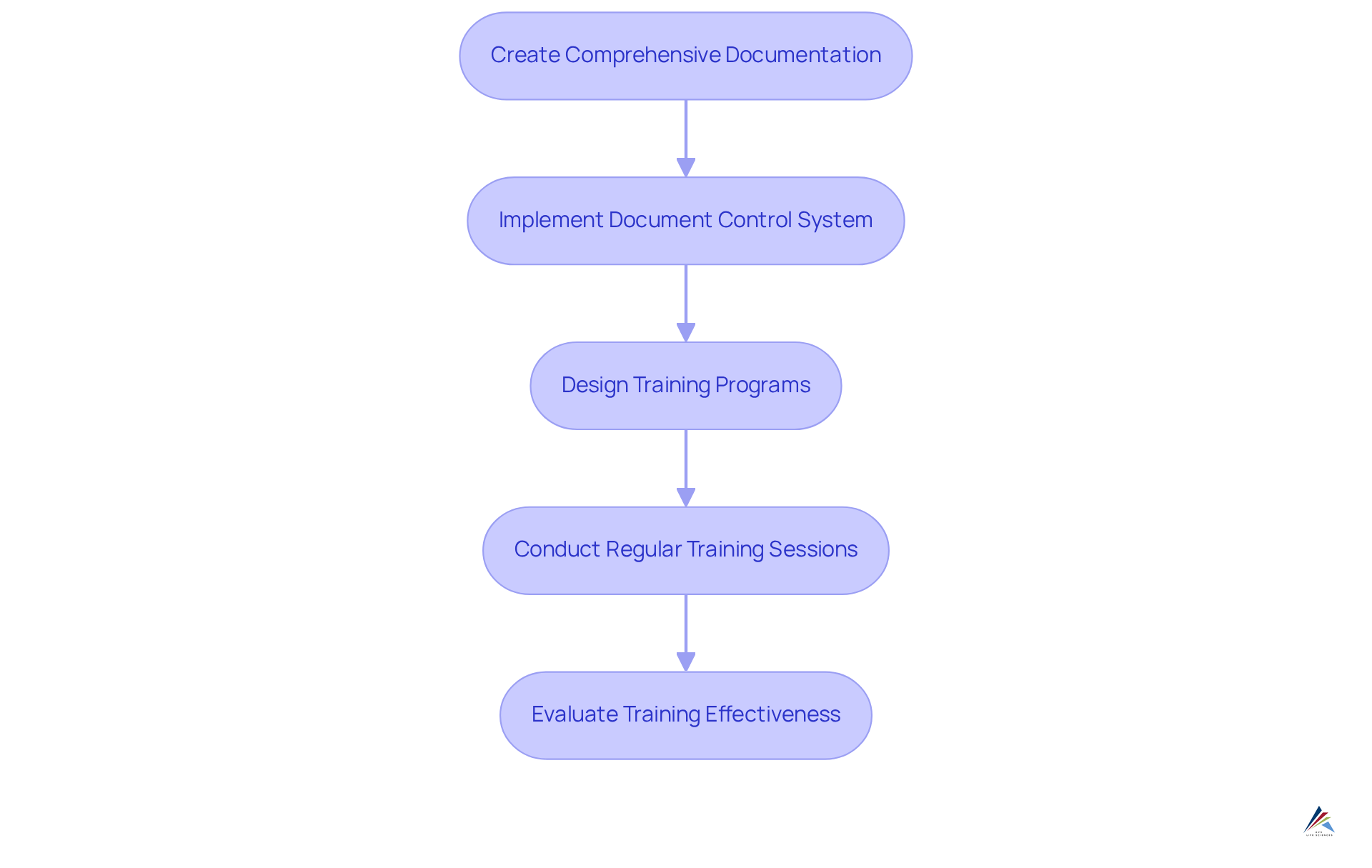
To establish effective documentation and training programs for ISO 22716 compliance, organizations must focus on several key steps:
-
Create Comprehensive Documentation: It is essential to develop detailed documents that outline all processes, including manufacturing, quality control, and corrective actions. Accessibility is crucial; ensure these documents are readily available to all staff to facilitate adherence to standards.
-
Implement a Document Control System: A robust document control system must be utilized to manage revisions and ensure that all employees are using the most current documents. This system is vital, as 66% of businesses face challenges with document approvals and reviews, often due to lengthy physical signing processes. Additionally, 83% of employees struggle with version control issues, underscoring the need for effective management.
-
Design Training Programs: Focused training initiatives should be developed that encompass ISO principles, company standard operating procedures (SOPs), and specific roles and responsibilities. Effective training is essential, as 51% of employees aged 18-34 and 57% of employees aged 35-44 would consider leaving their job due to inefficient document management, which could be improved by adhering to the ISO 22716 guidelines for private label skincare manufacturing.
-
Conduct regular training sessions is necessary to keep staff updated about regulatory requirements and to emphasize the significance of following the ISO 22716 guidelines for private label skincare manufacturing. Regular training helps mitigate the 21.3% productivity loss attributed to poor document management practices, as employees spend an average of 2 hours per day searching for documents.
-
Evaluate Training Effectiveness: It is necessary to implement assessments and gather feedback to evaluate the effectiveness of training programs. This evaluation process allows for necessary adjustments, ensuring that training remains relevant and impactful.
By prioritizing thorough documentation and efficient training, companies can develop a knowledgeable workforce committed to upholding ISO standards.
Implement Monitoring and Auditing Procedures for Continuous Improvement
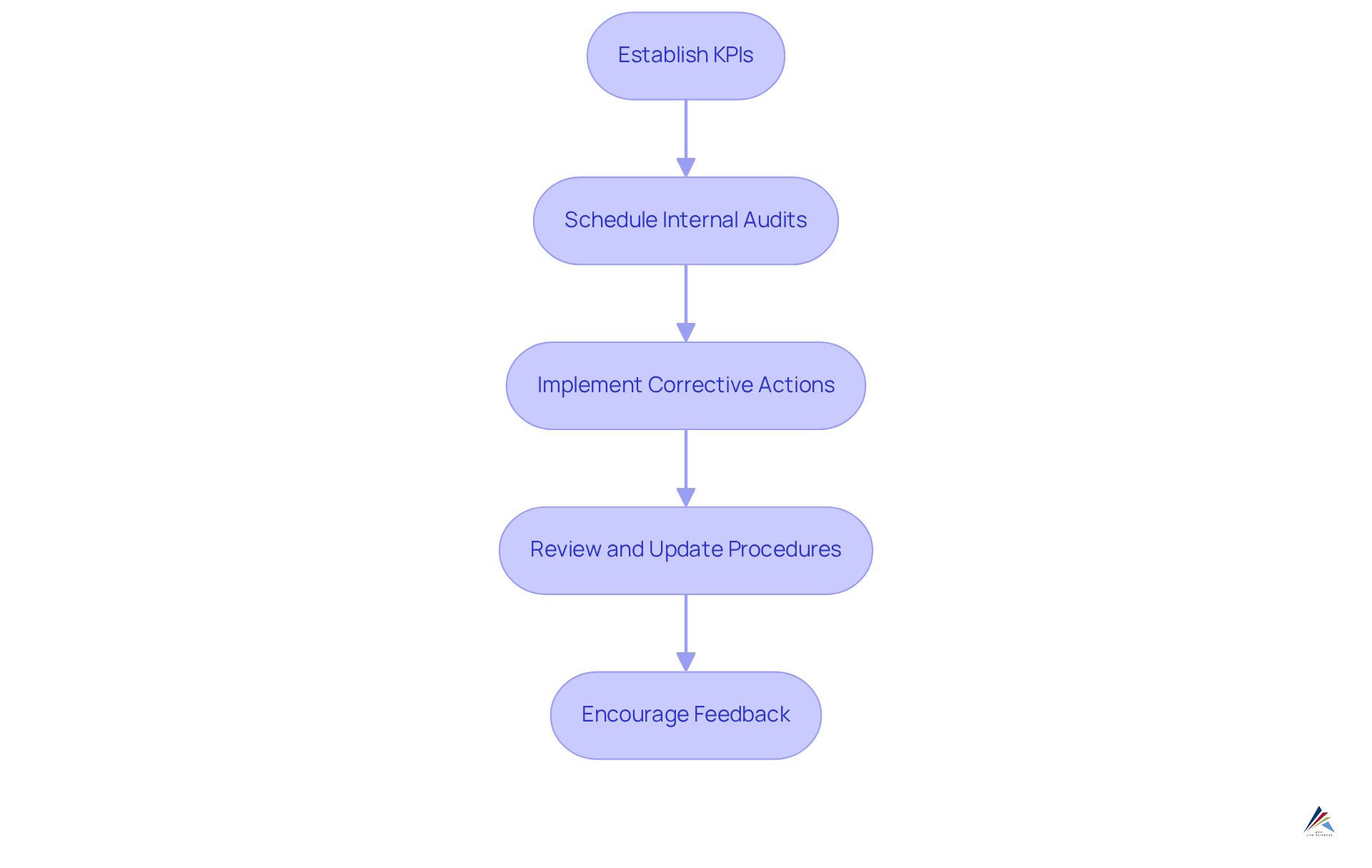
To implement effective monitoring and auditing procedures for ISO 22716 compliance, organizations must adhere to these essential steps:
-
Establish Key Performance Indicators (KPIs): Organizations should define specific KPIs that assess adherence effectiveness and product quality. These indicators must demonstrate compliance with internal policies and regulatory requirements, providing insights into the entity's adherence status. As highlighted in the case study 'Quantifying Compliance: Key Performance Indicators to Monitor,' careful monitoring of adherence metrics enables organizations to proactively reduce risks and uphold ethical standards.
-
It is crucial to schedule internal audits at appropriate intervals to evaluate adherence to the ISO 22716 guidelines for private label skincare manufacturing. This practice not only identifies areas for improvement but also reinforces a culture of accountability and transparency within the organization. Routine evaluations are vital for maintaining stakeholder confidence, as prompt reporting and efficient responses to regulatory incidents are essential.
-
Implement Corrective Actions: Developing a robust system for addressing non-conformities identified during audits is imperative. Ensure that corrective actions are documented, tracked, and communicated effectively to foster continuous improvement and mitigate potential liabilities. This aligns with insights from Claire Dossett, who emphasizes the importance of a thorough management system for adherence.
-
Review and Update Procedures: Organizations must regularly assess adherence procedures and documentation to ensure they remain relevant and effective. Continuous evaluation assists in adapting to changing regulatory demands, thereby enhancing overall adherence effectiveness. Monitoring adherence metrics is essential for recognizing trends and identifying areas requiring further focus.
-
Encourage Feedback: Creating open channels for staff to provide input on adherence processes is vital. Involving employees in this manner fosters a culture of ethical behavior and ongoing enhancement, which is crucial for maintaining high standards of adherence. The Integrity Platform engages employees at all touchpoints for ethical business transformation, promoting accountability and transparency within the entity.
By implementing these monitoring and auditing procedures, organizations can not only meet the ISO 22716 guidelines for private label skincare manufacturing but also enhance their overall compliance efforts. This proactive approach to risk management and ethical business practices is essential in today's regulatory environment.
Conclusion
Implementing the ISO 22716 guidelines is crucial for private label skincare manufacturers seeking to elevate product quality and ensure adherence to industry standards. By emphasizing Good Manufacturing Practices (GMP), organizations can establish a systematic approach that not only fulfills regulatory requirements but also cultivates consumer trust. The path to compliance necessitates a comprehensive understanding of key principles, the establishment of a robust framework, and the promotion of a culture centered on continuous improvement.
This article outlines critical steps to facilitate this compliance journey:
- Conducting a thorough gap analysis
- Clearly defining roles and responsibilities
- Developing standard operating procedures
- Implementing effective monitoring and auditing practices
Each phase is integral to achieving ISO 22716 compliance. Furthermore, prioritizing documentation and staff training ensures that all team members are well-equipped to uphold the established standards, thereby reinforcing the commitment to quality.
Ultimately, the importance of adhering to ISO 22716 transcends mere compliance; it embodies a commitment to excellence in skincare manufacturing. By adopting these guidelines, organizations not only mitigate risks but also position themselves as frontrunners in a competitive market. Taking decisive action now to implement these practices will pave the way for sustained success and foster a culture of accountability and quality assurance that benefits both the company and its consumers.
Frequently Asked Questions
What is ISO 22716?
ISO 22716 is a set of guidelines for private label skincare manufacturing that outlines Good Manufacturing Practices (GMP) for the cosmetics industry, focusing on quality management systems.
What are the key principles of ISO 22716?
The key principles of ISO 22716 include Quality Management, Risk Management, Personnel Training, and Documentation.
How does Quality Management function under ISO 22716?
Quality Management under ISO 22716 involves establishing a system that ensures products are consistently produced and controlled according to quality standards.
What is the role of Risk Management in ISO 22716?
Risk Management involves identifying and mitigating risks associated with the manufacturing process to ensure product safety.
Why is Personnel Training important in ISO 22716?
Personnel Training is important because it ensures that all staff are adequately trained and skilled in their roles to maintain the established standards.
What is the significance of Documentation in ISO 22716?
Documentation is significant as it provides thorough evidence of adherence to the guidelines and facilitates audits.
Why is it important to understand the principles of ISO 22716?
Understanding the principles of ISO 22716 is crucial for creating a compliant manufacturing environment that meets regulatory requirements and consumer expectations.
