4 Steps to Ensure 503b Compliance in Pharmaceutical Operations

Overview
To ensure 503b compliance in pharmaceutical operations, organizations must adopt a structured approach that addresses regulatory requirements, evaluates current practices, trains staff, and establishes monitoring processes. This article delineates these four essential steps, underscoring the critical importance of adhering to FDA standards and implementing robust quality control measures. By enhancing patient safety and product quality, organizations not only comply with regulations but also build trust and reliability in their operations. Engaging with AVS Life Sciences can provide the necessary expertise and resources to navigate these compliance challenges effectively.
Introduction
Navigating the intricate landscape of pharmaceutical operations necessitates a keen understanding of regulatory frameworks, particularly Section 503b of the Federal Food, Drug, and Cosmetic Act. This section delineates the stringent requirements for outsourcing facilities compounding sterile drugs and underscores the critical importance of compliance to ensure patient safety and product integrity.
As the industry confronts ongoing challenges, including medication shortages and inspection failures, the pressing question emerges: how can organizations effectively implement a robust compliance strategy that adapts to these evolving standards?
This guide provides a step-by-step approach to ensuring 503b compliance, empowering facilities to enhance their operational practices and safeguard public health.
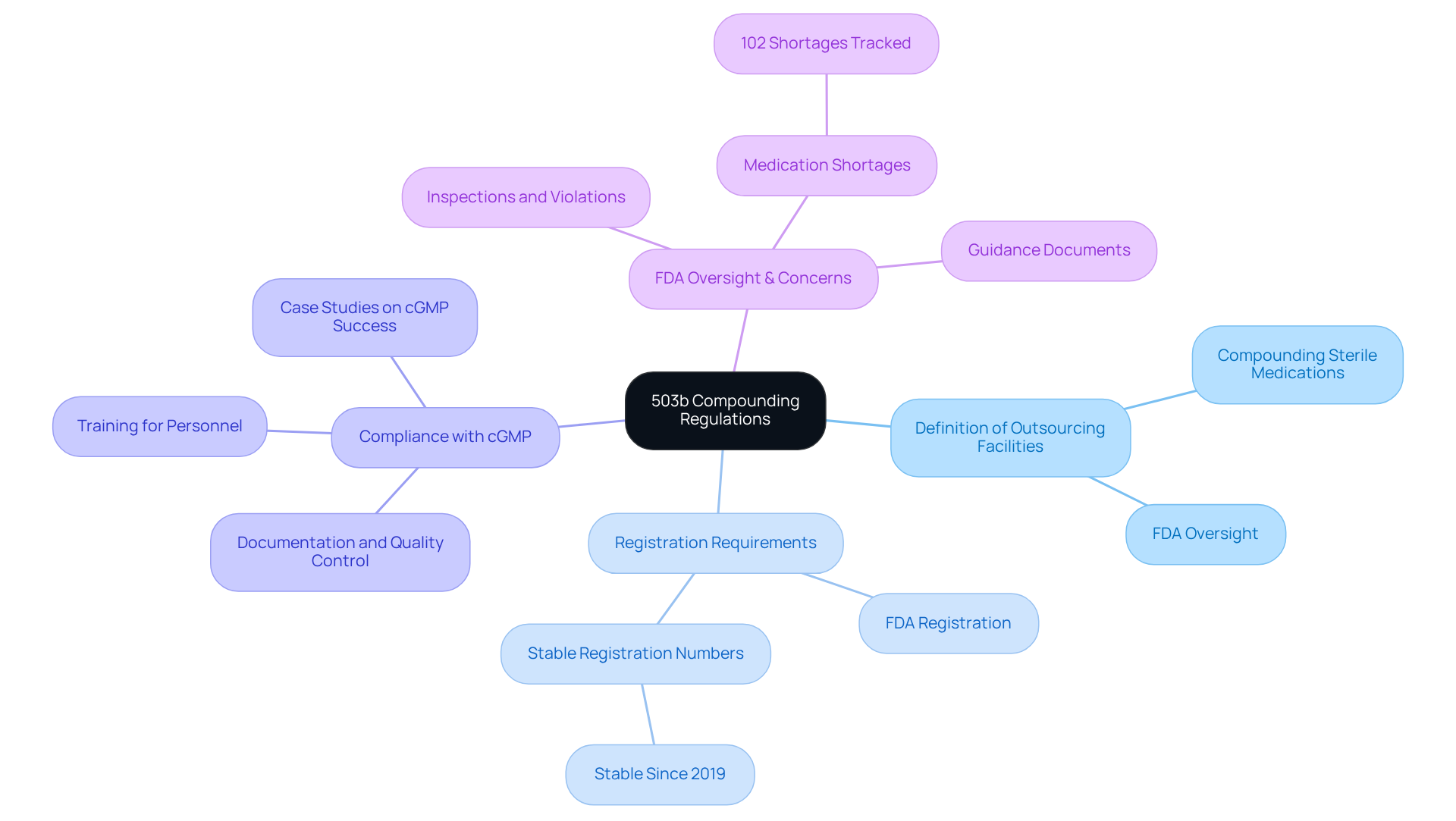
Understand 503b Compounding Regulations
To ensure adherence to the relevant guidelines, it is crucial to comprehend Section 503b of the Federal Food, Drug, and Cosmetic Act. This section delineates the requirements for outsourcing facilities, defined as entities that compound sterile drugs and are subject to FDA oversight. Key components include:
- Definition of Outsourcing Facilities: These facilities are responsible for compounding sterile medications and must adhere to stringent FDA regulations.
- Registration Requirements: Facilities must register with the FDA, providing comprehensive details about their operations and the products they offer. As of 2025, the FDA continues to monitor the number of registered outsourcing facilities, which has remained stable since 2019.
- Compliance with cGMP: Adherence to Current Good Manufacturing Practices (cGMP) is crucial. This encompasses maintaining thorough documentation, implementing robust quality control measures, and ensuring that all personnel receive adequate training. Case studies have demonstrated that successful implementation of cGMP in 503b compounding facilities is crucial for enhancing patient safety and product quality. provides expert solutions in GMP adherence, validation, and engineering, including the development of Standard Operating Procedures (SOPs) and addressing Data Integrity Deviations, ensuring that facilities meet these critical standards.
Furthermore, the FDA has voiced concerns regarding unsanitary conditions noted during inspections of compounding facilities, emphasizing the vital importance of adherence for patient safety. The FDA's oversight statistics indicate that as of July 31, 2024, there were 102 medication shortages being tracked, underscoring the importance of reliable compounding practices in addressing public health needs.
Examining the FDA's guidance documents and resources is essential for a thorough understanding of these regulations, which will establish the basis of your adherence efforts. The FDA has issued multiple guidances on regulatory issues related to compounding, emphasizing the need for ongoing adherence to established standards to mitigate risks associated with compounded medications. Moreover, insights from FDA officials concerning Section 503b requirements can offer valuable perspectives on regulatory expectations and the significance of upholding high standards in compounding practices. Collaborating with AVS Life Sciences can enhance your regulatory strategy, utilizing their established expertise in life sciences consulting.
Assess Current Compliance Practices
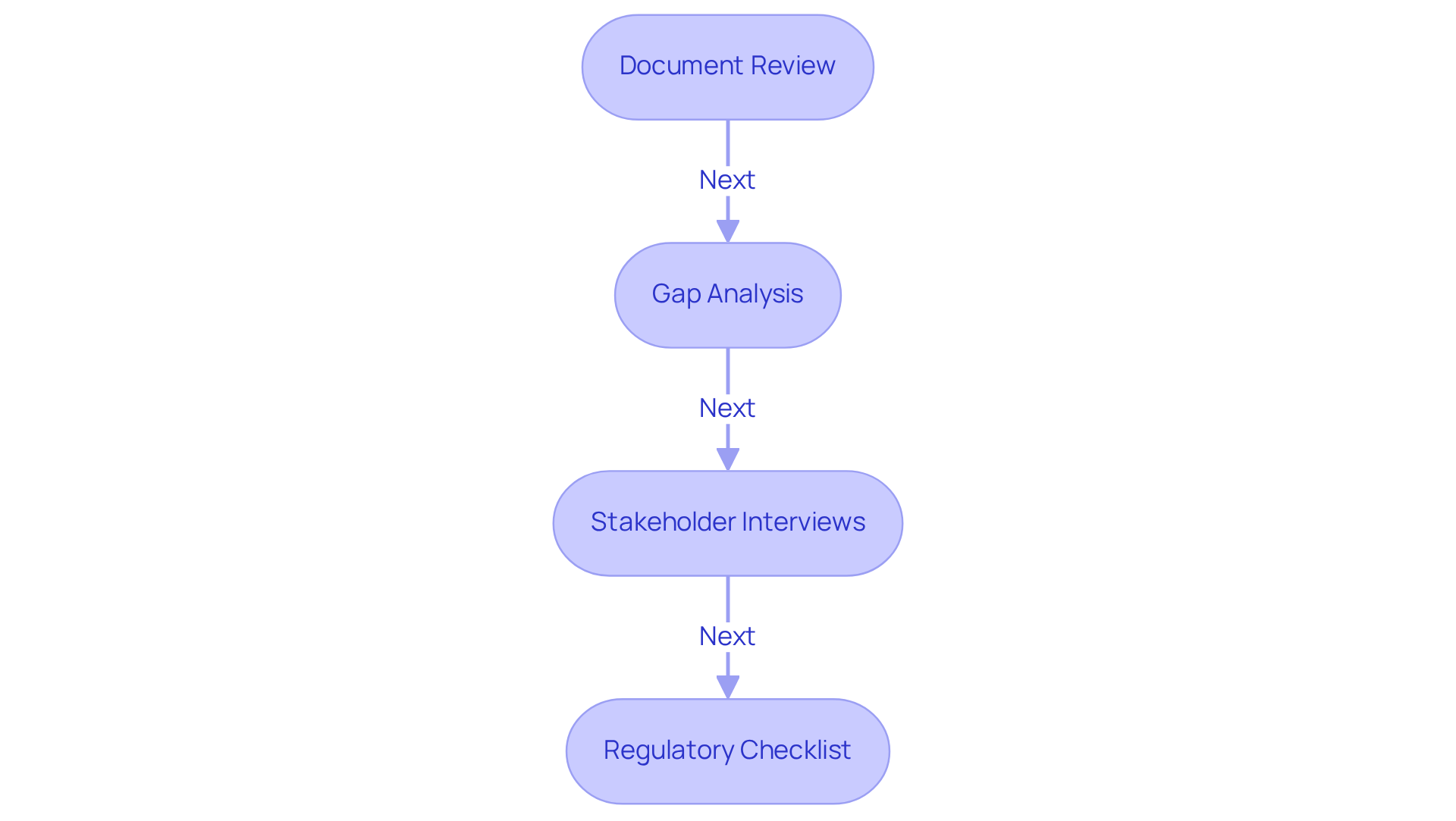
To ensure robust adherence to regulatory standards, a comprehensive assessment of your existing practices is imperative. Begin by following these essential steps to conduct an effective assessment:
- Document Review: Gather all relevant documentation, including (SOPs), training records, and previous audit reports. This foundational step is vital, as the FDA noted 56 violations related to inadequate documentation in 2022.
- Gap Analysis: Conduct a thorough comparison of your current practices against the 503b standards. Identify discrepancies or areas where documentation or procedures fall short. Engaging in an active gap assessment can significantly mitigate risks, as 70% of service organizations reported the necessity to demonstrate adherence to various frameworks in 2023.
- Stakeholder Interviews: Engage with staff members to gather insights on regulatory practices and the challenges they encounter. This step is crucial for grasping the practical implications of regulatory measures and cultivating a culture of transparency.
- Regulatory Checklist: Utilize a regulatory checklist tailored to specific standards to systematically evaluate each aspect of your operations. This organized approach not only clarifies adherence status but also highlights areas needing urgent attention or improvement.
By adhering to these steps, you will acquire a comprehensive understanding of your regulatory status, enabling you to effectively address gaps and enhance your organization's compliance with the relevant 503b standards.
Train Staff on Compliance Standards
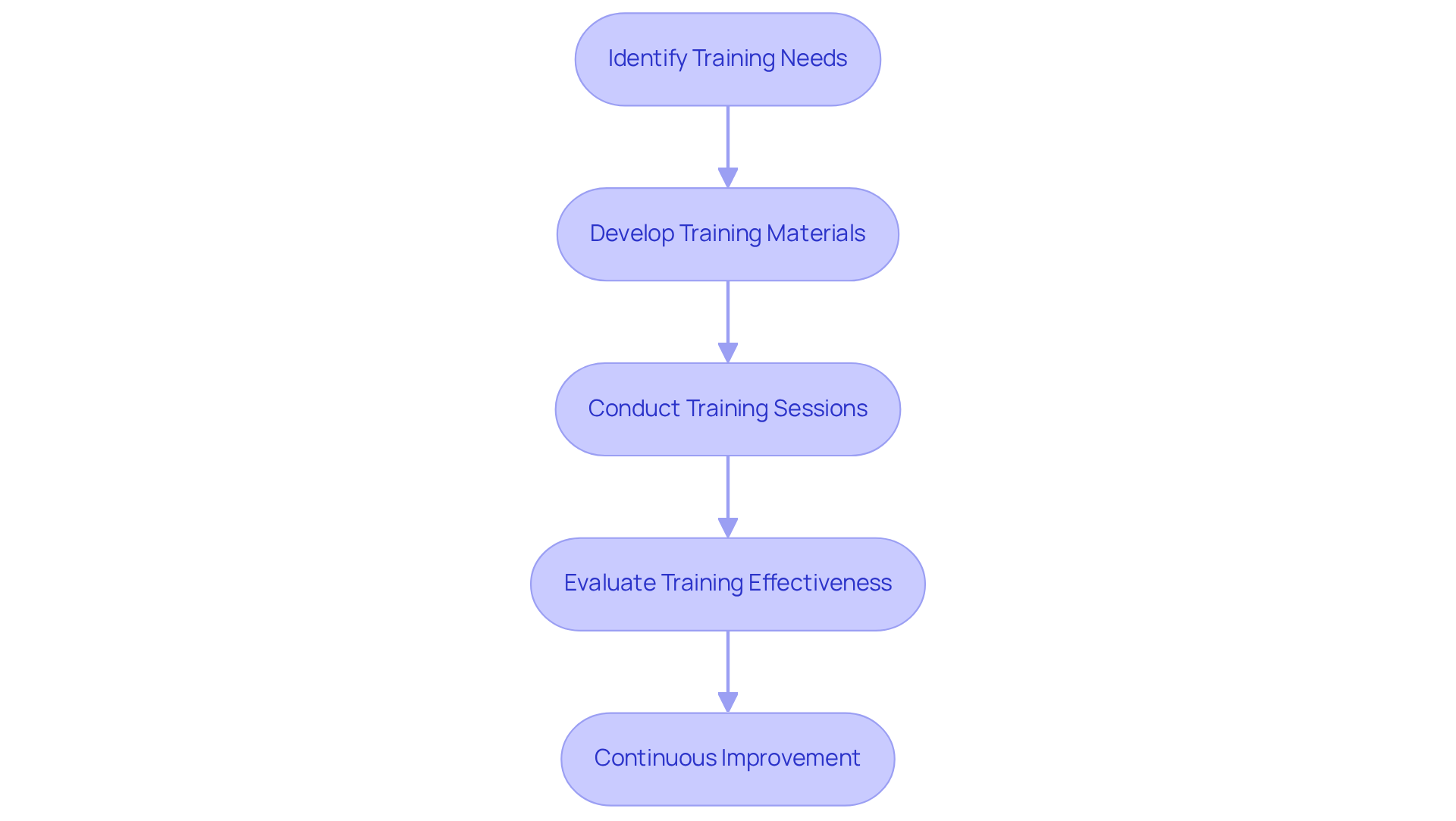
Establishing a comprehensive training program for all personnel involved in compounding activities is essential for maintaining compliance with specific standards. To achieve this, follow these steps:
- Identify Training Needs: Conduct a thorough assessment to identify regulatory knowledge gaps among staff members. This foundational step is vital for tailoring training to address the unique challenges encountered in the pharmaceutical environment.
- Develop Training Materials: Create or procure high-quality training materials that encompass guidelines for 503b, current Good Manufacturing Practices (cGMP) requirements, and internal Standard Operating Procedures (SOPs). Engaging and relevant content significantly enhances retention and understanding.
- Conduct Training Sessions: Schedule regular training sessions that mandate participation from all staff members. Employ a diverse approach that includes presentations, hands-on demonstrations, and assessments to reinforce learning. This multifaceted strategy has been shown to improve knowledge retention significantly, with experiential learning increasing retention rates by up to 75%.
- Evaluate Training Effectiveness: After each training session, assess staff understanding through quizzes or practical evaluations. Collect feedback to refine and enhance future training initiatives. Regular are crucial to keep personnel informed of any changes in regulations or internal procedures, ensuring continuous compliance.
By prioritizing these steps, organizations can foster a culture of adherence and continuous improvement, ultimately leading to enhanced operational results and a reduced risk of regulatory issues.
Establish Monitoring and Auditing Processes
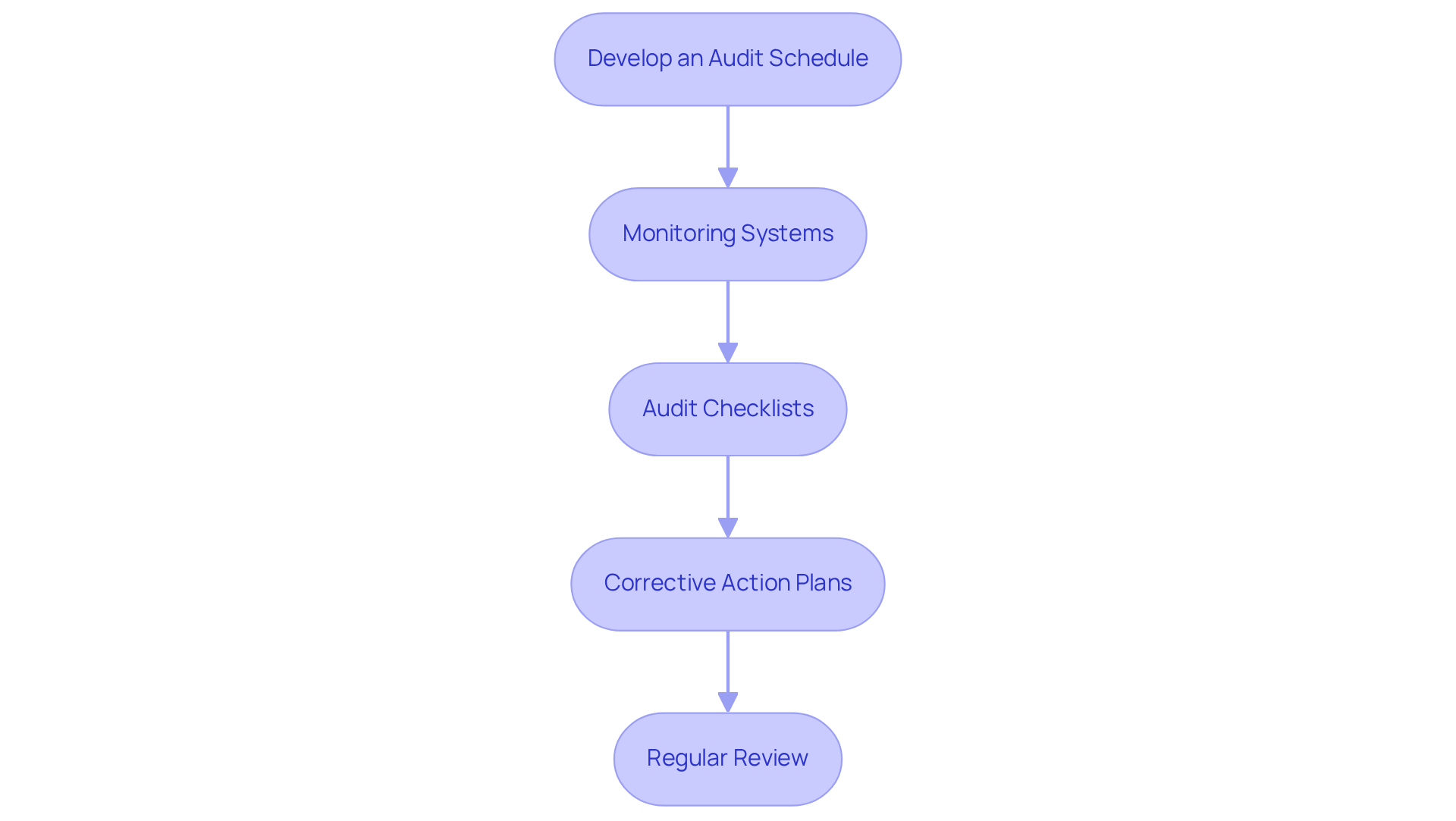
Implementing robust monitoring and auditing processes is essential to ensure compliance with 503b regulations.
- Develop an Audit Schedule: Establish a comprehensive audit schedule that incorporates both internal and external audits. This dual approach enables a thorough evaluation of adherence to and assists in identifying areas for enhancement.
- Monitoring Systems: Create systems for continuous monitoring of critical processes, including compounding procedures, equipment maintenance, and environmental controls. This proactive strategy guarantees that any deviations from regulations are identified early.
- Audit Checklists: Utilize detailed checklists during audits to address all regulatory areas thoroughly. These checklists should include aspects such as documentation, training, and operational practices to ensure thorough evaluations.
- : Following audits, develop corrective action plans to address any identified issues. Clearly assign responsibilities and establish timelines for resolution to ensure accountability and prompt action.
Regularly review and update your monitoring and auditing processes to remain aligned with evolving regulations and operational practices. This continuous improvement enhances compliance and operational efficiency.
Conclusion
Ensuring compliance with 503b regulations in pharmaceutical operations transcends mere regulatory obligation; it is a pivotal element in safeguarding patient health and enhancing the quality of compounded medications. By grasping the intricacies of Section 503b and adopting a structured approach to compliance, organizations can adeptly navigate the complexities of pharmaceutical regulations while cultivating a culture of safety and accountability.
This article delineates four essential steps to achieve robust 503b compliance:
- Understanding the regulatory framework
- Assessing current practices
- Training staff
- Establishing effective monitoring and auditing processes
Each step underscores the necessity of thorough documentation, continuous education, and proactive oversight to identify and rectify compliance gaps. Engaging with resources and expertise, such as those provided by AVS Life Sciences, empowers facilities to elevate their operational standards and align with FDA expectations.
Ultimately, the importance of 503b compliance extends beyond regulatory adherence; it embodies a commitment to patient safety and the integrity of pharmaceutical practices. Organizations are urged to prioritize these compliance steps, not only to fulfill regulatory requirements but to foster a culture of excellence that benefits both staff and patients alike. By embracing these practices, the pharmaceutical industry can ensure the delivery of safe, effective, and high-quality compounded medications to those who depend on them.
Frequently Asked Questions
What is Section 503b of the Federal Food, Drug, and Cosmetic Act?
Section 503b outlines the requirements for outsourcing facilities that compound sterile drugs and are subject to FDA oversight.
What defines an outsourcing facility?
An outsourcing facility is an entity responsible for compounding sterile medications and must comply with strict FDA regulations.
What are the registration requirements for outsourcing facilities?
Outsourcing facilities must register with the FDA, providing detailed information about their operations and the products they offer.
How has the number of registered outsourcing facilities changed since 2019?
As of 2025, the number of registered outsourcing facilities has remained stable since 2019.
What does compliance with cGMP entail?
Compliance with Current Good Manufacturing Practices (cGMP) includes maintaining thorough documentation, implementing quality control measures, and ensuring personnel receive adequate training.
Why is cGMP important in 503b compounding facilities?
Successful implementation of cGMP is crucial for enhancing patient safety and product quality in compounding facilities.
What concerns has the FDA raised regarding compounding facilities?
The FDA has expressed concerns about unsanitary conditions observed during inspections of compounding facilities, highlighting the importance of adherence to regulations for patient safety.
How many medication shortages were being tracked by the FDA as of July 31, 2024?
As of July 31, 2024, the FDA was tracking 102 medication shortages.
What resources does the FDA provide for understanding compounding regulations?
The FDA offers guidance documents and resources that are essential for understanding regulations related to compounding and establishing adherence efforts.
How can AVS Life Sciences assist with regulatory strategies?
AVS Life Sciences provides expertise in GMP adherence, validation, engineering, and developing Standard Operating Procedures (SOPs) to enhance regulatory strategies for compounding facilities.
