4 Steps to Enhance Manufacturing Quality for Compliance Officers

Overview
The article delineates four pivotal steps for compliance officers aimed at enhancing manufacturing quality. These steps encompass:
- Understanding quality management fundamentals
- Establishing quality standards and control processes
- Fostering a quality-driven culture
- Implementing continuous improvement alongside quality audits
Each step is fortified by practical strategies and methodologies, including:
- The definition of excellence standards
- The adoption of continuous improvement practices such as Lean and Six Sigma
- The promotion of employee engagement
Collectively, these efforts are designed to ensure compliance while elevating overall product quality within the manufacturing sector.
Introduction
Manufacturing quality transcends mere regulatory compliance; it stands as the bedrock of operational excellence and customer satisfaction. For compliance officers, mastering the complexities of quality management is imperative to ensure that products not only meet stringent standards but also cultivate a culture of continuous improvement. However, how can organizations adeptly navigate the intricate landscape of quality assurance to not only adhere to regulations but also enhance their overall production processes? This article explores four pivotal steps that empower compliance officers to elevate manufacturing quality, ensuring both conformity to guidelines and the delivery of superior products.
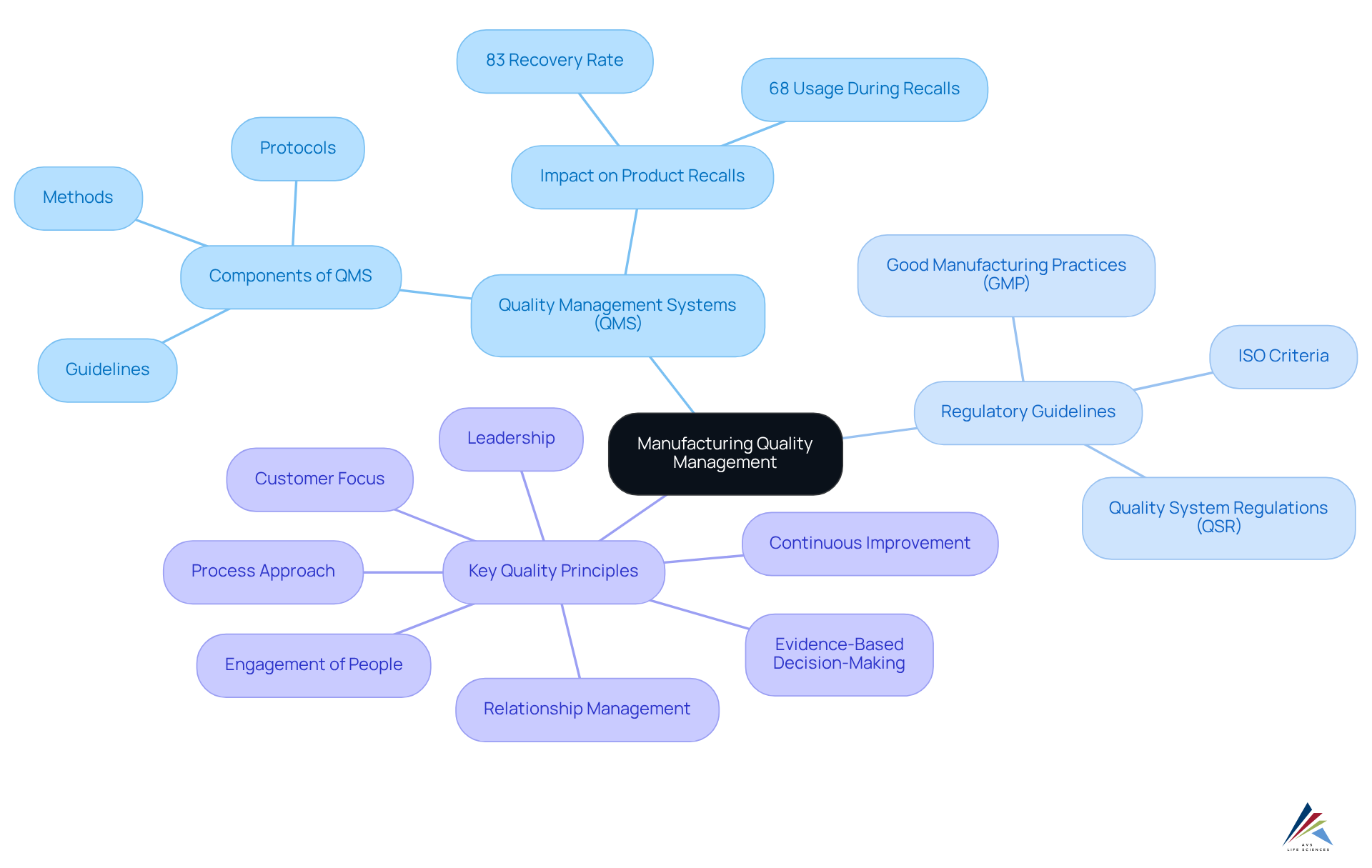
Understand the Fundamentals of Manufacturing Quality Management
To enhance production standards, oversight personnel must grasp the , which are essential for navigating the complexities of the industry. The key components include:
- [Quality Management Systems (QMS)](https://gosteelhead.com/quality-statistics-and-why-your-qms-matters): A comprehensive understanding of the structure and components of a QMS is imperative. This system encompasses guidelines, methods, and protocols designed to ensure product excellence and compliance with regulatory requirements.
- Regulatory Guidelines: Acquaintance with regulations such as [Good Manufacturing Practices (GMP)](https://etq.com/blog/4-stats-that-show-how-quality-management-impacts-product-recalls-2), ISO criteria, and Quality System Regulations (QSR) is crucial. These standards delineate the criteria necessary for production methods and aid organizations in maintaining compliance.
- Key Quality Principles: It is vital to emphasize principles such as customer focus, leadership, engagement of people, process approach, continuous improvement, evidence-based decision-making, and relationship management. These principles guide the development and implementation of effective management strategies aimed at achieving excellence.
Mastering these fundamentals empowers regulatory officers to significantly enhance manufacturing quality, ensuring compliance with regulations and fostering a culture of continuous improvement within their organizations.
Establish Quality Standards and Control Processes
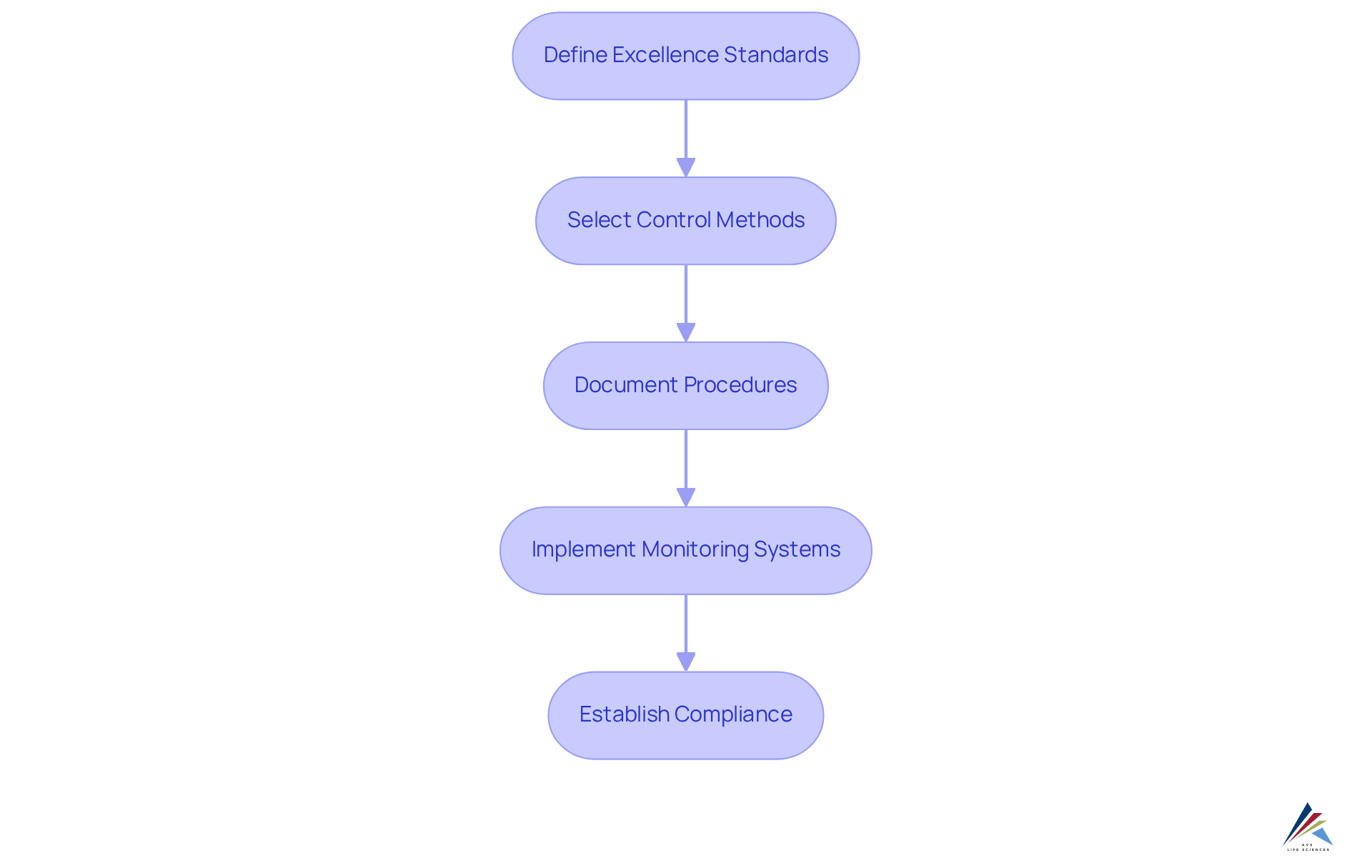
To establish effective quality standards and control processes, compliance officers must follow these essential steps:
- Define Excellence Standards: Clearly identify the specific excellence requirements for your products, aligning them with regulatory guidelines and customer expectations. This encompasses comprehensive specifications for materials, procedures, and final products, guaranteeing that all elements comply with , particularly those defined by GXP and FDA regulations.
- Select Control Methods: Implement suitable control techniques, such as Statistical Process Control (SPC), which utilizes control charts to monitor production processes. This approach assists in recognizing variations that could impact product excellence, ensuring adherence to established standards.
- Document Procedures: Create detailed documentation that describes the processes for standards control. This should encompass inspection techniques, testing procedures, and corrective measures for any non-conformance, facilitating transparency and accountability in management. Following excellent documentation practices and Standard Operating Procedures (SOPs) is essential for upholding regulations and ensuring data integrity.
- Implement Monitoring Systems: Utilize advanced technology and software solutions, such as Process Analytical Technology (PAT), to monitor performance metrics in real-time. PAT is anticipated to improve excellence, safety, efficiency, and automation, facilitating swift recognition of discrepancies from guidelines and permitting prompt corrective measures to uphold adherence and product integrity.
By setting clear criteria for manufacturing quality and implementing strong control procedures, compliance officers can significantly reduce the risk of violations and enhance overall product quality, ultimately leading to safer and more effective pharmaceutical products. Incorporating insights from industry experts can further reinforce the commitment to maintaining high standards in manufacturing.
Train Employees and Foster a Quality-Driven Culture
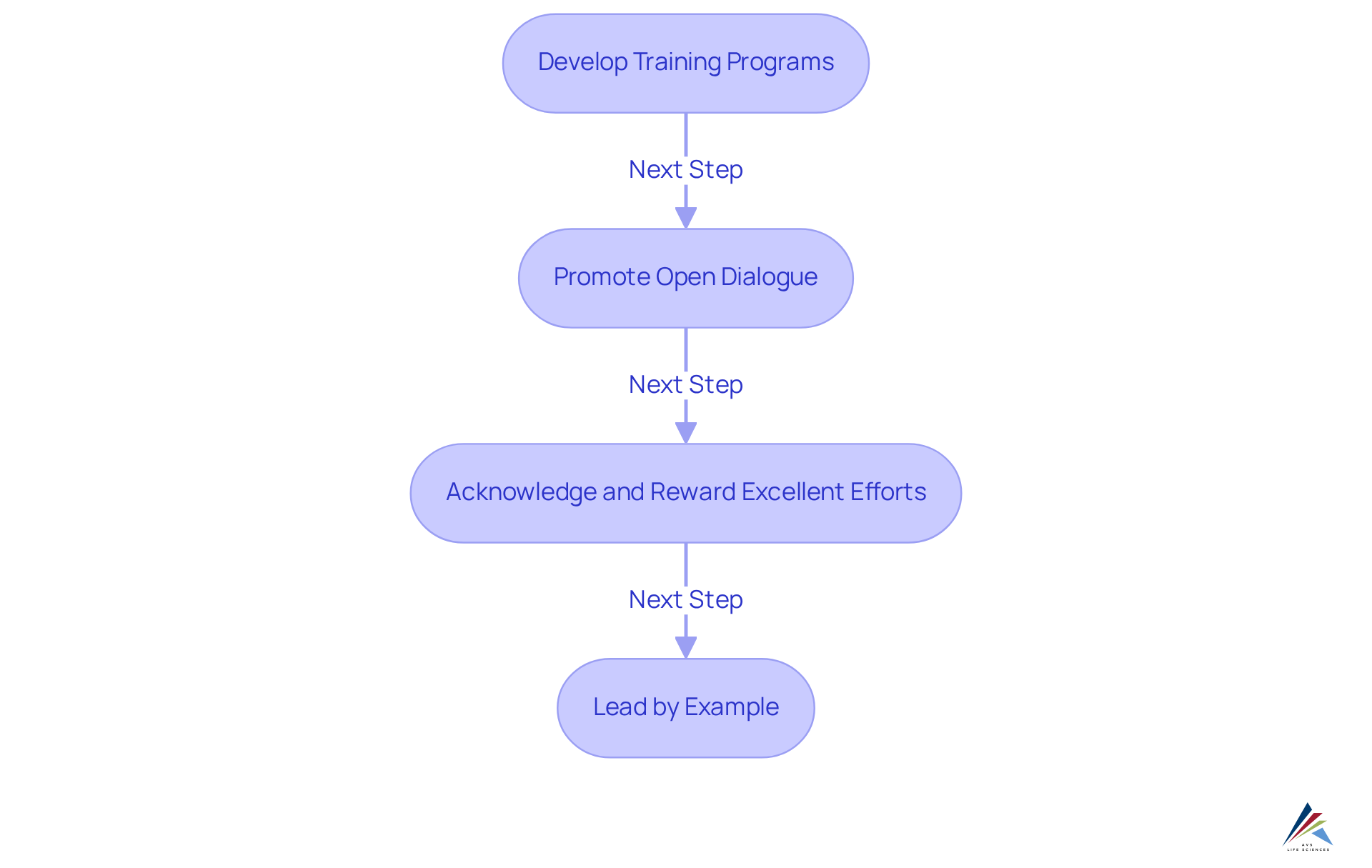
To cultivate a quality-driven culture, compliance officers must undertake the following steps:
- Develop Training Programs: Establish comprehensive training initiatives that encompass management principles, regulatory requirements, and specific control procedures tailored to employees' roles. Given that 70% of abilities are acquired through practical experiences, integrating hands-on training significantly enhances comprehension and application of excellence criteria. AVS Life Sciences offers expert consulting in validation and compliance, ensuring that training aligns with industry standards across biopharmaceuticals and medical devices.
- Promote Open Dialogue: Create an environment where employees feel empowered to voice concerns about standards and propose enhancements. Regular meetings and feedback sessions can facilitate this dialogue, fostering a culture where excellence is a collective responsibility. Research shows that organizations with robust communication practices experience improved operational efficiency and team alignment.
- Acknowledge and Reward Excellent Efforts: Implement recognition programs that celebrate employees' contributions to enhancement initiatives. Acknowledging these efforts underscores the significance of excellence in the workplace and encourages ongoing engagement. Companies that prioritize employee recognition often see higher retention rates, with 94% of employees indicating they would stay longer at a company that invests in their development.
- Lead by Example: Management should exemplify a commitment to excellence by adhering to established standards and actively participating in improvement initiatives. This leadership approach not only sets a positive example but also nurtures a . As W. Edwards Deming stated, 'Quality comes not from inspection, but from improvement of the production process.'
By investing in employee development and cultivating an environment of excellence, organizations can enhance adherence and stimulate continuous improvement, ultimately yielding superior manufacturing quality in the highly regulated life sciences sector. AVS Life Sciences provides essential guidance and resources to support these initiatives.
Implement Continuous Improvement and Quality Audits
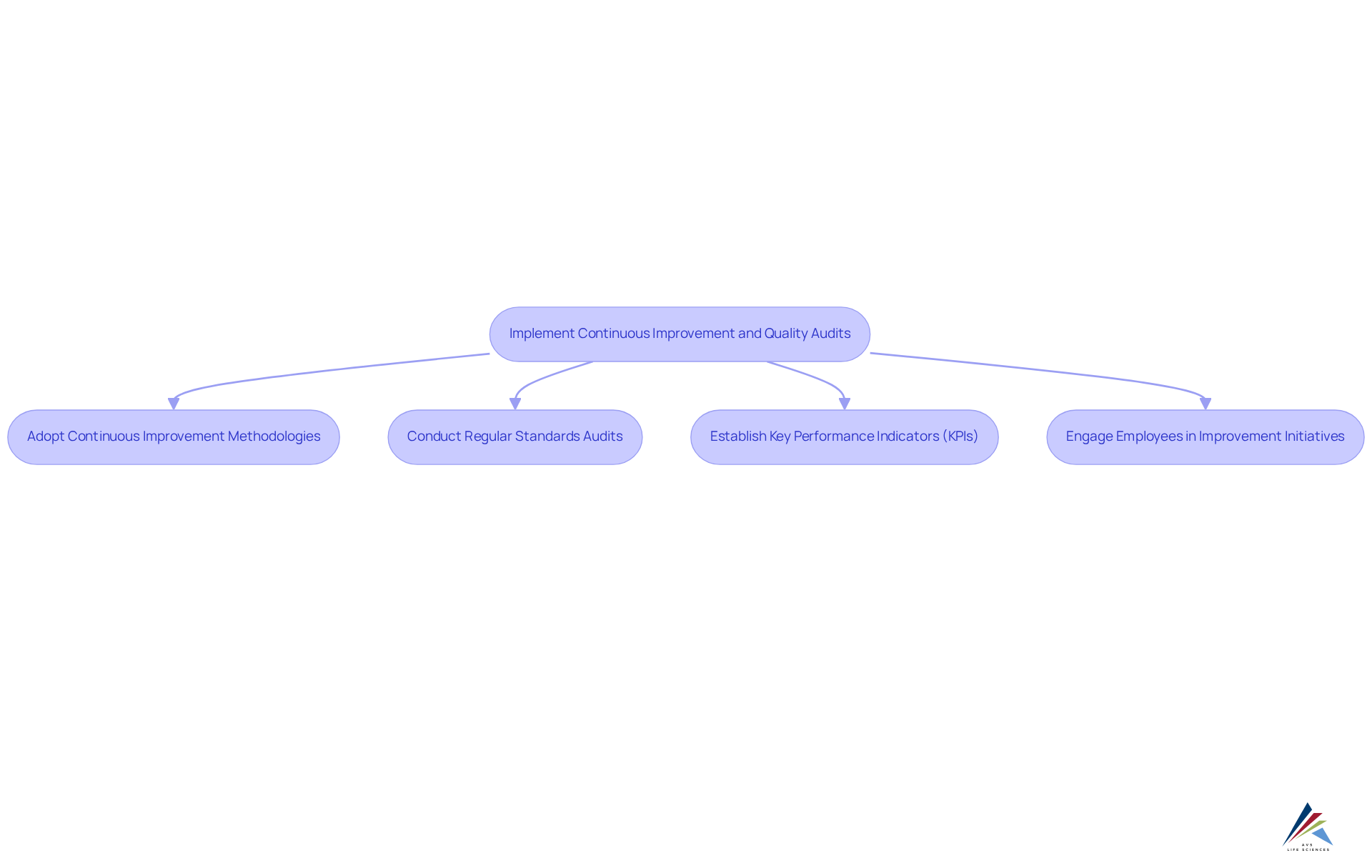
To ensure ongoing quality improvement and compliance, compliance officers must adopt effective strategies:
- Adopt Continuous Improvement Methodologies to enhance manufacturing quality by implementing strategies such as Lean, Six Sigma, or Kaizen to systematically identify and eliminate waste, reduce defects, and improve overall efficiency in manufacturing processes. These approaches can by up to 20% and accelerate time-to-market by 30%. The [AVS Life Sciences case study](https://avslifesciences.com/case-studies/qc-lab-study) exemplifies how these methodologies facilitated a successful upgrade from a Biosafety Level 1 to a Level 2 GMP facility, showcasing their real-world effectiveness.
- Conduct Regular Standards Audits: Arrange and execute routine assessments to evaluate adherence to set criteria and pinpoint opportunities for enhancement. Statistics indicate that organizations performing frequent audits experience a notable decrease in adherence problems, resulting in easier regulatory approvals. The AVS Life Sciences case study emphasizes how comprehensive documentation and assurance practices assisted a client in attaining compliance during their facility upgrade.
- Establish Key Performance Indicators (KPIs): Define KPIs that assess performance standards and monitor progress over time. Regularly examining these metrics aids in recognizing trends and aspects requiring focus, ensuring that standards are consistently achieved. Lessons learned from the AVS Life Sciences project underscore the importance of evaluating business processes to identify gaps that could lead to unreliable test results.
- Engage Employees in Improvement Initiatives: Encourage employees to participate in continuous improvement initiatives by providing training and resources that empower them to contribute ideas and solutions. Engaged employees are more likely to recognize inefficiencies and promote enhancements, fostering a culture of excellence throughout the organization. The collaborative efforts noted in the AVS Life Sciences case study illustrate how open conversations among team members can lead to improved management and accountability.
By implementing these continuous improvement practices and conducting thorough quality audits, compliance officers can ensure that manufacturing quality processes remain compliant and effective. Showcasing examples like the AVS Life Sciences case study illustrates the practical application of these methodologies, enhancing credibility and understanding.
Conclusion
Enhancing manufacturing quality represents a multifaceted endeavor that necessitates compliance officers mastering fundamental concepts, establishing rigorous standards, fostering a culture of excellence, and implementing continuous improvement practices. By grasping quality management systems, regulatory guidelines, and key quality principles, compliance officers are adeptly equipped to navigate the complexities of the industry, driving significant improvements in product quality and compliance.
This article delineates crucial steps, including:
- Defining excellence standards
- Selecting appropriate control methods
- Documenting procedures
- Utilizing advanced monitoring systems
Such actions not only mitigate compliance risks but also ensure that manufacturing processes align with industry standards. Furthermore, investing in employee training and fostering open communication cultivates a quality-driven culture, essential for sustaining high standards in manufacturing.
Ultimately, a commitment to continuous improvement and regular quality audits is vital for maintaining compliance and enhancing manufacturing quality. By adopting methodologies like Lean and Six Sigma, organizations can systematically address inefficiencies and elevate their operational standards. Compliance officers are encouraged to take proactive steps in implementing these strategies, recognizing that the pursuit of excellence in manufacturing quality not only benefits their organizations but also contributes to safer and more effective products for consumers.
Frequently Asked Questions
What is the importance of understanding manufacturing quality management fundamentals?
Understanding manufacturing quality management fundamentals is essential for oversight personnel to enhance production standards and navigate the complexities of the industry.
What are Quality Management Systems (QMS)?
A Quality Management System (QMS) is a comprehensive framework that includes guidelines, methods, and protocols designed to ensure product excellence and compliance with regulatory requirements.
What regulatory guidelines should be familiar to those in manufacturing quality management?
Those in manufacturing quality management should be familiar with regulations such as Good Manufacturing Practices (GMP), ISO criteria, and Quality System Regulations (QSR), which outline the necessary criteria for production methods and help maintain compliance.
What are the key quality principles in manufacturing?
Key quality principles include customer focus, leadership, engagement of people, process approach, continuous improvement, evidence-based decision-making, and relationship management. These principles guide the development and implementation of effective management strategies.
How does mastering these fundamentals impact manufacturing quality?
Mastering these fundamentals empowers regulatory officers to enhance manufacturing quality significantly, ensuring compliance with regulations and fostering a culture of continuous improvement within their organizations.
