4 Steps to Achieve Data Integrity by Design in Pharma

Overview
The article presents a comprehensive approach to achieving data integrity by design within the pharmaceutical industry, articulated through four strategic steps:
- Establishing clear policies
- Conducting training
- Implementing quality controls
- Employing technological solutions
It underscores the necessity of adhering to the ALCOA+ principles while proactively addressing prevalent challenges, such as insufficient training and legacy systems. These measures are essential for ensuring the accuracy and reliability of data, which ultimately supports regulatory compliance and enhances patient safety.
Introduction
In an industry where precision is paramount, the concept of data integrity stands as a cornerstone for pharmaceutical excellence. By embedding reliability into every stage of the drug development process, organizations not only comply with stringent regulations but also safeguard patient safety and product efficacy.
However, achieving data integrity by design presents significant challenges. How can companies effectively implement robust strategies that ensure accuracy and accountability in their information systems?
This guide explores essential steps and best practices for overcoming these hurdles, ultimately fostering a culture of quality and trust within the pharmaceutical sector.
Define Data Integrity in Pharmaceutical Context
In the pharmaceutical sector, ensuring accuracy, consistency, and reliability of information throughout its lifecycle—from development to manufacturing and testing—is essential for achieving data integrity by design. The foundational principles of information integrity, encapsulated in ALCOA+—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available—serve as indispensable guidelines. These principles ensure data integrity by design, guaranteeing that information is not only accurate but also traceable and verifiable, which is crucial for regulatory adherence and the maintenance of high product quality standards.
AVS Life Sciences plays a pivotal role in reinforcing these principles through its comprehensive quality management and regulatory compliance solutions. In the current regulatory landscape, particularly in 2025, data integrity by design is essential to ensure that the information informing decision-making processes is trustworthy and can withstand rigorous scrutiny by regulatory bodies. This unwavering commitment to information reliability, underpinned by AVS Life Sciences' expertise, ultimately supports the pharmaceutical sector's mission to deliver safe and effective products to the market.

Establish the Importance of Data Integrity by Design
Ensuring reliability through data integrity by design is essential in the pharmaceutical sector, as it guarantees that information quality is incorporated into processes from the very start. This proactive strategy significantly reduces the risk of errors and non-compliance, which can have dire consequences for patient safety and product efficacy. By incorporating data integrity by design into the principles of reliability in the design of systems and workflows, organizations promote a culture of quality that highlights precise information collection, processing, and reporting. This method not only assists in meeting regulatory obligations but also enhances overall operational efficiency and the dependability of the information generated.
Statistics show that an absence of information reliability can result in considerable mistakes, emphasizing the need for organizations to implement proactive information reliability strategies. Applying structures like ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available) can assist organizations in developing strong documentation practices, further improving information reliability. Efficient change management is crucial for adjusting to information accuracy demands and ensuring adherence to GXP standards and FDA regulations.
AVS Life Sciences provides extensive services, including Data Integrity Training, GMP Audits and Technical Writing, that assist organizations in attaining quality standards and validation throughout the drug development lifecycle. Entities that emphasize data integrity by design not only enhance compliance but also build trust with stakeholders, ultimately leading to better outcomes in product development and patient care. Moreover, independent information assessment techniques are essential for confirming accuracy and reliability, further improving the quality of the information generated, which is a key focus area for GXP evaluations in life sciences.
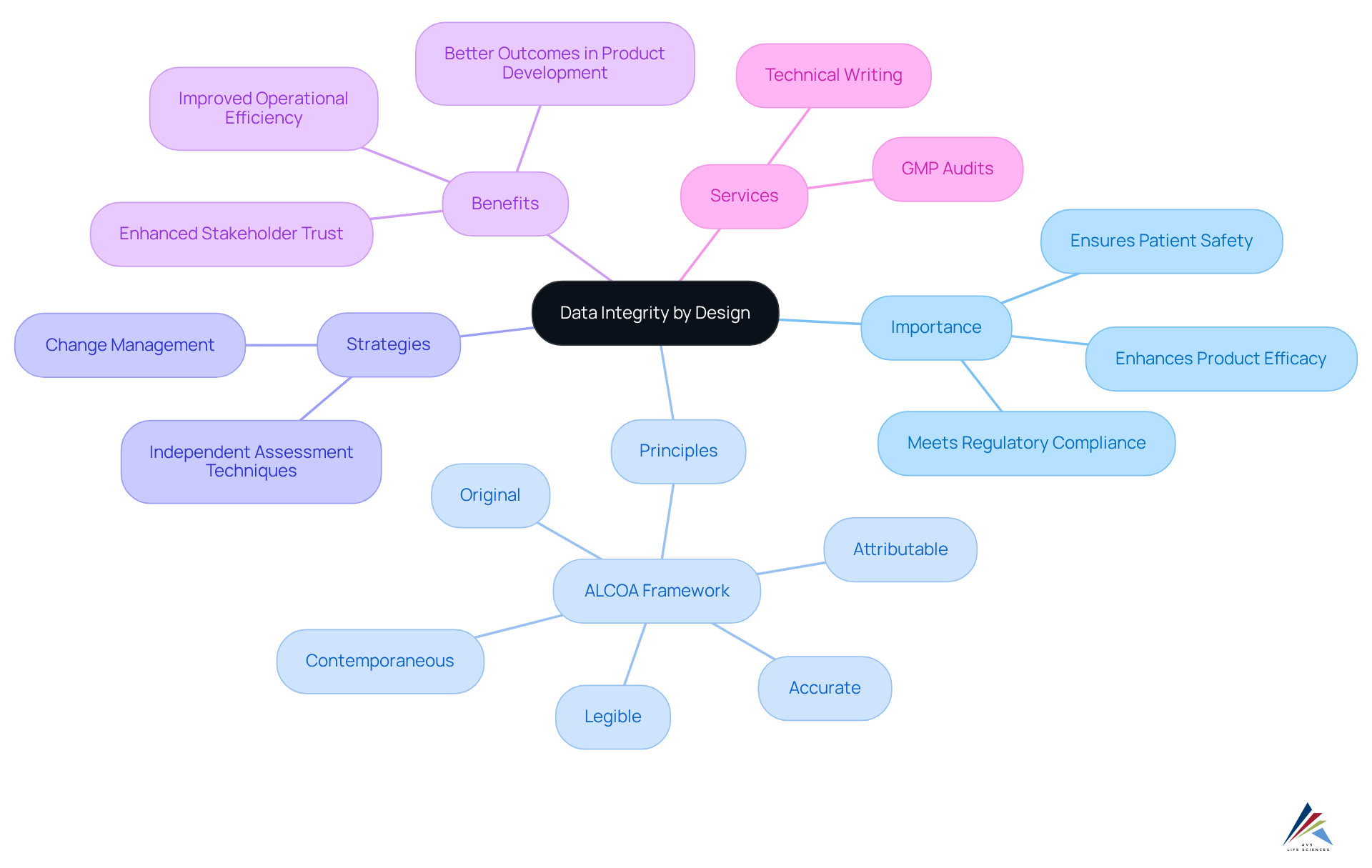
Implement Step-by-Step Strategies for Data Integrity
To implement data integrity effectively, organizations should follow these strategic steps:
- Establish Clear Policies and Procedures: Organizations must create thorough information reliability guidelines that explicitly outline roles, responsibilities, and expectations for handling information. It is crucial to ensure these policies are effectively communicated throughout the organization to promote understanding and adherence. The implementation of robust Computer System Validation (CSV) processes, as outlined in the detailed process checklist for CSV, can significantly enhance these policies by ensuring that software and systems operate as intended.
- Conduct Training Programs: Regularly educating employees on information accuracy concepts and the importance of adherence is essential. This initiative promotes a culture of accountability and increases awareness of the essential role of information reliability in protecting patient safety and ensuring adherence to regulations. As Maria Osipova states, "Data quality is crucial in the pharmaceutical industry, strengthening drug development efficacy, scientific research accuracy, and patient safety." Integrating CSV training can further enable staff to comprehend the significance of validation in preserving information integrity.
- Implement Information Quality Controls: Organizations should utilize automated systems to oversee information entry and processing. Conducting regular audits and validation checks ensures information accuracy and completeness, thereby minimizing the risk of human error and enhancing overall reliability. The stages of CSV, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), offer a structured method for validating quality controls.
- Employ Technological Solutions: Investing in advanced technology that ensures information accuracy, such as electronic lab notebooks and information management systems, is vital. These tools provide crucial audit trails and access controls, enabling thorough information examination and adherence to regulatory standards. The shift from manual record-keeping to advanced digital systems signifies a significant advancement in information management, as demonstrated in AVS Life Sciences' successful enhancement of a biotechnology GMP facility, which emphasized quality assurance and regulatory adherence.
- Regularly Review and Update Practices: It is imperative to continuously evaluate and enhance information quality methods to adjust to changing regulations and technological progress. This involves performing routine risk evaluations to identify possible vulnerabilities and making required modifications to ensure adherence. Current obstacles to information reliability include cybersecurity risks and the necessity for intricate management systems, which must be addressed to guarantee strong adherence. Leveraging insights from case studies, such as AVS Life Sciences' experience in enhancing quality control during facility upgrades, can provide valuable lessons for ongoing improvements.
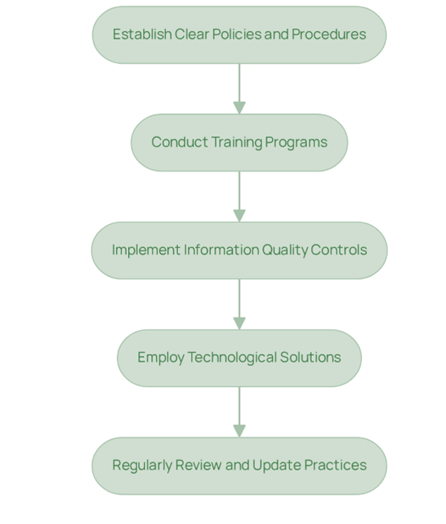
Troubleshoot Common Data Integrity Challenges
Organizations in the pharmaceutical industry frequently encounter significant challenges in maintaining information accuracy. Here are some common issues, accompanied by effective troubleshooting strategies, aligned with AVS Life Sciences' expertise in quality management and regulatory compliance:
- Insufficient Training: A considerable gap exists in employee understanding of information reliability concepts, with statistics indicating that many organizations struggle with training adherence. Solution: Implement regular training sessions and refresher courses, leveraging AVS Life Sciences' comprehensive training programs, to ensure that all staff are well-versed in information quality requirements, thereby fostering a culture of continuous learning.
- Legacy Systems: Outdated systems often fail to meet modern information reliability standards, leading to potential regulatory risks. Solution: Conduct a thorough evaluation of existing systems and prioritize upgrades through AVS Life Sciences' consulting services to align with current compliance requirements, ensuring robust audit trails are established.
- Poor Documentation Practices: Inconsistent documentation can severely compromise the reliability of information. Solution: Standardize documentation practices across the organization, utilizing AVS Life Sciences' quality management solutions to guarantee that all entries are complete, accurate, and timely, which is crucial for upholding high-quality standards.
- Lack of Access Controls: Unauthorized access poses a significant threat to information accuracy. Solution: Implement stringent access controls and regularly assess user permissions, guided by AVS Life Sciences' regulatory frameworks, to ensure that only authorized personnel can modify sensitive information.
- Failure to Conduct Regular Audits: In the absence of regular audits, issues related to information reliability may remain undetected. Solution: Establish a consistent review schedule to evaluate adherence to information accuracy guidelines, utilizing AVS Life Sciences' audit services to identify areas needing improvement and ensuring ongoing compliance with best practices.
By proactively addressing these challenges and incorporating AVS Life Sciences' solutions, organizations can significantly enhance their information reliability frameworks, ultimately leading to improved compliance, operational efficiency, and ensuring data integrity by design. Embracing ongoing improvement practices and modern technology, such as electronic signatures and audit trails, will further enhance data integrity by design efforts.
Conclusion
Ensuring data integrity by design in the pharmaceutical industry is paramount for maintaining the accuracy, consistency, and reliability of information throughout the entire lifecycle of drug development. By adhering to the foundational principles of ALCOA+—Attributable, Legible, Contemporaneous, Original, and Accurate, Complete, Consistent, Enduring, and Available —organizations can embed reliability into their processes from the outset, ultimately safeguarding patient safety and enhancing product quality.
The article outlines a comprehensive approach to achieving data integrity, emphasizing the importance of:
- Establishing clear policies
- Conducting regular training
- Implementing quality controls
- Leveraging technology
- Continuously reviewing practices
Each of these strategies contributes to a culture of quality that not only meets regulatory obligations but also builds trust with stakeholders and improves operational efficiency. Addressing common challenges such as insufficient training, outdated systems, and poor documentation practices is essential for maintaining high standards of information reliability.
In conclusion, the commitment to data integrity by design is not merely a regulatory requirement; it is a fundamental aspect of delivering safe and effective pharmaceutical products. Organizations must take proactive steps to enhance their data integrity frameworks, embracing modern technologies and best practices to navigate the complexities of the industry. By prioritizing these efforts, the pharmaceutical sector can ensure that the information guiding critical decisions is both trustworthy and compliant, ultimately leading to better outcomes for patients and the market as a whole.
Frequently Asked Questions
What is data integrity in the pharmaceutical context?
Data integrity in the pharmaceutical sector refers to the accuracy, consistency, and reliability of information throughout its lifecycle, from development to manufacturing and testing.
What are the foundational principles of data integrity?
The foundational principles of data integrity are encapsulated in ALCOA+, which stands for Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available.
Why are the ALCOA+ principles important?
The ALCOA+ principles are important because they ensure that data is not only accurate but also traceable and verifiable, which is crucial for regulatory adherence and maintaining high product quality standards.
How does AVS Life Sciences contribute to data integrity?
AVS Life Sciences reinforces data integrity principles through its comprehensive quality management and regulatory compliance solutions.
Why is data integrity by design essential in the current regulatory landscape?
Data integrity by design is essential to ensure that the information informing decision-making processes is trustworthy and can withstand rigorous scrutiny by regulatory bodies, especially in 2025.
What is the ultimate goal of ensuring data integrity in the pharmaceutical sector?
The ultimate goal of ensuring data integrity is to support the pharmaceutical sector's mission to deliver safe and effective products to the market.
